Abstract
目的
研究雌二醇其通过内质网应激通路影响巨噬细胞免疫特性的可能机制。
方法
提取C57小鼠腹腔巨噬细胞,IFN-γ 60 ng/mL作用下体外培养巨噬细胞,分组检测雌激素对巨噬细胞影响:实验组为雌二醇(1.0 nmol/L)处理;抑制剂组为雌二醇(1.0 nmol/L)和雌激素受体拮抗剂(Acolbifene, 4 nmol/L)同时作用;对照组加等量PBS处理,培养48 h后通过Western blot法检测比较巨噬细胞极化蛋白MHC-Ⅱ、iNOS和内质网应激标志性蛋白IRE1α、eIF2α和ATF6的表达水平,通过RT-PCR法检测巨噬细胞TGF-β、IL-6、IL-10、TNFα mRNA水平变化。随后分组检测内质网应激对巨噬细胞的影响:雌激素处理组;雌激素和内质网IRE1α信号抑制剂(4 μ 8 C,50 μmol/L)处理组;IRE1α激动剂组(TG,0.2 nmol/L)处理组;对照组加等量PBS处理,随后检测巨噬细胞的分化情况。
结果
和对照组比较,雌激素处理后巨噬细胞M1相关蛋白MHC-Ⅱ(P=0.021)和iNOS(P < 0.001)表达显著降低,促炎细胞因子TNF-α(P=0.003)和IL-6 mRNA(P=0.004)表达下调,抗炎的M2型细胞因子TGF-β(P=0.002)和IL-10(P=0.008)mRNA表达上升,同时发现内质网相关的IRE1α(P < 0.001)蛋白活化水平升高,其下游转录因子XBP-1(P < 0.001)表达上调,加入雌激素受体阻断剂能够改变这种变化。和单纯雌激素处理组比较,在培养基中加入雌激素的同时加入IRE1α抑制剂4 μ 8 C会导致巨噬细胞M1相关蛋白MHC-Ⅱ(P=0.002)和iNOS(P=0.003)表达显著上调,促炎细胞因子TNF-α(P=0.003)和IL-6 mRNA(P=0.024)表达上调,抗炎的M2型细胞因子TGF-β(P < 0.001)和IL-10(P < 0.001)mRNA表达下调,而激动IRE1α通路会矫正这种变化。
结论
雌激素能够通过上调IRE1α-XBP-1信号轴抑制巨噬细胞促炎表型的分化,从而对炎症反应发挥抑制作用。
Keywords: 雌激素, 巨噬细胞, 内质网应激通路
Abstract
Objective
To explore the mechanism by which estradiol modulates the immunophenotype of macrophages through the endoplasmic reticulum stress pathway.
Methods
Peritoneal macrophages isolated from C57 mice were cultured in the presence of 60 ng/mL interferon-γ (IFN-γ) followed by treatment with estradiol (1.0 nmol/L) alone, estradiol with estrogen receptor antagonist (Acolbifene, 4 nmol/L), estradiol with IRE1α inhibitor (4 μ 8 C), or estradiol with IRE1α agonist. After the treatments, the expression levels of MHC-Ⅱ, iNOS and endoplasmic reticulum stress marker proteins IRE1α, eIF2α and ATF6 in the macrophages were detected with Western blotting, and the mRNA levels of TGF-β, IL-6, IL-10 and TNF-α were detected with RT-PCR.
Results
Estrogen treatment of the macrophages significantly decreased the expressions of M1-related proteins MHC-Ⅱ (P=0.021) and iNOS (P < 0.001) and the mRNA expressions of TNF-α (P=0.003) and IL-6 (P=0.004), increased the mRNA expression of TGF-β (P=0.002) and IL-10 (P=0.008), and up-regulated the protein expressions of IRE1α (P < 0.001) and its downstream transcription factor XBP-1 (P < 0.001). Addition of the estrogen inhibitor obviously blocked the effect of estrogen. Compared with estrogen treatment alone, combined treatment of the macrophages with estrogen and the IRE1α inhibitor 4 μ 8 C significantly up-regulated the protein expressions of MHC-Ⅱ (P=0.002) and iNOS (P=0.003) and the mRNA expressions of TNF-α (P=0.003) and IL-6 (P=0.024), and obviously down-regulated the mRNA expression of TGF-β (P < 0.001) and IL-10 (P < 0.001); these changes were not observed in cells treated with estrogen and the IRE1α agonist.
Conclusion
Estrogen can inhibit the differentiation of murine macrophages into a pro-inflammatory phenotype by up-regulating the IRE1α-XBP-1 signaling axis, thereby producing an inhibitory effect on inflammatory response.
Keywords: estrogen, macrophages, endoplasmic reticulum stress pathway
雌激素是人体内重要的激素之一,不仅在女性第二性征的发育中起重要作用,而且在维持体内免疫环境的平衡起着重要作用,其对免疫细胞的影响也越来越引起人们的关注。巨噬细胞作为免疫细胞的重要组成部分,在体液免疫和细胞免疫的进程中发挥关键作用[1-3],主要分为促炎的M1型和抗炎的M2型,M1型巨噬细胞高表达一氧化氮合酶和肿瘤坏死因子等促炎介质,在体内能够引起炎症反应[4];M2型巨噬细胞高表达IL-10和精氨酸酶1,在组织修复和抑制炎症反应方面发挥积极作用[5]。有研究显示雌激素会引起巨噬细胞表型的分化[6, 7],但其内在的作用机制尚缺乏文献报道,需要进一步研究。
内质网应激是指在组织缺氧、损伤、炎症等环境下,细胞为了缓解压力以及恢复内质网功能,产生的一个信号级联反应[8],已有研究证实其在巨噬细胞产生细胞因子方面发挥调节作用[8, 9],也有研究者发现在肿瘤组织中内质网应激在调控巨噬细胞极化中发挥重要作用[10],这些研究表明内质网应激在巨噬细胞极化及发挥生物功能上发挥了重要作用。作为巨噬细胞分化及免疫功能研究方面的两个热点,雌激素和内质网应激两者在巨噬细胞分化中起到何种作用呢?两者是否相互联系?目前国内外对此研究较少,其中的机制及联系尚不明确。本研究旨在探讨雌激素通过内质网应激途径影响巨噬细胞极化的可能机制。
1. 材料和方法
1.1. 实验动物
雌性C57BL/6J小鼠,8~10周龄,购自南方医科大学实验动物中心,使用许可证号:SYXK(粤)2016- 0167,动物生产许可证号:SCXK(粤)2016-0041。饲养于SPF级动物房。所有动物处理和程序均经南方医科大学动物保护和使用委员会批准(L2016068)。
1.2. 实验试剂
DMEM培养基(Hycolone,SH30022.01),胎牛血清(FBS,Gibco,10099-141),青-链霉素溶液(Gibco,15140-122),RAPI蛋白裂解液(碧云天,P0013C),PMSF(碧云天,ST506),雌二醇(Sigma,E2758),雌激素受体拮抗剂(MCE, HY-16023A),逆转录试剂盒(Theromfish,K1622),SYBR荧光定量PCR试剂盒(Theromfish,11762500),5 × loadding buffer(弗德,FD002),PVDF膜(默克,32031613),ECL显影试剂盒(弗德,FD8000),IRE1α抗体(novas,NB100-2324),pIRE1α抗体(novas,NB100-2323),eIF2α抗体(abcam,ab5369),P-eIF2α抗体(abcam,ab32157),ATF6抗体(novas,NBP1-40256),IRE1α激动剂TG(Santa Cruz,67526-95-8),IRE1α抑制剂4 µ 8 C(MCK, HY-19707)。实验仪器实时荧光定量PCR仪(Stepone plus),流式细胞仪(BD,FACS Calibur)。
1.3. 实验方法
1.3.1. 小鼠巨噬细胞分离
小鼠颈椎脱臼处死,75%酒精浸泡5 min,10 mL无菌注射器吸取5 mL培养基,小心的注射到小鼠腹腔中,按摩小鼠腹部2~3 min,剪开腹部浅层皮肤,无菌注射器吸取腹腔液于离心管中,500 g离心5 min,磷酸缓冲盐溶液(PBS)冲洗2次,随后接种到培养皿中37 ℃培养,通过流式细胞术和免疫荧光对巨噬细胞进行鉴定。
1.3.2. 细胞分组
细胞正常培养24 h后,0.25%的胰酶消化细胞,完全培养基终止消化后以1×106孔,接种到6孔板中,分别给予雌激素(1 nmol/L)、雌激素(1 nmol/L)+ 雌激素受体拮抗剂(4 nmol/L),雌激素(1 nmol/L)+ IRE1α抑制剂(50 µmol/L)、IRE1α激动剂(0.2 nmol/L)处理,空白对照组加等量PBS处理,随后正常培养。
1.3.3. 流式细胞术分析鉴定
胰酶消化巨噬细胞,10% 胎牛血清(FBS)完全培养基终止消化,800 r/min离心5 min,收集细胞,PBS重悬细胞至106,加入PE-F4/80抗体,室温避光孵育15 min,加入PBS重悬清洗,1000 r/min离心5 min,重复2次,100 µL PBS重悬细胞,上机检测。
1.3.4. 免疫荧光鉴定巨噬细胞
将巨噬细胞接种到爬片上,培养48 h后,吸弃培养基,预冷的PBS洗2次,加入PE-F4/80抗体,室温避光孵育15 min,加入PBS重悬清洗2次,在载玻片上滴加DAPI封片剂,将爬片倒扣于载玻片上,荧光显微镜下观察。
1.3.5. 实时定量PCR检测
细胞培养至48 h后,吸弃培养基,预冷的PBS冲洗2次,每孔加入1 mLTrizol试剂,充分裂解细胞,按照说明书操作提取细胞总RNA,测定浓度后进行反转录。后续cDNA产物通过SYBR试剂进行实时定量PCR检测,计算目的基因的相对表达量(表 1)。
表 1.
基因的引物序列
Primer sequences for the target genes
| Target gene | Sequences |
| IL-6 | Forward: GAGTCCGCAGCAGGTG |
| Reverse: TGTCAGAGTCCATGGGA | |
| TNF-α | Forward: GGCGGTGCCTATGTCTCA |
| Reverse: CCTCCACTTGGTGGTTTGT | |
| IL-10 | Forward: TTTCAAACAAAGGACCAG |
| Reverse: GGATCATTTCCGATAAGG | |
| TGF-β | Forward: GGCGGTGCTCGCTTTGTA |
| Reverse: TCCCGAATGTCTGACGTATTGA | |
| XBP-1 | Forward: GAGTCCGCAGCAGGTG |
| Reverse: GTGTCAGAGTCCATGGGA | |
| ATF6 | Forward: GATGGCTGTCCAGTACACA |
| Reverse: GCAGATGATCCCTTCGAAAT | |
| eIF2α | Forward: AATCAATGTCGCTAACAAGG |
| Reverse: TAAAGTTGTAGGTTAGGCGT | |
| GAPDH | Forward: CAATGTGTCCGTCGTGGATCT |
| Reverse: GTCCTCAGTGTAGCCCAAGATG |
1.3.6. 蛋白印迹分析检测
配制蛋白裂解液,1 mL RAPI裂解液加入10 µL 100 mmol/L蛋白酶-磷酸酶抑制剂和10 µL 100 nmol/L的PMSF。细胞培养至48 h后,吸弃培养基,预冷的PBS冲洗2次,每孔加入300 µL配制好的裂解液,冰上裂解30 min充分裂解细胞,吸取细胞匀浆于洁净的EP管中,4 ℃ 14 000 r/min离心15 min,小心的吸取上清加入5×Loadding buffer,煮沸变性5 min。制备10%分离胶,5%浓缩胶,80~120 V电泳,PVDF膜90 V恒压转膜90 min,5%脱脂奶粉封闭30 min,加入一抗稀释液配制的一抗,4 ℃摇床孵育过夜,TBST清洗5 min×3次,加入二抗,室温孵育1 h。TBST清洗5 min×3次,ECL显影液显影曝光,ImagJ软件分析条带灰度值。
1.4. 统计学分析
采用统计学软件SPSS 20.0统计分析实验数据,计量资料使用均值±标准差表示。组间比较采用单因素方差分析以及独立样本t检验,P < 0.05时认为差异具有统计学意义。所有实验进行3次以上重复。
2. 结果
2.1. 巨噬细胞的流式鉴定
免疫荧光对F4/80染色的巨噬细胞进行分析,结果显示分离出的细胞主要为F4/80+细胞(图 1A)。同时采用流式细胞术对细胞纯度进行分析鉴定,结果显示F4/80+ 细胞率达98.8%(图 1B)。
图 1.
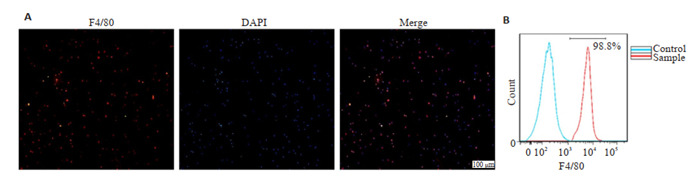
巨噬细胞免疫荧光染色鉴定结果
Identification of the isolated macrophages by immunofluorescence staining. A: Immunofluorescence analysis of the macrophages with F4/80 staining. B: Flow cytometry of the macrophages.
2.2. 雌激素对炎症条件下巨噬细胞极化的影响
Western blot结果显示,与对照组比较,雌激素处理抑制巨噬细胞促炎相关蛋白MHC-Ⅱ(P=0.021)和iNOS(P < 0.001)的表达,加入雌激素受体拮抗剂中断雌激素信号后,这种抑制作用显著被矫正(图 2A)。对3组巨噬细胞总RNA进行定量分析,同样发现雌激素处理后促炎的TNF-α(P=0.003)、IL-6(P=0.004)表达下调,而TGF-β(P=0.002)、IL-10(P=0.008)等抑炎分子表达上调,而雌激素受体阻断剂能够矫正这种变化(图 2B)。
图 2.

各处理组巨噬细胞促炎相关蛋白及相关因子mRNA表达情况
Expression of pro-inflammatory proteins and mRNA expressions of macrophage polarization-related proteins in the macrophages. A: Expression levels of iNOS and MHC-Ⅱ detected by Western blotting. B: mRNA expressions of macrophage polarization-related proteins. *P < 0.05.
2.3. 雌激素对巨噬细胞内质网应激的影响
Western blot结果显示,与对照组比较,雌激素处理后IRE1α信号显著激活(P < 0.001),加入雌激素受体拮抗剂中断雌激素信号后,其活化被抑制,而其它两条信号ATF6(P=0.986)和eIF2α(P=0.975)没有显著变化(图 3A)。实时荧光定量PCR分析同样发现刺激处理后巨噬细胞的IRE1α下游XBP1(P < 0.001)显著上调,而雌激素受体抑制剂能够抑制其上调,另外两条通路ATF6(P=0.985)和eIF2α(P=0.976)无显著变化(图 3B)。
图 3.
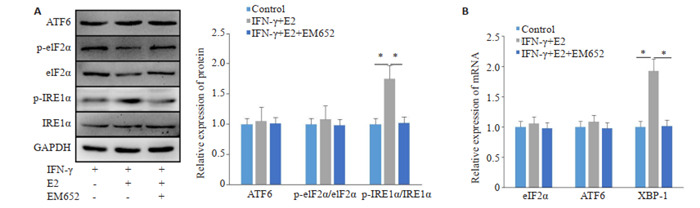
各处理组巨噬细胞内质网应激相关分子表达情况
Expression of endoplasmic reticulum stress-related molecules in the macrophages. A: Expression of ATF6, IRE1α and eIF2α proteins. B: The mRNA expressions ofATF6, IRE1α And eIF2α. *P < 0.05.
2.4. 雌激素通过IRE1α通路对巨噬细胞极化的影响
同时存在雌激素和IRE1α抑制剂时,巨噬细胞M1相关蛋白MHC-Ⅱ(P=0.002)和iNOS(P=0.003)表达显著上调,促炎细胞因子TNF-α(P=0.003)和IL-6 mRNA(P=0.024)表达上调,抗炎的M2型细胞因子TGF-β(P < 0.001)和IL-10(P < 0.001)mRNA表达下调,而激动IRE1α通路会矫正这种变化,同时给予雌激素并激动IREα会导致类似的变化(图 4)。
图 4.

各处理组巨噬细胞极化相关蛋白及促炎因子表达情况
Expression of macrophage polarizationrelated proteins and proinflammatory cytokines in each group. A: Expressions of macrophage polarization- related proteins. B: mRNA expressions of ATF6, IRE1α and eIF2α. *P < 0.05.
3. 讨论
雌激素是类固醇激素,具有广泛的生理功能。其存在主要有三种形式:雌二醇、雌酮和雌三醇,其中以雌二醇为主[11]。有研究发现在体内雌激素能够和雌激素受体结合,在调节骨骼发育、心血管系统保护和体内平衡中起着重要作用[12]。雌激素能够通过和免疫细胞上的雌激素受体结合,调控免疫细胞的增殖和分化,并最终影响免疫细胞的功能来发挥调控作用[13-15]。在局部炎症反应中,雌激素能够刺激浆细胞产生免疫球蛋白[16],直接上调B细胞活化分子CD22、SHP-1和Bcl-2的表达,同时下调B细胞凋亡分子PD-1的表达[17-19]。除此之外,雌激素能够通过增加调节T细胞的数量和占比,由此对先天免疫产生抑制作用[21],调控T细胞中趋化因子受体的表达,进而抑制单核细胞和中性粒细胞促炎细胞因子的分泌[22],调控自然杀伤细胞的细胞毒性影响损伤组织的炎症进程[23]。而在炎症反应过程中,内质网应激发挥了重要作用。虽然雌激素在免疫方面的研究较多,但是关于雌激素对巨噬细胞的影响及其机制尚缺乏相关报道。
在本研究中,我们首先通过体外培养巨噬细胞,并在炎症环境下给予雌激素处理,发现雌激素能够下调巨噬细胞向促炎表型的分化,为了验证这一变化是由雌激素通路引起的,我们添加了雌激素受体抑制剂做对照,结果发现巨噬细胞的分化变化得到了矫正。有学者研究显示雌激素在调节癌症患者癌变部位未折叠蛋白反应中起到重要作用[24-26],而未折叠蛋白反应是引起内质网应激的重要信号。那么雌激素影响巨噬细胞的分化是否和内质网应激之间存在某种联系呢?内质网应激发生后主要通过UPR下游的ATF6、IRE1α、eIF2α三条主要的信号通路调节免疫过程[27]。
为了验证我们的假设我们检测了雌激素处理后巨噬细胞中内质网应激关键蛋白ATF6、IRE1α、eIF2α的变化情况,结果显示IRE1α的活化及其下游功能分子XBP1s的转录与雌激素密切相关。IRE1α及其下游的XBP1在调节细胞因子的合成和释放中起着重要作用[28],最新的研究发现XBP1能够促进了NF-κB的激活,进而引起主要在巨噬细胞中的炎症细胞因子的释放[29]。根据之前的报道,内质网应激相关反应主要参与促炎作用[30]。然而,我们发现IRE1α抑制导致IFN-γ处理的巨噬细胞促炎分化,这一观点与最近发现的ER应激负调节干扰素引起的先天免疫相一致[31]。这可能和雌激素作用存在某种关联性。那么雌激素调控的巨噬细胞的分化是否与IRE1α信号的变化有直接联系呢?为探究这一问题,我们对巨噬细胞在给予雌激素作用的同时抑制IRE1α通路随后对巨噬细胞进行蛋白水平和基因水平的检测,发现通过抑制IRE1α信号能够矫正巨噬细胞受雌激素影响导致的促炎分化的抑制情况,而直接激活IRE1α信号同样能够抑制巨噬细胞的促炎分化。这提示我们雌激素对巨噬细胞促炎症表型分化的抑制作用有可能是通过激活内质网应激中的IRE1α-XBP1信号通路实现的,这与学者发现的IRE1α-XBP1能够在M2型巨噬细胞上表达激活的结论相辅相成。
综上所述,本研究阐释了雌激素通过激活IRE1α- XBP1信号抑制巨噬细胞的促炎分化的可能机制,为雌激素对巨噬细胞引起的炎症反应的治疗提供新的治疗策略和理论基础。
Biographies
卓灵剑,医师,E-mail: 277682948@qq.com
王烁辰,主治医师,E-mail: 277682948@qq.com
Funding Statement
国家自然科学基金(81671905);南方医科大学南方医院院长基金(2021C024、2021C040)
Supported by National Natural Science Foundation of China (81671905)
Contributor Information
卓 灵剑 (Lingjian ZHUO), Email: 277682948@qq.com.
王 烁辰 (Shuochen WANG), Email: 277682948@qq.com.
李 想 (Xiang LI), Email: lixiang920402@163.com.
References
- 1.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Hannemann N, Apparailly F, Courties G. New insights into macrophage heterogeneity in rheumatoid arthritis. Jo Bone Spine. 2021;88(1):105091. doi: 10.1016/j.jbspin.2020.105091. [DOI] [PubMed] [Google Scholar]
- 3.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol Mech Dis. 2020;15(6):123–47. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. 2015;20(8):816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dou C, Ding N, Zhao CR, et al. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J Bone Miner Res. 2018;33(5):899–908. doi: 10.1002/jbmr.3364. [DOI] [PubMed] [Google Scholar]
- 7.Qi XY, Zhang BC, Zhao Y, et al. Hyperhomocysteinemia promotes insulin resistance and adipose tissue inflammation in PCOS mice through modulating M2 macrophage polarization via estrogen suppression. Endocrinology. 2017;158(5):1181–93. doi: 10.1210/en.2017-00039. [DOI] [PubMed] [Google Scholar]
- 8.Rashid HO, Yadav RK, Kim HR, et al. ER stress: Autophagy induction, inhibition and selection. Autophagy. 2015;11(11):1956–77. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Chen X, Lee AH, et al. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411–8. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batista A, Rodvold JJ, Xian S, et al. IRE1α regulates macrophage polarization, PD-L1 expression, and tumor survival. PLoS Biol. 2020;18(6):e3000687. doi: 10.1371/journal.pbio.3000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008;22(9):3328–36. doi: 10.1096/fj.08-107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):557–68. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Doyle HH, Murphy AZ. Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res. 2017;95(1/2):487–99. doi: 10.1002/jnr.23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–9. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirandola L, Wade R, Verma R, et al. Sex-driven differences in immunological responses: challenges and opportunities for the immunotherapies of the third millennium. Int Rev Immunol. 2015;34(2):134–42. doi: 10.3109/08830185.2015.1018417. [DOI] [PubMed] [Google Scholar]
- 16.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. JAllergy Clin Immunol. 1999;103(2):282–8. doi: 10.1016/S0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 17.Dinesh RK, Hahn BH, Singh RP. PD- 1, gender, and autoimmunity. Autoimmun Rev. 2010;9(8):583–7. doi: 10.1016/j.autrev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato S, Tuscano JM, Inaoki M, et al. CD22 negatively and positively regulates signal transduction through the B lymphocyte antigen receptor. Semin Immunol. 1998;10(4):287–97. doi: 10.1006/smim.1998.0121. [DOI] [PubMed] [Google Scholar]
- 19.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2(1):13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 20.Merino R, Ding L, Veis DJ, et al. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13(3):683–91. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arruvito L, Sanz M, Banham AH, et al. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178(4):2572–8. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 22.Mo RR, Chen J, Grolleau-Julius A, et al. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174(10):6023–9. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 23.Hao S, Zhao JL, Zhou JJ, et al. Modulation of 17 β-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol. 2007;7(13):1765–75. doi: 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6(2):635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Saatcioglu F. Targeting the unfolded protein response in hormone-regulated cancers. Trends Cancer. 2020;6(2):160–71. doi: 10.1016/j.trecan.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Ma TT, Huang C, et al. Flavonoids from Litsea coreana decreases TNF-α secretion from peritoneal macrophages in adjuvantinduced arthritis rats via UPR pathway. Am J Chin Med. 2014;42(4):905–19. doi: 10.1142/S0192415X14500578. [DOI] [PubMed] [Google Scholar]
- 28.Zhou CM, Luo LM, Lin P, et al. Annexin A2 regulates unfolded protein response via IRE1-XBP1 axis in macrophages during P. aeruginosa infection. J Leukoc Biol. 2021;110(2):375–84. doi: 10.1002/JLB.3A1219-686RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reverendo M, Mendes A, Argüello RJ, et al. At the crossway of ERstress and proinflammatory responses. FEBS J. 2019;286(2):297–310. doi: 10.1111/febs.14391. [DOI] [PubMed] [Google Scholar]
- 30.Ho HJ, Huang DY, Ho FM, et al. Inhibition of lipopolysaccharideinduced inducible nitric oxide synthase expression by endoplasmic Reticulum stress. Cell Signal. 2012;24(11):2166–78. doi: 10.1016/j.cellsig.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 31.di Conza G, Ho PC. ER stress responses: an emerging modulator for innate immunity. Cells. 2020;9(3):695. doi: 10.3390/cells9030695. [DOI] [PMC free article] [PubMed] [Google Scholar]


