Abstract
Nociception is a fundamental acute protective mechanism that prevents harm to an organism. Understanding the integral processes that control nociceptive processing are fundamental to our appreciation of which cellular and molecular features underlie this process. There is an extensive understanding of how sensory neurons interpret differing sensory modalities and intensities. However, it is widely appreciated that the sensory neurons do not act alone. These work in harmony with inflammatory and vascular systems to modulate pain perception. The spinal cord has an extensive interaction with the capillary network in the form of a blood spinal cord barrier to ensure homeostatic control of the spinal cord neuron milieu. However, there is an extensive appreciation that disturbances in the blood spinal cord barrier contribute to the onset of chronic pain. Enhanced vascular permeability and impaired blood perfusion have both been highlighted as contributors to chronic pain manifestation. Here, we discuss the evidence that demonstrates alterations in the blood spinal cord barrier influences nociceptive processing and perception of pain.
Keywords: Pain, Endothelial, Spinal cord, VEGF, Vessel, Permeability
1. Introduction
Nociception and the development of chronic pain states are inherently dependent upon the generation, transmission and modulation of sensory information by the somatosensory system. Pain is a fundamental process for all organisms and acts as a dynamic protective mechanism to prevent harm to tissues whilst allowing us to understand our surrounding environment. Unfortunately, these sensory neuronal systems are sensitive to manipulation, with alterations in their function within the peripheral and/or central nervous system responsible for the manifestation of long lasting, inescapable, chronic pain. This sensory neuronal plasticity is key to understanding how pain perception is controlled. Despite extensive investigations into the sensory neural circuits involved in controlling these nociceptive processes, our understanding of how our pain perception transitions to pathological chronic pain states still remains elusive.
The somatosensory nervous system is not only a neuronal system but is a dynamic heterogenic population of differing cell types that work in harmony to control neurological processes. These include resident glia, an extensive vascular network and interactions with the immune system. Recent evidence highlights that remodelling of the capillary function and structure within the somatosensory system is culpable for alterations in sensory neuronal function, pain perception and the transition to chronic pain states. Here we will explore this sensory neuro-vascular interaction by focussing upon the role of the blood spinal cord barrier (BSCB) in modulating the somatosensory nervous system and exploring the evidence that highlights how changes in vascular dynamics can lead to chronic pain states.
2. Structure and role of the blood spinal cord barrier
The BSCB is an integral regulator of the microenvironmental homeostasis within the spinal cord. The spinal cord is an essential system that is segregated into discrete neural centres, the dorsal and ventral horns, which dictate the physiological processes associated with somatosensation and motor function. To match the demands of these fundamental physiological processes, the vascular system is required to deliver a blood supply to the spinal cord that matches the required energy expenditure of the nervous system. The spinal cord blood supply is provided via large arterial vessel inputs, posterior and anterior spinal arteries as well as posterior and anterior spinal veins (Kim et al., 2019). However, these conduit vessels only make up a proportion of the vasculature in the spinal cord, with the capillary bed acting as the distribution network between these large vessels (Fig. 1A). Importantly, capillaries play a vital role in maintaining a continuous blood supply to conserve spinal cord neuronal integrity and function, roles which are impacted during times of pathology leading to sensory neurological complications. These functions include regulation of vascular permeability and resulting accessibility to the spinal cord parenchyma and maintenance of spinal cord blood supply. Thus the effective function of the spinal cord is dependent upon the uninterrupted provision of the required nutritional support and protection from potential harmful entities. This is provided by the BSCB that regulates the movement of cells and solutes from the blood to the spinal cord tissues. This process is controlled by structures and mechanisms that mediate trans- and paracellular diffusion using a highly refined filtration system that is regulated by differing intercellular junctional proteins as well as a glycocalyx layer on the luminal surface (Noble et al., 1996; Kutuzov et al., 2018).
Fig. 1.
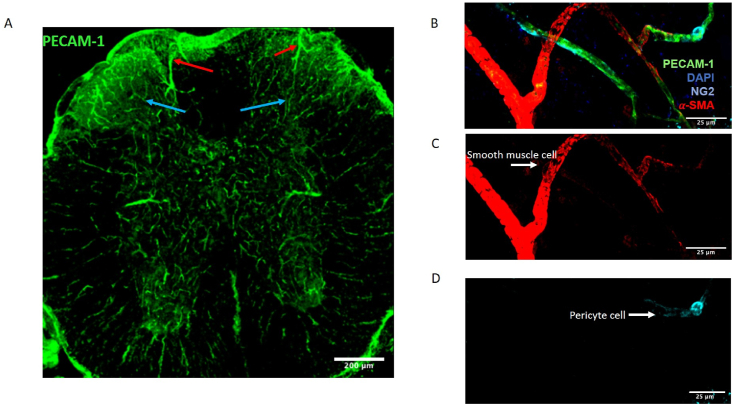
Blood vessel network within the mouse spinal cord
The mouse lumbar spinal cord has a vast blood vessel network that covers the entirety of the neural tissue. [A] This is represented by the vessel endothelium (CD31 labelled, green). This includes differing of hierarchical order of vessels, including larger arterioles (Red arrow) that flow into smaller microvessels (Blue arrow). [B] Higher magnification (scale bar = 25 μm) image of this vessel organisation (endothelium labelled Green with CD31) in the spinal cord. [C] Larger arterioles are labelled with alpha smooth muscle actin (α-SMA, Red), with smaller capillaries solely labelled with endothelial marker CD31. [D] Furthermore, mural cells are identifiable with pericyte marker neuron-glia marker 2 (NG2, Blue) highlighting pericyte cells encapsulating only the capillaries. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The protective nature of the Blood Brain Barrier (BBB) and the BSCB was first discovered by Goldmann and Ehrlich who demonstrated that the BBB and the BSCB prevented penetration of varying solutes of differing molecular weights (Saunders et al., 2014). Their work was subsequently validated by others through varying methodological approaches (Ved et al., 2018; Beggs et al., 2010). The BSCB is a biological interface residing between the spinal cord neural tissue and blood supply, controlling the exchange of solutes such as nutrients, oxygen and provides tissue protection by preventing access of foreign entities including pathogens and pharmacological agents. The BSCB differs to other physiological tissues (e.g. the kidney) that typically consist of fenestrated capillaries such as the kidney Bowman's capsule, to enable effective vascular permeability and filtration (Morita and Miyata, 2012). The BSCB differs in that it is a continuous capillary with no fenestrations formed between the endothelial cells that make up the lumen of the capillary, minimising BSCB permeability (Morita and Miyata, 2012) (Fig. 2A). In addition, the intercellular junctions between neighbouring endothelial cells are highly interactive with junctional and surface proteins. These include vascular endothelial cadherin (VE-CAD) and platelet endothelial cell adhesion molecule 1 (PECAM1 or alternatively referred to as CD31) forming interactions with tight junctional proteins e.g. zona occluden 1 (ZO1), Claudins and Occludin (Occ), to control the adhesion and passage of blood constituents (e.g. solutes, cells) across this barrier. The importance of these junctional and adherence proteins play in maintaining the BSCB is demonstrated by the loss of these junctional proteins, impacting upon the integrity and function of the BSCB, and presented typically as an increase in permeability (Nitta et al., 2003). Furthermore, a reduction of claudin 5 has been shown to induce a reduction in tight junctional proteins in the CNS to enable CNS targetted therapeutic intervention (Dithmer et al., 2017). Maintenance of the vascular integrity is mediated by an array of angiogenic modulators that include, though not restricted to, the Vascular Endothelial Growth Factor (VEGF) family of proteins, fibroblast growth factors and angiopoietin 1, to influence endothelial cell function (Mukwaya et al., 2021). These angiogenic molecules drive proangiogenic processes that initiates endothelial cell proliferation and sprouting, which further leads to elevations in vascular permeability. The extensive nature of the archetypal angiogenic family; VEGF-A protein(Moens et al., 2014) is multifactorial with differing protein variants, having differences in cellular and physiological function (Bates et al., 2018). Fluctuations in VEGF-A, a master regulator of angiogenic function, expression is associated with distinct endothelial cell activity relating to physiological function and pathogenesis(Fallah et al., 2019). These signal predominantly through VEGFR2 to influence BSCB vascular integrity, a receptor found to be expressed in the spinal cord (Glaesel et al., 2020). Elevated levels of the proangiogenic VEGF-A xxxa isoform family within the central nervous system increases vascular permeability and cellular infiltration in part through downregulation of tight junctional proteins (Ved et al., 2017) and increase in adhesion molecules(Thichanpiang et al., 2014). This is typified by application of exogenous VEGF-A165a leading to increased abundance of evans blue within the spinal cord compared to control (Ay et al., 2008). In addition, these junctional proteins and adhesion molecules are highly susceptible to degradation upon exposure to proteinase activity from metalloproteinases(Zhang et al., 2017). Metalloproteinases play an important role in the formation of the architecture of tissues as well as contributing to a number of cellular processes such as angiogenesis(Gu et al., 2019), which occur in the spinal cord and are highly associated to the manifestation of chronic pain (Gu et al., 2019). The presence of metalloproteinases have been shown to support the adhesion and infiltration of neutrophils through the breakdown of associations between junctional proteins (Zhang et al., 2017; Lee et al., 2014) as well as increasing endothelial cell expression of immunological adhesion molecules such as Intercellular Adhesion Molecule 1 (ICAM1) (Montague-Cardoso et al., 2020a). This loss of tight junctional proteins is heavily implicated in neuroinflammatory processes of the CNS due to influx of inflammatory cell types that have been heavily associated with a number of neurological disorders. This includes chronic pain, multiple sclerosis and amyotrophic lateral sclerosis(Weiss et al., 2009).
Fig. 2.
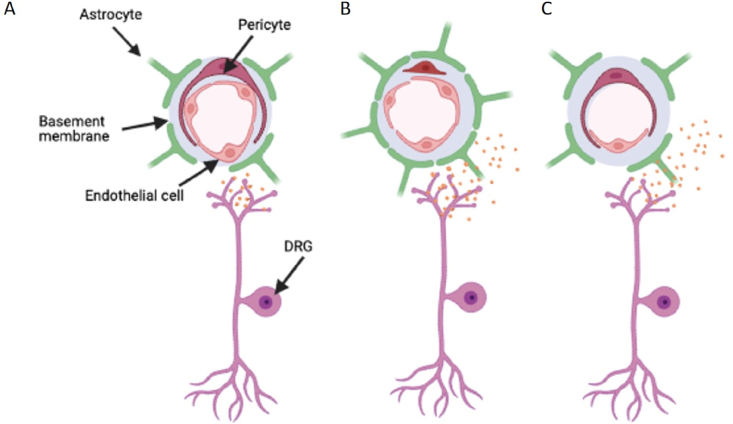
Disturbances in the blood spinal cord barrier
The blood spinal cord barrier (BSCB) plays an essential role in monitoring the spinal cord microenvironment. [A] The capillary network consists of the endothelial cells that form the lining of the vessel lumen, controlling passage of varying solutes and cells through into the spinal cord via stringent filtering processes that line the endothelium (Glycocalyx and tight junctional proteins). Additionally, these capillaries interact with surrounding mural cells, pericytes and astrocytes, to further regulate the BSCB integrity and function. [B] Enhanced vascular permeability is strongly associated with increases in the inflammatory profile of the spinal cord during chronic pain ie inflammatory pain and traumatic sensory nerve lesion. This is typified by the opening of interendothelial cell junctions due to diminished tight junction expression. Furthermore, astrogliosis, activation of dorsal horn astrocytes, accompanies an enhanced interaction with the endothelium. Upregulation of astrocytic foot processes depicted by elevated GFAP expression influence BSCB permeability through diminished expression of tight junctional proteins and elevated infiltration of cells and solutes. Similarly, damage to the peripheral C fibre nociceptors ie through traumatic nerve injury induces enhanced BSCB permeability through increased release of neurogenic inflammatory mediators. [C] Conversely, the endothelium degenerates, presented as a reduction in endothelium and the number of blood vessels. This is associated with reduced tissue perfusion of the spinal cord. This is associated with cytotoxicity of neuropathological conditions such as hyperglycaemia, driving endothelial cell death.
Despite the impermeable nature of the BSCB, transport mechanisms still reside to allow transfer of fundamental nutrients across this surface with passive diffusion previously discussed. However, this will only allow the movement of small molecules, therefore larger molecules require additional assistance via channel mediated transport. As highlighted, the passive nature through the BSCB is regulated by intercellular junctional proteins but there are transport proteins such as glucose transporter 1 (GLUT1) that are present to enable the efficient delivery of essential larger molecular weight nutrients(Winkler et al., 2015). Additionally, as part of this channel mediated system, efflux mechanisms are also found in the BBB and BSCB on the luminal surface of the capillary, to further refine the selectivity of the BSCB to the penetration of varying solutes (Campos et al., 2012). These efflux mechanisms utilise transporters to further minimise the accessibility of the neural tissues by exporting agents that penetrate the CNS. The expression of these efflux transporters impairs delivery and accumulation of differing agents including pharmacological treatments for neurodegenerative conditions(Foran et al., 2016). These efflux transporters include ATP binding cassettes such as P-Glycoprotein (P-gp) and multi drug resistance protein (MRP) (Campos et al., 2012; Wang et al., 2014). The increased expression of these efflux transporters enhances the BSCB's ability to pump out and thus decreasing the abundance of those substances in the spinal cord (Wang et al., 2014; Jablonski et al., 2012). This obviously hinders pharmacological intervention when targeting the CNS or specifically the spinal cord. In an attempt to overcome this limitation, delivery approaches have utilised combination therapy to suppress P-gp efflux activity, whilst simultaneously administrating treatments to act upon the spinal cord tissues such as to treat spinal muscular atrophy (Foran et al., 2016) and multiple sclerosis (Jablonski et al., 2012). This approach minimises the amount of the pharmacological agent pumped out of the spinal cord enabling effective actions of the treatment upon the tissue.
In addition to the extensive role that the endothelium plays in acting as blood delivery conduit to the spinal cord, it also acts as a gatekeeper, controlling the passage of differing blood constituents including immune cells and solutes to the spinal cord parenchyma. These processes are only efficient due to the interaction with several other auxiliary cell types that similarly contribute to the maintenance of the spinal cord neural tissue. These cell types include astrocytes, smooth muscle cells and pericytes as well as in instances the resident glia and circulating immune cell complement (Li et al., 2017; Winkler et al., 2012) (illustrated in Fig. 1B–D and Fig, 2A). Smooth muscle cells and pericytes make up the mural cell grouping that support the BSCB networks. The locality of these specialised cells covers the hierarchal order of the macrovascular and microvascular system that is used to regulate function and regulate the structure of the BSCB (Fig. 1B–D). Smooth muscle cells (SMC) ensheath arterial vessels controlling blood flow and haemodynamic capabilities through utilising contractile proteins e.g. alpha smooth muscle actin (αSMA) and desmin, to regulate vessel diameter and therefore arterial blood perfusion. Pericytes predominate in the capillary networks and have a multi-dynamic role in regulating microvascular stability and function. Pericytes (pericyte positive labelling includes Neural glial antigen 2 (NG2 or CSPG4); Platelet derived growth factor receptor β (PDGFRβ) (Berthiaume et al., 2018)) are widely characterised as having a ‘bump on a log’ structure, comprising of a cell soma with long processes extending out wrapping around the endothelium (Berthiaume et al., 2018). The role of the pericyte is integral for the maintenance of the capillary structures such as the BBB. Pathogenic loss or chemical ablation of the pericyte population leads to a disintegration of the BSCB (Sauer et al., 2017) and BBB (Nikolakopoulou et al., 2019) capillary networks and is associated with the initiation of neurodegenerative processes including Alzheimer's disease (Kisler et al., 2017). Despite the view of distinct locality as well as molecular and functional clustering of these cellular populations, there is conjecture around defining the role of the pericyte. SMC function is to control vessel perfusion through manipulating vascular lumen diameter. However, it has been identified that pericytes not only possess a paracrine endothelial survival function(De Palma et al., 2005) but also modulate perfusion of the capillaries via vessel contractility capabilities (Li et al., 2017) through the expression of comparative contractile proteins such as αSMA (Peppiatt et al., 2006).
Glia have classically been known as the ‘glue’ supporting the structure of the nervous system. The spinal cord is made up of many cells that contribute to the vascular architecture, forming the BSCB. Over the last few decades, the complex roles glia have in maintaining CNS homeostasis have been ascertained. There are three major classes of glial cells: microglia, originating from the haematopoietic system(Sun et al., 2019); oligodendrocytes that produce myelination (Gritsch et al., 2014); and astrocytes that comprise part of the BSCB (Gao et al., 2010; Linnerbauer et al., 2020). The BSCB consists of the capillary lumen formed from endothelial cells surrounded by the basement membrane and the pericyte that encapsulates the endothelial cells. Astrocytes act in a supportive role, interacting with both neuronal and vascular systems. Astrocytes are thought to interact extensively with neuronal soma (Gao et al., 2010), whilst supplying fundamental resources such as metabolites essential for neurotransmitter synthesis to maintain efficient neurological function. Astrocytes typically extend radial processes - astrocyte endfeet, commonly attributable to aquaporin 4 (AQP4) and potassium channel proteins (e.g. Kir 4.1) (Lu et al., 2020), which interact extensively with their surroundings such as with the endothelium. Considering their involvement in the neurovasculature, astrocytes have been suggested to play major roles in the pathogenesis of neuropathic pain. Furthermore, astrogliosis and astrocytic activation in the CNS have been attributed to alterations in BSCB function (Gordh et al., 2006). These alterations include the hypertrophy of cell bodies and processes, as well as upregulation of the astrocyte specific cytoskeletal protein glial fibrillary acidic protein (GFAP), which is indicative of astrocyte activity (Gordh et al., 2006; Giannoni et al., 2016). The current understanding of astrocyte involvement in chronic pain is limited and needs further investigation.
2.1. Experimental approaches for investigating the BSCB
Appreciation of the BSCB in relation to healthy physiological function and structure is integral prior to understanding of how CNS disease alters the BSCB. Investigating the BSCB is a complex undertaking due to the heterogenic cellular nature of this system, whilst also considering the difficulties in accessing the BSCB due to it being embedded in the spinal vertebra. To allow mechanistic evaluation, numerous in vitro approaches have investigated single cell type or co-culture setups that incorporate differing aspects of the BSCB. In vitro assays designed to interrogate endothelial cell activity have been applied to BSCB endothelial cells(Watson PM Thom et al., 2013). Watson et al. explored a monolayer of BSCB endothelial cells through evaluating tight junctional proteins using fluorescent immunocytochemistry, whilst measuring functional measures of tight junctions in monolayer endothelial permeability via transwell fluorescent tracer permeability and transepithelial/transendothelial electrical resistance (TEER) (Watson PM Thom et al., 2013).
In vivo monitoring of BSCB integrity is highly important to understand the progression of many diseases. One of the most common methods to investigate BSCB integrity in vivo is through the use of fluorescent tracers. Sodium fluorescein (SF) and Evans Blue (EB) are the most used tracers and can be used to measure solute abundance in tissues (Ved et al., 2018), other methods include histological evaluation using light or fluorescence microscopy (Xanthos et al., 2012) or intravital imaging of the spinal cord (Farrar et al., 2012). Previously, Xanthos et al. used a variety of techniques to explore changes in the BSCB including techniques involving the measurement of IgG accumulation, monitoring levels of Occludin protein levels and the evaluation of fluorescent dyes including EB and SF (Xanthos et al., 2012). More recently a study by Sauer et al. (2017) used these tracers to evaluate BSCB after chronic constriction injury(Sauer et al., 2017). Similarly, another class of solute permeability tracer includes protein luciferases and are used in the same way as EB and provide very similar data on BSCB permeability. Once injected the luciferin is oxidised leading to the emission of light that can be imaged using fluorescence microscopy. Radiolabelled tracers such as [14C]-alpha-aminoisobutyric acid, [3H]-D-mannitol and [14C]-carboxyl-inulin (Prockop et al., 1995; Popovich et al., 1996) have also been utilised in variety of studies investigating the BSCB. Once the radiolabelled substrate is administered the levels of isotope activity can be monitored and correlated between serum and the CNS.
Non-invasive techniques are also readily used to investigate BSCB integrity. The majority of these techniques involve paramagnetic contrast agents and magnetic resonance imaging (MRI). These small molecular weight agents (e.g. gadolinium) are administered through intravenous injection and can infiltrate damaged BSCB, with dynamic contrast-enhanced MRI used to image and evaluate the integrity of the BCSB. Cohen and colleagues used a similar method to evaluate spatial and temporal alterations in the BSCB permeability in an experimental model of spinal cord injury (Cohen et al., 2009) and autoimmune encephalomyelitis (Schellenberg et al., 2007). These processes typically provide a presentation of a neuropathology, but only in relation to a snapshot of time. An example of in vivo methodologies to evaluate BSCB function and integrity is intravital microscopy (IVM) of the spinal cord(Farrar et al., 2012). This method allows the surveillance of cell or tissue specific biological processes in live animals at a high resolution (Masedunskas et al., 2012). IVM has been utilised with real-time epifluorescence (EPI-IVM) (Sathiyanadan et al., 2014) and two-photon laser scanning microscopy (2P-IVM) (Locatelli et al., 2018) and necessitates surgical window preparations. Dray and colleagues used 2P-IVM to identify the effects of neovascularisation on axonal outgrowth following spinal cord injury (Dray et al., 2009) and has been used to identify enhanced microglial cell migration in response BSCB injury (Davalos and Akassoglou, 2012).
Overall, these methods used to study BSCB integrity and function are of utmost importance though they are limited in aspects. In vitro methods are often likely to be limited by the injury to the SC and do not always provide proof of functional physiological change.In vivo methods are limited by the fact that they do not specify which BSCB components have been compromised. Therefore it seems the most effective approach to investigate BSCB integrity and function is a combination of both.
2.2. Endothelial remodelling in spinal cord neuropathology
The microvasculature comprises largely of endothelial cells (EC), but the microvessel constituents also comprise of mural cells including smooth muscle cells and pericytes that act in harmony with the endothelium to regulate tissue perfusion. However, dysfunction in this coordinated communication is implicated in age and metabolic disturbances in cerebrovascular disease (Kisler et al., 2017; Reeson et al., 2015; Taylor et al., 2015). Pericytes are located to the capillary network and are widely regarded as integral in maintaining capillary architecture and function, with pericyte dysfunction or death termed ‘drop off’, a characteristic of vascular disease (Nikolakopoulou et al., 2019; Kisler et al., 2017; Cai and Boulton, 2002; Diéguez-Hurtado et al., 2019). A concept widely acknowledged to occur in diabetic vascular disease. Recent evidence highlights that pericyte mediated contractility underlies reduced neural tissue perfusion (Li et al., 2017; Berthiaume et al., 2018) and underpins neurodegenerative diseases including Alzheimer's disease (Hall et al., 2014; Nortley et al., 2019).
In relation to the BSCB, the adaptive nature of the capillary network is key to enabling sensory neurons to function in line with the metabolic demands of the neurological tissue. However, alterations in the BSCB through endothelium remodelling is an integral process that underlies the development of a number of neuropathological processes that arise in differing neurological disorders (Kim et al., 2019; Uchida et al., 2019). A primary example is spinal cord injury (SCI), characterised by insult or injury to the spinal cord, leads to complete or incomplete cessation of neuronal sensory communication to the spinal region. Whether the SCI is complete or incomplete is dependent on the viability of sensory and motor functions at the distal level of insult (Nas et al., 2015). The lateral and anterior spinothalamic tracts of the white matter are hubs that communicate somatosensory signals such as touch, temperature and pain information and are the primary sites of injury. Primary damage to the SC is instantaneous and involves impact damage, extensive cell death, and inflammation (Varma et al., 2013). There is also severe haemorrhaging to the grey matter due to alterations in vascularity (Rowland et al., 2008). With such damage includes extensive BSCB leakage, neurotransmitter accumulation and eventually neuronal cell death. The severe damage caused by SCI is responsible for altered pain perception and is a source of chronic pain in many individuals (Jensen et al., 2005). In recent years, SCI and neurodegenerative conditions including multiple sclerosis and amyotrophic lateral sclerosis (ALS) have been shown to have common pathologies. Astrocytes, in particular in ALS and SCI, at the site of degenerating areas elicit morphological and functional changes that result in compromised or total loss of function (Nicaise et al., 2015), and are suggested targets for therapeutic intervention in both neurodegeneration and SCI (Ban et al., 2019). In humans, alterations in BSCB have been mentioned in various diseases including ALS, though a recent study has suggested dysfunction of the BSCB is independent of motor neuron pathology, interleukin 6 (Waters et al., 2021). Degenerative cervical myelopathy (DCM) is the most common form of spinal cord injury in humans, it involves tightening of the cervical spinal canal which causes spinal cord compression and results in neuropathic pain (Milligan and Ryan, 2019). A recent review concluded BSCB injury and subsequent leakage was involved in DCM pathology (Blume et al., 2020).
In relation to pathological pain development, enhanced vascular permeability has been extensively documented and concurrently implicated in a number of differing diseases including cancer (Wang et al., 2020a)), arthritis (Beazley-Long et al., 2018), as a result of physical injury to the sensory nervous system such as traumatic nerve injury (Echeverry et al., 2011) or the treatment of certain diseases (e.g. chemotherapy (Montague-Cardoso et al., 2020b)). Furthermore, other factors have been associated with alterations in nociception. These include differences in nociceptive behaviour in rodent strains in comparison to human contexts and also the recognition of gender differences (Mogil, 2020). Despite this body of work, conjecture remains with regards pain hypersensitivity between rodents and humans. There have been studies that have highlighted aligned nociceptive responses to heat detection and noxious thresholds between human and rodents (Dunham et al., 2015). However, gender disparities do need to be considered with regards to pain perception. It is widely presented that females, both rodent and human, are more sensitive to the prevalence of pain behaviours (Mogil, 2012, 2020). However, in contrast, males present stronger responses to analgesic interventions (Mapplebeck et al., 2018) as well as amelioration of chronic pain through cessation of inflammatory processes (Sorge et al., 2015). This dichotomy in relation to the investigations into acute and chronic pain behaviours demonstrates the gender demographic needs to be considered when considering pain treatment and drug discovery programmes. How this integrates with regards the BBB has been acknowledged, with differences in the makeup of the brain capillary network differing with strain and gender (Schaffenrath et al., 2021). In addition, measures of vascular permeability within the BBB is also altered with gender (Schaffenrath et al., 2021). Acknowledgement of sex-specific differences acting upon BBB integrity mostly surrounds the beneficial role of estrogen on neurodegenerative diseases (Lee et al., 2019; Medina et al., 2003; Zárate et al., 2017; Jesmin et al., 2010). This highlights a role of sexual dysmorphism on neuronal function, however there is yet to be data which supports sex-specific influence on the BSCB and neurodegeneration.
Alterations in the BSCB have presented as a result of direct damage to the peripheral sensory nervous system e.g. directly upon sensory nerve afferents through traumatic nerve injury (Beggs et al., 2010) as well as through alternative modes of sensory nerve afferent damage such as chemotherapy (Montague-Cardoso et al., 2020a). In rodent models of diabetic neuropathic pain, within the spinal cord there is a reduction in the endothelium (Ved et al., 2018), indicative of curtailed blood perfusion. This highlights that disturbances in the BSCB is implicated in chronic pain development.
2.3. Activation of nociceptors and increased BSCB permeability
Activation of peripheral c fibre nociceptors induce localised neurogenic inflammation. This is represented typically by skin redness and swelling due to increased local oedema that arises due to elevated localised vascular perfusion and permeability. Neurogenic inflammation as a result of nociceptor activation via physical noxious stimuli, through electrical or optogenetic stimulation induces vascular permeability in discrete localised tissues with peripheral sensory nerve terminal innervation (Bharali and Lisney, 1992) (Michoud et al., 2021). This arises due to activated nociceptors releasing neurogenic inflammatory modulators that include calcitonin gene related peptide and substance P (Lynn, 1996). These act by altering capillary function e.g. in the skin or joint through modulating vascular permeability mechanisms and/or vasodilation(Michoud et al., 2021). However, the dorsal root ganglia sensory neurons not only extend sensory neuronal projections to the viscera or hindlimbs, but also to the sensory aspect of the spinal cord, the dorsal horn. The subdivisions termed lamina of the dorsal horn handle distinctive nociceptive and somatosensory inputs, which arrive from the sensory afferents (Ved et al., 2018; Greenspon et al., 2018). Within the dorsal horn there are local neural circuits encompassing local interneurons and descending projections from neurons residing in higher centres. These decipher this incoming information from the periphery to determine whether this is further relayed onto higher brain centres for the perception of pain. This information is ultimately transmitted by projections neurons that classically encode sensory inputs from both innocuous and noxious stimuli and are localised to the superficial lamina I (predominate as Neurokinin 1 receptor 1) and deeper lamina IV-V (Todd, 2010). During times of chronic pain these projection neurons have heightened levels of activity referred to as sensitisation (Li et al., 1999) which is accompanied by molecular (Luo et al., 2008; Lu et al., 2015) and structural (Tan et al., 2012) alterations that underlie these neuronal maladaptations. Alterations in spinal cord activity during pathology suggests a role for altered BSCB integrity and function to influence nociceptive hyperexcitability. However, exploration of how dorsal horn neurons directly respond to disturbances in BSCB integrity and functions are lacking. In contrast, the role nociceptors play in controlling vascular permeability has been investigated. Activating nociceptors electrically or chemically with a TRPV1 agonist capsaicin induces a decrease in tight junction protein expression in the BSCB and accompanies an increase in BSCB permeability (Beggs et al., 2010; Sauer et al., 2017). Furthermore, in a number of experimental chronic pain scenarios, traumatic surgical intervention (Campbell and Meyer, 2006) as well as models of inflammatory pain, nociceptors are activated and modulate BSCB permeability (Beggs et al., 2010; Sauer et al., 2017; Echeverry et al., 2011; Li et al., 2020). This is demonstrated following traumatic injury, a direct injury to sensory nerve fibres causing reductions in Claudin 1 and Occludin in the spinal cord for the duration of the study (up to 14 days) (Sauer et al., 2017) (Fig. 2B). This data has been generated by utilising an array of methodological approaches to evaluate BSCB permeability including fluorescent tracers such as EB, immunological cell infiltration and quantification of key features of the BSCB ie tight junctional molecules (Sauer et al., 2017) as discussed earlier. These disturbances in BSCB function through increased vascular permeability have been found to be attributable to C fibre nociceptor mediated activity and deemed TRPV1 dependent (Beggs et al., 2010). This falls in line with the propensity for increased C fibre activity to develop in models of chronic pain, whether to evoked stimuli (Dunham et al., 2008) and/or ongoing nociceptor activity (Kelly et al., 2012; Djouhri et al., 2006; Hulse, 2016).
2.4. Inflammation induced BSCB endothelial remodelling
Inherently, the accumulation of circulating inflammatory cell types in the spinal cord and associated increase in pro-inflammatory state within the dorsal horn is thought to be instrumental in the onset of pain (Echeverry et al., 2011). This is exemplified by Costigan and colleagues who showed that following a traumatic nerve injury in immuno-compromised mice that lacked T lymphocytes, did not present an accumulation of infiltrating inflammatory cells in the spinal cord (Costigan et al., 2009) as typically identified in rodent models of chronic pain (Hu et al., 2017/10; Li et al., 2016). This was accompanied by those rodents lacking T lymphocytes also not presenting neuropathic pain phenotypes(Costigan et al., 2009). This highlights a pronounced involvement of pro-inflammatory mechanisms as a mediator of inducing chronic pain states. Furthermore, a circulatory pro-inflammatory cell complement interacts strongly with the BSCB and in particular the endothelium, to induce endothelial remodelling. This dependence upon an engaged immuno-vascular interaction to promote immune cell infiltration into the spinal cord is demonstrated by the depletion of the neutrophil population resulting in the preservation of the BSCB (Aubé et al., 2014).
Pathological vascular alterations arise in part due to the endothelial-immune interaction, with circulatory cell types such as macrophages (Aubé et al., 2014) and neutrophils (Aubé et al., 2014). These cells release prominent inflammatory agents, including metalloproteinase 3 (Lee et al., 2014), metalloproteinase 9 (Lee et al., 2012), tumour necrosis factorα (TNFα) (Lee et al., 2019), interleukin 6 (IL6), and monocyte chemoattractant protein 1(MCP1) (Yao et al., 2016; Wang et al., 2020b), which are associated with increased BSCB vascular permeability. These inflammatory mediators are widely regarded to activate sensory neuron activity in the peripheral and the central somatosensory system. However, they are also known mediators and contributors to an inflammatory spinal cord environment and initiate a pronounced remodelling of the endothelium (Echeverry et al., 2011). The release of these mediators influence architectural and functional properties of the endothelium by impairing the BSCB integrity leading to extravasation. This is typically demonstrated by enhanced vascular permeability due to loss of endothelial cell tight junctional proteins ZO1 and Occ (Lee et al., 2014; Wang et al., 2020b) (Fig. 2B). This increased opportunity for cellular passage and transmigration of inflammatory cells into the spinal cord tissues is in conjunction with elevated endothelial cell surface interactions/adhesions with circulating inflammatory cells (Montague-Cardoso et al., 2020a). This includes the increasing expression of cell adhesion molecules on the endothelial lumen including ICAM1 (Sweitzer et al., 2002; Nordal and Wong, 2004), which can occur in rodent models of neuropathic pain such as chemotherapy induced neuropathic pain (Montague-Cardoso et al., 2020a). This interaction is presented typically as an increased adherence to enable greater inflammatory cell attachment/rolling on the endothelial lumen (Montague-Cardoso et al., 2020a). Furthermore, remodelling of the BSCB endothelium can be suppressed. By inhibiting the inflammatory mediator cocktail, i.e. through provision of neutralising antibodies (MCP1) or through TGFb1 or interleukin 10, dampens the increased vascular permeability that develops following peripheral nerve injury (Echeverry et al., 2011).
Prominent endothelial cell interactions within the immune system control the chronic maladaptation of the vasculature in the spinal cord. Increased inflammation of neural tissues is accompanied by the infiltration of several immunological cell types. As mentioned, this elevated inflammatory cell invasion is concomitantly associated with increased abundance of local inflammatory mediators, which include TNFα. These factors have been shown to induce alterations in the endothelium to activate molecular pathways that support cellular adhesion and breakdown of the endothelial monolayer or in this instance the BSCB. Inflammatory mediators such as interleukin1β (Il1β) (Wang et al., 2020b) and TNFα (Lee et al., 2019) also induce endothelial remodelling through manipulating angiogenic signalling cascades that control the blood vessel architecture and function in the spinal cord. In rodent models of inflammatory pain there is a significant glio-vascular interaction typified by strong associations between CD11b cell types and the endothelium in the dorsal horn of arthritic rodents (Beazley-Long et al., 2018). This interaction is strongly dependent upon VEGFR2 signalling in the endothelium. Cessation of VEGFR2 signalling whether through pharmacological inhibition or endothelium specific VEGFR2 knockout rodent models, prevented arthritic pain (Beazley-Long et al., 2018). This was attributable to the suppression of endothelial derived VEGFR2 signalling induced by circulating monocyte activity in arthritic rodents. In arthritis patients, circulating monocytes expression profiles of TNF α, IL6 and VEGF-A (Kim et al., 2020; McInnes and Schett, 2007) are elevated and initiate elevated endothelial adhesion through activating endothelial VEGFR2-ICAM1 dependent vascular remodelling (Thichanpiang et al., 2014; Beazley-Long et al., 2018). This mirrors chemotherapy induced neuropathic pain that presents elevated ICAM1 expression in the BSCB to drive monocyte adherence(Montague-Cardoso et al., 2020a; Montague-Cardoso and Malcangio, 2021). However, it is important to note that part of the nociceptive system works in harmony with resident microglia (CD11b positive cells) to maintain environmental and functional homeostasis within the dorsal horn. This is highlighted by the revitalisation of the microglia cellular complement within the spinal cord that is dependent upon a pronounced infiltration of monocytes into the spinal cord (Yao et al., 2016; Wang et al., 2020b). However, a shift in the spinal cord parenchyma inflammatory profile can tip towards the induction of chronic pain states. Increased transmigration of the CD11b positive circulating cells that may differentiate to act as resident microglia, induce chronic pain (Sun et al., 2019; Li et al., 2016), as described in inflammatory arthritis (Beazley-Long et al., 2018).
In relation to mural cell involvement, astrocytes, pericytes, neurons, and microglia in the neurovascular unit (NVU) are involved in the efficient control and maintenance of the BSCB. These interact extensively with the microvessels supporting vessel integrity and function. In times of neuro-sensory pathology such as traumatic nerve injury and chemotherapy induced pain, astrogliosis is highly prominent in the dorsal horn (Gordh et al., 2006; Wang et al., 2020b). The association of astrocytes in relation to chronic pain, is attributable to alterations in AQP4 channel distribution in the spinal cord. AQP4 expression is highly abundant in laminae I and II, which are superficially located in the dorsal horn, and therefore attributable to the modulation of nociceptive processing (Lu et al., 2020). AQP4 dependent astrocytic mechanisms have been shown to regulate neuronal activity, survival, regeneration and neurotransmitter release in pathological conditions. Involvement of astrocytic AQP4 signalling in nociception is further demonstrated through AQP4 deficiency attenuating inflammatory pain and neuropathic pain, but has no effect in acute pain (Lu et al., 2020). Consequently, increased astrocytic activity is associated to increased BSCB extravasation depicted by increased EB accumulation and reductions in tight junctional markers (Wang et al., 2020b). Additional, astrocyte associated mechanisms contributing to chronic pain have been demonstrated through traumatic injury to tissues such as skin, joints, or muscles. This drives astrogliosis in the spinal cord leading to the release of inflammatory mediators such as cytokines and chemokines e.g. MCP-1 and IL-1 through activation of ERK and or JNK (Gao et al., 2010; Liao et al., 2011). These pro-inflammatory mediators have previously been mentioned in modulating BSCB function and integrity. Similarly, the pericytes control vascular permeability, with the presence of pericytes protecting the BSCB. This is depicted by a loss of ZO1 and Occ and elevated BSCB permeability in a PDGFRβ knockout model which drives pericyte loss (Winkler et al., 2012).
2.5. Reductions in spinal cord blood flow and degeneration of the endothelium
Alterations in nociception in rodent models encapsulate modulation of the BSCB with relation to enhanced vascular permeability. However, maintaining the health and integrity of the BSCB endothelium is a significant factor in nociceptive processing. The spinal cord microvessels provide integral function in meeting the essential energy demands of the dorsal horn neurons, which allow the required provision of important solutes such as glucose and oxygen. A dysfunction in the delivery of these molecules as a consequence of endothelium degeneration and consequently diminished vascular perfusion of neural tissues is prominent in the development of neurological disease (Bracko et al., 2021). As highlighted previously, the vasculature is highly adaptable, responding to differing tissue demands and cellular/tissue stressors. During vascular related pathologies such as retinopathy (Gupta et al., 2013) and nephropathy (Kumar Vr et al., 2016), the vasculature is highly angiogenic - demonstrated through an expansion of the capillary network (Korn and Augustin, 2015) and heightened vascular permeability. However, in some instances vascular regression can dominate (Korn and Augustin, 2015). This describes the degeneration or retraction of the endothelium, diminishing the vascular network and consequently reducing blood perfusion of neural tissues. This is highly prevalent in neurodegenerative disease including Alzheimer's (Bracko et al., 2021). In relation to nociception, impaired vascular perfusion is strongly implicated in peripheral sensory neuropathy such as peripheral limb disease in diabetic patients (Hsiao et al., 2016; Richner et al., 2019). At the level of the spinal cord, modulation of nociceptive processing has been demonstrated to be dependent upon vascular perfusion (Ved et al., 2018) (Fig. 2C). Endothelial cells are susceptible to hyperglycaemia, with high levels of glucose leading to endothelial cell apoptosis (Oltean et al., 2014) and blood vessel functional abnormalities that are displayed as reductions in vascular perfusion of the lumbar spinal cord (Ved et al., 2018). These studies highlight that endothelial cell survival was dependent upon the cytoprotective actions of VEGF-A isoform VEGF-Axxxb mediated signalling through VEGFR2 (Oltean et al., 2014). Interestingly, these studies demonstrate that in a rodent model of diabetes there is an association between BSCB damage and the onset of neuropathic pain (Ved et al., 2018). However, these studies have multiple variables including alterations in blood glucose. To specifically investigate the interaction between the BSCB integrity and nociception, the reliance of the endothelial cell upon VEGFR2 signalling for cell survival and function(Beazley-Long et al., 2018) was assessed. By removing VEGFR2 signalling from the BSCB endothelium using a rodent transgenic model demonstrated distinctly that degeneration of the BSCB leads to heat hypersensitivity (Ved et al., 2018).
3. Conclusion
This review discusses how alterations to the BSCB have a significant contribution in the modulation of nociceptive processing and the onset of chronic pain states. It is presented here that disturbances to the BSCB endothelium results in the development of neuropathic as well as inflammatory pain. As a result of enhanced BSCB permeability infiltration of inflammatory cell types into the spinal cord is enabled and the consequent release of pro-inflammatory agents induce pain hypersensitivity. Additionally, a degradation of the endothelium presented as a reduced capillary network within the dorsal horn also leads to the development of neuropathic pain states. Here the preclinical data is presented to highlight the role of the BSCB in chronic pain manifestation. There is minimal understanding of how the BSCB is altered in chronic pain states and how it influences nociception. This will allow for further insight into the pathological circumstances and maladaptations that occur in chronic pain states that as present remain undefined and therefore present future avenues for analgesia development.
Funding
This work was supported by the European Foundation for the Study of Diabetes/Boehringer Ingelheim European Research Programme in Microvascular Complications of Diabetes (BI18_5 to RPH).
CRediT authorship contribution statement
Awais Younis: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Lydia Hardowar: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Sarah Barker: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, All authors approved the final version of the manuscript. Richard Philip Hulse: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
RPH, AY, LW and SB contributed to the conception or design of the work. RPH, AY, LW and SB drafted the manuscript and all authors approved the final version of the manuscript. The authors would like to thank Graham Hickman of the Imaging Suite at Nottingham Trent University for their support and assistance in this work’.
References
- Aubé B., Lévesque S.A., Paré A., Chamma É., Kébir H., Gorina R., et al. Neutrophils mediate blood–spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J. Immunol. 2014;193(5):2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- Ay I., Francis J.W., Brown R.H. VEGF increases blood-brain barrier permeability to Evans blue dye and tetanus toxin fragment C but not adeno-associated virus in ALS mice. Brain Res. 2008;1234:198–205. doi: 10.1016/j.brainres.2008.07.121. [DOI] [PubMed] [Google Scholar]
- Ban J., Sámano C., Mladinic M., Munitic I. Glia in amyotrophic lateral sclerosis and spinal cord injury: common therapeutic targets. Croat. Med. J. 2019 Apr;60(2):109–120. doi: 10.3325/cmj.2019.60.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D.O., Beazley-Long N., Benest A.V., Ye X., Ved N., Hulse R.P., et al. Physiological role of vascular endothelial growth factors as homeostatic regulators. Compr. Physiol. 2018 Jun;8(3):955–979. doi: 10.1002/cphy.c170015. [DOI] [PubMed] [Google Scholar]
- Beazley-Long N., Moss C.E., Ashby W.R., Bestall S.M., Almahasneh F., Durrant A.M., et al. 2018. VEGFR2 Promotes Central Endothelial Activation and the Spread of Pain in Inflammatory Arthritis. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S., Liu X.J., Kwan C., Salter M.W. Vol. 6. 2010. p. 74.http://www.ncbi.nlm.nih.gov/pubmed/21044346 (Peripheral Nerve Injury and TRPV1-Expressing Primary Afferent C-Fibers Cause Opening of the Blood-Brain Barrier). Mol Pain [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume A.A., Hartmann D.A., Majesky M.W., Bhat N.R., Shih A.Y. Pericyte structural remodeling in cerebrovascular health and homeostasis. Front. Aging Neurosci. 2018;10:210. doi: 10.3389/fnagi.2018.00210. 2018/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharali L.A., Lisney S.J. The relationship between unmyelinated afferent type and neurogenic plasma extravasation in normal and reinnervated rat skin. Neuroscience. 1992;47(3):703–712. doi: 10.1016/0306-4522(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Blume C., Geiger M., Brandenburg L.-O., Mueller M., Mainz V., Kalder J., et al. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: a prospective comparative cohort study. Eur. Spine J. 2020 May 1:29. doi: 10.1007/s00586-020-06298-7. [DOI] [PubMed] [Google Scholar]
- Bracko O., Cruz Hernández J.C., Park L., Nishimura N., Schaffer C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer's disease. J. Cerebr. Blood Flow Metabol. 2021;41(7):1501–1516. doi: 10.1177/0271678X20982383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye. 2002;16(3):242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- Campbell J.N., Meyer R.A. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C.R., Schröter C., Wang X., Miller D.S. ABC transporter function and regulation at the blood-spinal cord barrier. J. Cerebr. Blood Flow Metabol. 2012;32(8):1559–1566. doi: 10.1038/jcbfm.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D.M., Patel C.B., Ahobila-Vajjula P., Sundberg L.M., Chacko T., Liu S.-J., et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009 Apr;22(3):332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M., Moss A., Latremoliere A., Johnston C., Verma-Gandhu M., Herbert T.A., et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 2009;28(46):14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Akassoglou K. In vivo imaging of the mouse spinal cord using two-photon microscopy. JoVE : JoVE. 2012 Jan;(59) doi: 10.3791/2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M., Venneri M.A., Galli R., Sergi Sergi L., Politi L.S., Sampaolesi M., et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. 2005/09/20. [DOI] [PubMed] [Google Scholar]
- Diéguez-Hurtado R., Kato K., Giaimo B.D., Nieminen-Kelhä M., Arf H., Ferrante F., et al. Loss of the transcription factor RBPJ induces disease-promoting properties in brain pericytes. Nat. Commun. 2019;10(1):2817. doi: 10.1038/s41467-019-10643-w. 2019/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dithmer S., Staat C., Müller C., Ku M.C., Pohlmann A., Niendorf T., et al. Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery. Ann. N. Y. Acad. Sci. 2017;1397(1):169–184. doi: 10.1111/nyas.13359. [DOI] [PubMed] [Google Scholar]
- Djouhri L., Koutsikou S., Fang X., McMullan S., Lawson S.N. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006;26(4):1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray C., Rougon G., Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proceed. National Academy of Sciences of the United States of America. 2009 Jun;106(23):9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham J.P., Kelly S., Donaldson L.F. Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur. J. Neurosci. 2008;27(12):3151–3160. doi: 10.1111/j.1460-9568.2008.06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham J.P., Hulse R.P., Donaldson L.F. A novel method for delivering ramped cooling reveals rat behaviours at innocuous and noxious temperatures: a comparative study of human psychophysics and rat behaviour. J. Neurosci. Methods. 2015;249 doi: 10.1016/j.jneumeth.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Echeverry S., Shi X.Q., Rivest S., Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J. Neurosci. 2011;31(30):10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah A., Sadeghinia A., Kahroba H., Samadi A., Heidari H.R., Bradaran B., et al. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019 Feb;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Farrar M.J., Bernstein I.M., Schlafer D.H., Cleland T.A., Fetcho J.R., Schaffer C.B. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nat. Methods. 2012;9(3):297–302. doi: 10.1038/nmeth.1856. 2012/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran E., Kwon D.Y., Nofziger J.H., Arnold E.S., Hall M.D., Fischbeck K.H., et al. CNS uptake of bortezomib is enhanced by P-glycoprotein inhibition: implications for spinal muscular atrophy. Neurobiol. Dis. 2016 Apr;88:118–124. doi: 10.1016/j.nbd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.-J., Zhang L., Ji R.-R. Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010 Nov;58(15):1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni P., Arango-Lievano M., Neves I.D., Rousset M.C., Baranger K., Rivera S., et al. Cerebrovascular pathology during the progression of experimental Alzheimer's disease. Neurobiol. Dis. 2016;88:107–117. doi: 10.1016/j.nbd.2016.01.001. 2016/01/17. [DOI] [PubMed] [Google Scholar]
- Glaesel K., May C., Marcus K., Matschke V., Theiss C., Theis V. miR-129-5p and miR-130a-3p regulate VEGFR-2 expression in sensory and motor neurons during development. Int. J. Mol. Sci. 2020 May;21(11) doi: 10.3390/ijms21113839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordh T., Chu H., Sharma H.S. Spinal nerve lesion alters blood-spinal cord barrier function and activates astrocytes in the rat. Pain. 2006 Sep;124(1–2):211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Greenspon C.M., Battell E.E., Devonshire I.M., Donaldson L.F., Chapman V., Hathway G.J. Lamina-specific population encoding of cutaneous signals in the spinal dorsal horn using multi-electrode arrays. J. Physiol. 2018 doi: 10.1113/JP277036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsch S., Lu J., Thilemann S., Wortge S., Mobius W., Bruttger J., et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat. Commun. 2014;5:5472. doi: 10.1038/ncomms6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H.W., Xing F., Jiang M.J., Wang Y., Bai L., Zhang J., et al. Upregulation of matrix metalloproteinase-9/2 in the wounded tissue, dorsal root ganglia, and spinal cord is involved in the development of postoperative pain. Brain Res. 2019;1718:64–74. doi: 10.1016/j.brainres.2019.05.007. May. [DOI] [PubMed] [Google Scholar]
- Gupta N., Mansoor S., Sharma A., Sapkal A., Sheth J., Falatoonzadeh P., et al. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013;7:4–10. doi: 10.2174/1874364101307010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. 2014/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao H.T., Lin Y.C., Wang J.C., Tsai Y.C., Liu Y.C. Hypoxia inducible factor-1α inhibition produced anti-allodynia effect and suppressed inflammatory cytokine production in early stage of mouse complex regional pain syndrome model. Clin. Exp. Pharmacol. Physiol. 2016;43(3):355–359. doi: 10.1111/1440-1681.12536. [DOI] [PubMed] [Google Scholar]
- Hu L.Y., Zhou Y., Cui W.Q., Hu X.M., Du L.X., Mi W.L., et al. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav. Immun. [Internet] 2017/10/16. 2018;68 doi: 10.1016/j.bbi.2017.10.011. https://www.ncbi.nlm.nih.gov/pubmed/29051087 132–45. Available from: [DOI] [PubMed] [Google Scholar]
- Hulse R.P. Identification of mechano-sensitive C fibre sensitization and contribution to nerve injury-induced mechanical hyperalgesia. Eur. J. Pain. 2016;20(4) doi: 10.1002/ejp.779. [DOI] [PubMed] [Google Scholar]
- Jablonski M.R., Jacob D.A., Campos C., Miller D.S., Maragakisc N.J., Pasinellia P., et al. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol. Dis. 2012;47(2):194–200. doi: 10.1016/j.nbd.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.P., Hoffman A.J., Cardenas D.D. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005 Dec;43(12):704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- Jesmin S., Mowa C.N., Sultana S.N., Mia S., Islam R., Zaedi S., et al. Estrogen receptor alpha and beta are both involved in the cerebral VEGF/Akt/NO pathway and cerebral angiogenesis in female mice. Biomed. Res. 2010 Dec;31(6):337–346. doi: 10.2220/biomedres.31.337. [DOI] [PubMed] [Google Scholar]
- Kelly S., Dunham J.P., Murray F., Read S., Donaldson L.F., Lawson S.N. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis Cartilage. 2012;20(4):305–313. doi: 10.1016/j.joca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Kim Y.Y., Chao J.R., Kim C., Jung H., Kim B., Kang T.C., et al. Comparing the superficial vasculature of the central nervous system in six laboratory animals: a hypothesis about the role of the “circle of willis. Anat. Rec. 2019;302(11):2049–2061. doi: 10.1002/ar.24146. [DOI] [PubMed] [Google Scholar]
- Kim J.W., Kong J.S., Lee S., Yoo S.A., Koh J.H., Jin J., et al. Angiogenic cytokines can reflect the synovitis severity and treatment response to biologics in rheumatoid arthritis. Exp. Mol. Med. 2020;52(5):843–853. doi: 10.1038/s12276-020-0443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K., Nelson A.R., Rege S.V., Ramanathan A., Wang Y., Ahuja A., et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017;20(3):406–416. doi: 10.1038/nn.4489. 2017/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C., Augustin H.G. Mechanisms of vessel pruning and regression. Dev. Cell. 2015;34(1):5–17. doi: 10.1016/j.devcel.2015.06.004. 2015/07/08. [DOI] [PubMed] [Google Scholar]
- Kumar Vr S., Darisipudi M.N., Steiger S., Devarapu S.K., Tato M., Kukarni O.P., et al. Cathepsin S cleavage of protease-activated receptor-2 on endothelial cells promotes microvascular diabetes complications. J. Am. Soc. Nephrol. : JASN (J. Am. Soc. Nephrol.) 2016 Jun;27(6):1635–1649. doi: 10.1681/ASN.2015020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzov N., Flyvbjerg H., Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain Barrier. Proceed. National Academy of Sciences of the United States of America. 2018;115(40):E9429–E9438. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim H.S., Choi H.Y., Oh T.H., Ju B.G., Yune T.Y. Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J. Neurochem. 2012;121(5):818–829. doi: 10.1111/j.1471-4159.2012.07731.x. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Choi H.Y., Ahn H.J., Ju B.G., Yune T.Y. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am. J. Pathol. 2014 doi: 10.1016/j.ajpath.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Lee T.H., Hsieh S.T., Chiang H.Y. Fibronectin inhibitor pUR4 attenuates tumor necrosis factor α-induced endothelial hyperpermeability by modulating β1 integrin activation. J. Biomed. Sci. 2019;26(1):1–15. doi: 10.1186/s12929-019-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Simone D.A., Larson A.A. Windup leads to characteristics of central sensitization. Pain. 1999;79(1):75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Li Z., Wei H., Piirainen S., Chen Z., Kalso E., Pertovaara A., et al. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016;58:107–117. doi: 10.1016/j.bbi.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Li Y., Lucas-Osma A.M., Black S., Bandet M.V., Stephens M.J., Vavrek R., et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat. Med. 2017;23(6):733–741. doi: 10.1038/nm.4331. 2017/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.L., Huang Y., Zhou Y.L., Teng R.H., Zhou S.Z., Lin J.P., et al. C-X-C motif chemokine 10 contributes to the development of neuropathic pain by increasing the permeability of the blood–spinal cord barrier. Front. Immunol. 2020;11(March):1–9. doi: 10.3389/fimmu.2020.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y.-H., Zhang G.-H., Jia D., Wang P., Qian N.-S., He F., et al. Spinal astrocytic activation contributes to mechanical allodynia in a mouse model of type 2 diabetes. Brain Res. 2011 Jan;1368:324–335. doi: 10.1016/j.brainres.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Linnerbauer M., Wheeler M.A., Quintana F.J. Astrocyte crosstalk in CNS inflammation. Neuron. 2020 Nov;108(4):608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli G., Theodorou D., Kendirli A., Jordão M.J.C., Staszewski O., Phulphagar K., et al. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat. Neurosci. 2018 Sep;21(9):1196–1208. doi: 10.1038/s41593-018-0212-3. [DOI] [PubMed] [Google Scholar]
- Lu J., Luo C., Bali K.K., Xie R.G., Mains R.E., Eipper B.A., et al. A role for Kalirin-7 in nociceptive sensitization via activity-dependent modulation of spinal synapses. Nat. Commun. 2015;13(6):6820. doi: 10.1038/ncomms7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Pang C., Chen Y., Wu N., Li J. Aquaporin 4 is involved in chronic pain but not acute pain. Behav. Brain Res. 2020 Sep;393:112810. doi: 10.1016/j.bbr.2020.112810. [DOI] [PubMed] [Google Scholar]
- Luo C., Seeburg P.H., Sprengel R., Kuner R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain. 2008;140(2):358–367. doi: 10.1016/j.pain.2008.09.008. http://www.ncbi.nlm.nih.gov/pubmed/18926636 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- Lynn B. Neurogenic inflammation caused by cutaneous polymodal receptors. Prog. Brain Res. 1996;113:361–368. doi: 10.1016/s0079-6123(08)61098-5. [DOI] [PubMed] [Google Scholar]
- Mapplebeck J.C.S., Dalgarno R., Tu Y., Moriarty O., Beggs S., Kwok C.H.T., et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018 Sep;159(9):1752–1763. doi: 10.1097/j.pain.0000000000001265. [DOI] [PubMed] [Google Scholar]
- Masedunskas A., Milberg O., Porat-Shliom N., Sramkova M., Wigand T., Amornphimoltham P., et al. Intravital microscopy: a practical guide on imaging intracellular structures in live animals. BioArchitecture. 2012;2(5):143–157. doi: 10.4161/bioa.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I.B., Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007 Jun;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Medina R.A., Meneses A.M., Vera J.C., Guzman C., Nualart F., Astuya A., et al. Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology. 2003 Oct;144(10):4527–4535. doi: 10.1210/en.2003-0294. [DOI] [PubMed] [Google Scholar]
- Michoud F., Seehus C., Schönle P., Brun N., Taub D., Zhang Z., et al. Epineural optogenetic activation of nociceptors initiates and amplifies inflammation. Nat. Biotechnol. 2021 Feb;39(2):179–185. doi: 10.1038/s41587-020-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J., Ryan K. Degenerative cervical myelopathy. Clin. Rev. 2019;65 [PMC free article] [PubMed] [Google Scholar]
- Moens S., Goveia J., Stapor P.C., Cantelmo A.R., Carmeliet P. The multifaceted activity of VEGF in angiogenesis - implications for therapy responses. Cytokine Growth Factor Rev. 2014 Aug;25(4):473–482. doi: 10.1016/j.cytogfr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020;21(7):353–365. doi: 10.1038/s41583-020-0310-6. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- Montague-Cardoso K., Malcangio M. Changes in blood–spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. PAIN Rep. 2021 Jan;6(1):e879. doi: 10.1097/PR9.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague-Cardoso K., Pitcher T., Chisolm K., Salera G., Lindstrom E., Hewitt E., et al. Changes in vascular permeability in the spinal cord contribute to chemotherapy-induced neuropathic pain. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague-Cardoso K., Pitcher T., Chisolm K., Salera G., Lindstrom E., Hewitt E., et al. Changes in vascular permeability in the spinal cord contribute to chemotherapy-induced neuropathic pain. Brain Behav. Immun. 2020;83:248–259. doi: 10.1016/j.bbi.2019.10.018. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res. 2012;349(2):589–603. doi: 10.1007/s00441-012-1421-9. [DOI] [PubMed] [Google Scholar]
- Mukwaya A., Jensen L., Lagali N. Relapse of pathological angiogenesis: functional role of the basement membrane and potential treatment strategies. Exp. Mol. Med. 2021 Feb;53(2):189–201. doi: 10.1038/s12276-021-00566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nas K., Yazmalar L., Şah V., Aydın A., Öneş K. Rehabilitation of spinal cord injuries. World J. Orthoped. 2015 Jan;6(1):8–16. doi: 10.5312/wjo.v6.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C., Mitrecic D., Falnikar A., Lepore A.C. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J. Stem Cell. 2015 Mar;7(2):380–398. doi: 10.4252/wjsc.v7.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakopoulou A.M., Montagne A., Kisler K., Dai Z., Wang Y., Huuskonen M.T., et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 2019;22(7):1089–1098. doi: 10.1038/s41593-019-0434-z. 2019/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. JCB (J. Cell Biol.) 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble L.J., Mautes A.E.M., Hall J.J. Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. J. Comp. Neurol. 1996;376(4):542–556. doi: 10.1002/(SICI)1096-9861(19961223)376:4<542::AID-CNE4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Nordal R.A., Wong C.S. Intercellular adhesion molecule-1 and blood-spinal cord barrier disruption in central nervous system radiation injury. JNEN (J. Neuropathol. Exp. Neurol.) 2004;63(5):474–483. doi: 10.1093/jnen/63.5.474. [DOI] [PubMed] [Google Scholar]
- Nortley R., Korte N., Izquierdo P., Hirunpattarasilp C., Mishra A., Jaunmuktane Z., et al. Amyloid beta oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science. 2019;(6450):365. doi: 10.1126/science.aav9518.. 2019/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltean S., Qiu Y., Ferguson J.K.K., Stevens M., Neal C., Russell A., et al. Vascular endothelial growth factor-a165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J. Am. Soc. Nephrol. 2014 Aug;26(8):1889–1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112) doi: 10.1038/nature05193. 2006/10/13. 700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich P.G., Horner P.J., Mullin B.B., Stokes B.T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996 Dec;142(2):258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Prockop L.D., Naidu K.A., Binard J.E., Ransohoff J. Selective permeability of [3H]-D-mannitol and [14C]-carboxyl-inulin across the blood-brain barrier and blood-spinal cord barrier in the rabbit. The j. spinal cord med. 1995 Oct;18(4):221–226. doi: 10.1080/10790268.1995.11719399. [DOI] [PubMed] [Google Scholar]
- Reeson P., Tennant K.A., Gerrow K., Wang J., Weiser Novak S., Thompson K., et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J. Neurosci. 2015;35(13):5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner M., Ferreira N., Dudele A., Jensen T.S., Vaegter C.B., Gonçalves N.P. Functional and structural changes of the blood-nerve-barrier in diabetic neuropathy. Front. Neurosci. 2019;13:1–9. doi: 10.3389/fnins.2018.01038. JAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland J.W., Hawryluk G.W.J., Kwon B., Fehlings M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- Sathiyanadan K., Coisne C., Enzmann G., Deutsch U., Engelhardt B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur. J. Immunol. 2014 Aug;44(8):2287–2294. doi: 10.1002/eji.201344214. [DOI] [PubMed] [Google Scholar]
- Sauer R.S., Kirchner J., Yang S., Hu L., Leinders M., Sommer C., et al. Blood–spinal cord barrier breakdown and pericyte deficiency in peripheral neuropathy. Ann. N. Y. Acad. Sci. 2017;1405(1):71–88. doi: 10.1111/nyas.13436. [DOI] [PubMed] [Google Scholar]
- Saunders N.R., Dreifuss J.J., Dziegielewska K.M., Johansson P.A., Habgood M.D., Møllgård K., et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front. Neurosci. 2014;8:1–26. doi: 10.3389/fnins.2014.00404. DEC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffenrath J., Huang S.F., Wyss T., Delorenzi M., Keller A. Characterization of the blood–brain barrier in genetically diverse laboratory mouse strains. Fluids Barriers of the CNS. 2021;18(1):1–15. doi: 10.1186/s12987-021-00269-w. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg A.E., Buist R., Yong V.W., Del Bigio M.R., Peeling J. Magnetic resonance imaging of blood-spinal cord barrier disruption in mice with experimental autoimmune encephalomyelitis. Magn. Reson. Med. 2007 Aug;58(2):298–305. doi: 10.1002/mrm.21289. [DOI] [PubMed] [Google Scholar]
- Sorge R.E., Mapplebeck J.C.S., Rosen S., Beggs S., Taves S., Alexander J.K., et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015 Aug;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.J., Tang L., Zhao X.P., Xu J.M., Xiao Y., Li H. Infiltration of blood-derived macrophages contributes to the development of diabetic neuropathy. J. Immunol. Res. 2019 doi: 10.1155/2019/7597382. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer S.M., White K.A., Dutta C., DeLeo J.A. The differential role of spinal MHC class II and cellular adhesion molecules in peripheral inflammatory versus neuropathic pain in rodents. J. Neuroimmunol. 2002 Apr;125(1–2):82–93. doi: 10.1016/s0165-5728(02)00036-x. [DOI] [PubMed] [Google Scholar]
- Tan A.M., Samad O.A., Fischer T.Z., Zhao P., Persson A.K., Waxman S.G. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. J Neurosci. 2012;32(20):6795–6807. doi: 10.1523/JNEUROSCI.1017-12.2012. http://www.ncbi.nlm.nih.gov/pubmed/22593049 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.L., Trudeau D., Arnold B., Wang J., Gerrow K., Summerfeldt K., et al. VEGF can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol. Dis. 2015;78(1):11. doi: 10.1016/j.nbd.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Thichanpiang P., Harper S.J., Wongprasert K., Bates D.O. TNF-alpha-induced ICAM-1 expression and monocyte adhesion in human RPE cells is mediated in part through autocrine VEGF stimulation. Mol. Vis. 2014;20:781–789. [PMC free article] [PubMed] [Google Scholar]
- Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. http://www.ncbi.nlm.nih.gov/pubmed/21068766 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Sumiya T., Tachikawa M., Yamakawa T., Murata S., Yagi Y., et al. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol. Neurobiol. 2019 doi: 10.1007/s12035-018-1207-5. [DOI] [PubMed] [Google Scholar]
- Varma A.K., Das A., Wallace G., 4th, Barry J., Vertegel A.A., Ray S.K., et al. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 2013 May;38(5):895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved N., Hulse R.P., Bestall S.M., Donaldson L.F., Bainbridge J.W., Bates D.O. Vascular endothelial growth factor-A165b ameliorates outer-retinal barrier and vascular dysfunction in the diabetic retina. Clin. Sci. 2017;131(12) doi: 10.1042/CS20170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved N., Da Vitoria Lobo M.E., Bestall S.M., Vidueira C L., Beazley-Long N., Ballmer-Hofer K., et al. Diabetes-induced microvascular complications at the level of the spinal cord: a contributing factor in diabetic neuropathic pain. J. Physiol. 2018;596(16):3675–3693. doi: 10.1113/JP275067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Campos C.R., Peart J.C., Smith L.K., Boni J.L., Cannon R.E., et al. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 2014;34(25):8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Xu K., Wang Y., Mao Y., Huang Y., Liang Y., et al. Spinal cannabinoid receptor 2 activation reduces hypersensitivity associated with bone cancer pain and improves the integrity of the blood-spinal cord barrier. Reg. Anesth. Pain Med. 2020;45(10):783–791. doi: 10.1136/rapm-2019-101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Xu K., Wang Y., Mao Y., Huang Y., Liang Y., et al. Spinal cannabinoid receptor 2 activation reduces hypersensitivity associated with bone cancer pain and improves the integrity of the blood-spinal cord barrier. Reg. Anesth. Pain Med. 2020;45(10):783–791. doi: 10.1136/rapm-2019-101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S., Swanson M.E.V., Dieriks B.V., Zhang Y.B., Grimsey N.L., Murray H.C., et al. Blood-spinal cord barrier leakage is independent of motor neuron pathology in ALS. Acta Neuropathol. Commun. 2021;9(1):1–17. doi: 10.1186/s40478-021-01244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson Pm Thom G., Ginman U., Lundquist S., Webster C.I. PJC. Modelling the endothelial blood-CNS barriers: a method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci. 2013;14(59):1186. doi: 10.1186/1471-2202-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N., Miller F., Cazaubon S., Couraud P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta. 2009;1788(4):842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Winkler E.A., Sengillo J.D., Bell R.D., Wang J., Zlokovic B.V. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cerebr. Blood Flow Metabol. 2012;32(10):1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D., et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18(4):521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthos D.N., Püngel I., Wunderbaldinger G., Sandkühler J. Effects of peripheral inflammation on the blood-spinal cord barrier. Mol. Pain. 2012;8(1):1. doi: 10.1186/1744-8069-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Echeverry S., Shi X.Q., Yang M., Yang Q.Z., Wang G.Y.F., et al. Dynamics of spinal microglia repopulation following an acute depletion. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep22839. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate S., Stevnsner T., Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front. Aging Neurosci. 2017;9:430. doi: 10.3389/fnagi.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Tang Q., Zheng G., Wang C., Zhou Y., Wu Y., et al. Metformin ameliorates BSCB disruption by inhibiting neutrophil infiltration and MMP-9 expression but not direct TJ proteins expression regulation. J. Cell Mol. Med. 2017;21(12):3322–3336. doi: 10.1111/jcmm.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]