Highlights
-
•
A bilayer film was prepared with incorporation of Ascorbic Acid and Hyssopus officinalis essential oil.
-
•
Antioxidant activity and total phenolic content of films were increased with Hyssopus officinalis oil and Ascorbic Acid addition.
-
•
Mechanical properties of bilayer films were changed via addition of Hyssopus officinalis oil and Ascorbic Acid.
-
•
Water vapor permeability and water solubility of bilayer samples were determined.
PubMed CID: Isopinocamphone (PubChem CID: 84532), 3-Pianone (PubChem CID: 11038), Pinocamphone (6425107), Thymol (PubChem CID: 6989), Carvacrol (PubChem CID: 10364), Cymene (PubChem CID: 10703), myrtenol (PubChem CID: 10582), Alpha- pinene (PubChem: 6654), trans-β-Ocimene (PubChem CID: 5281553), Cis-Verbenol (PubChem CID: 164888), Eucalyptol (PubChem: 2758).
Abbreviations: EO, Essential Oil; HO, Hyssopus officinalis; AA, Ascorbic Acid
Keywords: Food preservation, Active packaging, Antioxidant bilayer film, Hyssopus officinalis, Mechanical properties
Abstract
In this study, a bio-based bilayer edible film based on gelatin/frankincense, with the incorporation of different concentrations of Ascorbic acid (AA) (0, 1, 2%) into the inner layer (gelatin) and Hyssopus officinalis (HO) (0, 0.75, 1.5%) essential oil in the outer layer (frankincense) was prepared. A significant increase (p < 0.05) in b* and a remarkable decrease in whiteness and lightness of the films were seen via increasing the HO ascribed to the Total Phenolic Content of HO and non-enzymatic browning. Although there was a significant decrease (p < 0.05) in Tensile Strength with the addition of HO, Elongation at Break was increased significantly as a function of HO, which is correlated with a dense and compact network in SEM images. The maximum thickness of film emulsified with 1.5%HO + 2%AA ascribed to the accumulation of solid content. The improvement in Water Contact Angle (℃) and a reduction in Water Vapor Permeability (gr/s mPa) have occurred due to the hydrophobic nature of HO.
1. Introduction
Food quality and safety is considered a vital characteristic of food products determined by several attributes, including hygienic, nutritional, and organoleptic properties (Shirani et al., 2020). Food packaging is one of the determinant factors influencing food quality and consumer acceptance. Conventional packaging methods were widely centered on fossil fuels-based materials to produce synthetic polymers, including polyvinylchloride, polystyrene, polyamide, polypropylene, ethylene vinyl alcohol, and other synthetic polymers due to good mechanical and physical attributes (Espitia et al., 2014, Ojagh et al., 2010). Biopolymer films based on biodegradable polymers, including proteins, polysaccharides, and lipids obtained from plants, animals, and microorganisms, contribute to an ideal eco-friendly, renewable, and sustainable candidate for replacing traditional petroleum-derived products packaging materials (Sani et al., 2021). Additionally, these biomaterials are considered appropriate carriers for additive compounds, including antioxidant, antimicrobial, phytochemicals, and flavorings agents (Ojagh et al., 2010). Active films are generally recognized as self-standing covering layers of food products manipulated with biologically active materials (Espitia et al., 2014). Biopolymer-based packaging enables increasing shelf life or improves foodstuffs' safety and organoleptic properties by manipulating the surrounding conditions of packaged food and maintaining food quality (Priyadarshi et al., 2018).
Over the last decade, many studies have performed on applying different additives to the polymeric films based on their antioxidant and antimicrobial capacities. These additives must be approved by Food and Drug Administration (FDA), not have harmful effects on health and left any negative effects on sensorial attributes of the food stuffs. One of the most generally used proteins for fabricating films is gelatin, a meat protein isolated by hydrolyzation of the insoluble collagen present in the bones, skin, and tendons made over animal slaughtering and processing (Sani et al., 2021). Gelatin is obtained from hydrolyzation of the insoluble collagen by heating the collagen in an alkaline solution or strong acid at high temperatures like 80 ℃, followed by purifying and converting to powder which has a particular role in the food industry. At high concentrations, the gelatin can construct solid-like features owing to the 3D network created by gelatin molecules (Gennadios, 2002, Mohamed et al., 2019, Sani et al., 2021). Many studies show that most protein-based films, such as gelatin, are dry and fragile (Yakimets et al., 2005) and in comparison to the Petro-based polymers have a low gas barrier and high sensitivity to humidity due to their inherent hydrophilic behavior (Rivero et al., 2009). However, gelatin films could be developed in suitable thickness and mechanical properties for food industry goals (Sani et al., 2021).
Principally, protein-based and carbohydrate-based films are good barriers to oxygen because their network is based on hydrogen and also its tight-packed structure. Nevertheless, hydrophilic characteristics of polymeric films makes them present limited water vapor permeability (Bermúdez-Oria et al., 2017). Previously, gelatin has been used to fabricate edible films with other polymers (Pereda et al., 2011, Vejdan et al., 2016). Films centered on only one polymer have limitations, e.g., poor physical and mechanical properties, which constrict their use in practical bio-packaging applications. To tackle this problem, the emergence of edible films based on one polymer in combination with other biopolymers has become a study hotspot recently (Baron et al., 2017).
Frankincense 1known as an aromatic resin isolated from Boswellia carterii in East Africa and China and commonly applied as a medicinal plant in India, China, Arabian Peninsula, and Northeast Africa. Frankincense contains three main constituents: the content of the essential oil is 5–9%, 65–85% is an ethanol-soluble gum, and the rest polymer is water-soluble content (Rijkers et al., 2006). Different studies have been approved that the terpenes which are considered as natural phenolic compounds are abundant in Frankincense. Briefly, α- Pinene, Trans-β-Ocimeneare, Hepthene trimethyl, and Cis-Verbenol are major compounds in Frankincense, based on gas chromatography information which were conducted in our previous research2 (Pirnia et al.2020)(Sharma et al., 2009).
Hyssopus officinalis (HO) has been used to treat asthma, bronchitis, herpes simplex, and HIV. HO essential oil is mainly composed of pinocamphon, α-pinene, β-pinene, iso pinocamphon, myrtenil-methyl, and pinocarvan (Said-Al Ahl et al., 2015). The antifungal and antioxidant properties of Hyssopus officinalis L. subsp. pilifer essential oil and deodorized extracts were proven by Dzamic et al. (2013). Likewise, the antimicrobial and antioxidant potential of HO has been demonstrated by previous studies (Džamić et al., 2013, Kizil et al., 2010, Soleimani et al., 2011).
Ascorbic Acid, identified as vitamin C is a strong antioxidant found mainly in fresh fruits and vegetables. acid ascorbic can scavenge the free radicals by transferring the electrons. Moreover, AA acts as an oxygen scavenger and chelator of pro-oxidative metal ions. In addition, it is widely used as a natural preservative food stuffs. Proficient antioxidant capacity and a cheap mass-production make AA an appropriate ingredient in the novel packaging attitudes (Kowalczyk, 2016).
Incorporation of Gelatin/Frankincense bilayer edible films with vitamin C and Hyssopus officinalis oil was hypothesized to present advantage antioxidant and preservation attributes, and to the best of our knowledge, it has never been studied before. Therefore, the overall objective of the present study is to design gelatin/frankincense-based bilayer films with the addition of vitamin C in the inner layer (gelatin) and HO in the outer layer (frankincense). Subsequently, the treated and control films' physical, mechanical, structural, barrier properties and antioxidative capacity have been screened and discussed.
2. Materials and methods
1.1. Materials
The Frankincense used in this research was bought from a market at Jiroft-Iran. Glycerol (analytical grade) and tween 80 (Fluka, Sigma-Aldrich, USA) were applied for film- developing. Folin-Cio-calteu reagent, sodium carbonate, ascorbic acid (AA), and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co (St. Louis, MO). Hyssopus officinalis essential oil (HO) was provided by Barij Company in Kashan-Iran. Ethyl alcohol (≥99.7% CH3OH, 46.07 g/mol) was obtained from (tamad Kala Co, Iran). Supplementary materials including NaOH, sodium, calcium chloride, and bovine gelatin were purchased from Merck Corporation, Germany.
1.2. Purification of polysaccharides of frankincense gum
Purification of polysaccharides from frankincense has been performed by a continuous process. 10 gr of frankincense powder was applied for extracting by 250 mL ethanol 95% (v/v) at 80 ℃ for 4 h. The extract was filtered, then the rest was kept in 250 mL water at 80℃ during the night. The solid residue has been removed by filtration, and supernatant has been concentrated to around 120 mL. Proteins of the samples have been discarded with 5 mL of Sevag reagent (1:4n-butanol: chloroform). Next, polysaccharides were purified, and crude polysaccharides were dissolved in distilled water and afterwards filtered via a 0.2 mm syringe filter (Zhang et al., 2020).
1.3. Film elaboration
The films were prepared according to the casting procedure. Firstly, gelatin film-forming solution was prepared by dissolving 3% gelatin in distilled water at room temperature, and a magnetic stirrer was employed with 750 rpm at 55-60℃ for 30 min to increase the dissolving. After heating, the gelatin film solution was cooled to 21 ± 1℃, and then ascorbic acid (AA) was added at various concentrations (0, 1%, and 2% (v/v) of film solution). At the same time, the 2 N NaOH was used to adjust the pH up to 8. Also, a Vacuum pump desiccator has been used to remove the air bubbles at film solution. Subsequently, a second film-forming solution was prepared by dissolving 6% frankincense (polysaccharides) at the mentioned condition.
Hyssop officinalis oils were integrated into frankincense film suspensions at the different concentrations (0, 0.75%, and 1.5% (v/v) of the solution). Tween 80 at levels of 0.2% (v/v) of HO was loaded to prevent the dissolution of the essential oils. Additionally, 35% (w/w) of the Glycerol was added to the solutions as a plasticizer. In this study, gelatin film was applied as the inner layer of the bilayer film, while frankincense suspension was poured to form a layer on it. Thin Films were shaped by casting 25 g of the gelatin forming film emulsion onto the Petri dishes (with a diameter of 20 cm) and then kept in the oven at 35-40℃ for 24 h to be dried and have an adhesive surface. Then, frankincense film-forming solution was poured on top of the first layer (gelatin film). Finally, plates were kept in the oven at 35℃ for 24–36 h. Film samples then were stored at room temperature.
1.4. Film characterization
1.4.1. Scanning Electronic Microscopy
Firstly, the film was cryo-fractured by plunging in liquid nitrogen (5 mm × 5 mm) for 2 min and then mounted on aluminum stubs using double-sided tape. Later, the portion was covered with a thin gold layer (20 nm) for 5 min by a sputter coater. At last, the samples were observed by an SEM apparatus at 20 kV and magnification of 4000. Microstructure and surfaces images of G/F bilayer films were investigated by Scanning Electronic Microscopy (SEM) (Philips XL30, Netherlands) (Rivero et al., 2009).
1.4.2. Mechanical characteristics
Firstly, the samples were cut into 2.5 × 10 cm2 pieces and were placed in a desiccator with Mg2NO3 to reach the RH of 53%. The film strips were kept at 25℃ for 48 h. Next, the tensile strength (TS, MPa) and the elongation to break (EB, %) were measured by an Instron Universal Testing Machine (Model A1 700, Gotech, Taiwan). Average of six replication were applied. This procedure has been described in ASTM D882-01 (ASTM, 1996)(Hosseini et al., 2009).
| (1) |
| (2) |
F max: the maximum applied force W: film width (mm) T: film thickness (m).
L: final length (mm), L0: initial length(mm) A: distance between the blades of a machine.
1.4.3. Film thickness
The film thickness was determined with a hand-held digital micrometer (Mitutoyo No. 293–766, Tokyo, Japan) with an accuracy of 0.001 mm. The average of five points of film pieces was stated as the film thickness(Zareie et al., 2020).
1.4.4. Water vapor permeability
The water vapor permeability of the film samples was measured based on ASTM Method E96-95 (ASTM, 2016) together with some adjustments. Briefly, the films were wrapped over the tubes having anhydrous calcium chloride (0% RH). The permeation Tubes were then sited in a desiccator comprising saturated NaCl (RH 75%), and then they were weighed over the 72 h at 12 h intervals and was plotted as a function of time until the steady-state was witnessed in the weight loss against time. All of the tests were carried out in triplicate for each film. The WVP of the films was calculated the next equations:
| (3) |
| (4) |
WVTR: Water Vapor Transmission Rate S: Slope of the regression model A: the permeation area (m2).
X: Film thickness (mm) ΔP: water vapor pressure difference across the film (KPa).
1.4.5. Moisture content
The moisture content of the film strips was determined with an oven-drying method (105℃ for 24 h). Measurements were carried out in triplicate (Zareie et al., 2020).
| (5) |
Mt: moisture content of the sample (%) Wi: Initial sample weight (g) Wd: Dried sample weight (g).
1.4.6. Water contact angle (WCA)
Surface hydrophobicity of films in terms of Water Contact Angle (WCA) was evaluated using a Goniometer (Krüss Drop Shape Analyzer, Germany, Version 1.4.1.2) which was applied with image analysis software (Image J). A droplet of water (2.0 μl) was dropped on the film surface and captured at the moment and over 60 s. The angle of water and film surface was conducted by software (Abdelhedi et al., 2018). The tests were performed in triplicate and the average value was expressed as a WCA.
1.4.7. Color and transparency measurement
The film strips' colorimetric parameters (a*, b*, and L*) were assessed using a colorimeter (Chroma meter CR-410, Japan). A white color plate was firstly used as a standard to calibrate the colorimeter (L*= 88.49, a*= 0.85, b*= − 4.78), and the Transparency, Whiteness Index (WI), and overall color difference (ΔE) were then determined as below:
WI = 100- [(100-L*)2 + a*2 + b*2]0.5 (6).
| (7) |
T%= L* (8).
L*: Lightness (0 for black to 100 for white) b*: (Yellow to blue) a*: (Red to green).
1.4.8. Total phenolic content
Total phenol content (TPC) of the samples was assessed based on an approach expressed by Siripatrawan and Harte (2010) via Folin-Ciocalteu calorimetrically technique (Siripatrawan & Harte, 2010). Briefly, an extract of film samples was prepared by soaking 25 mg of the film in 3 mL of distilled water, 0.1 mL of which was mixed well with 7 mL distilled water and 0.5 mL Folin reagent (Merck company, Germany). The solution was incubated for 8 min at room temperature. Then, 1.5 mL sodium carbonate and 0.9 mL distilled water were added, and the mixture was kept in the dark for 2 h at room temperature. The solution was evaluated for absorbance at 765 nm using a spectrometer in the last stage. The TPC value of samples was expressed as mg gallic acid equivalent/gr film.
1.4.9. Antioxidant characteristic
The antioxidant capacity of the film samples was established considering the report of Shojaee-Aliabadi et al. (2012)(Shojaee-Aliabadi et al., 2013). Concisely, 25 mg of film sample was dissolved in 5 mL of distilled water to prepare the extract, 0.1 mL of the film extract solution were added to 3.9 mL of the DPPH solution (0.1 mM methanol solution), after that 60 min incubation in dark at room temperature. The absorbance was assessed at 517 nm using a Perkin- Elmer spectrophotometer and the methanol was used as a reference. The percentage of DPPH radical-scavenging activity was obtained using the following equation:
| (9) |
A reference: absorbance of reference A sample: absorbance of sample.
1.4.10. Water solubility
Firstly, film samples were cut into pieces around three 2 cm × 3 cm, weighed, and submerged in 50 mL of distilled water for 6 h under constant stir at room temperature. Subsequently, the film strips were oven-dried at 105℃ to reach stable dry weight (This stage was previously used to assess the samples' dry weight and water content). Tests were performed in triplicate (Ojagh et al., 2010). The water solubility value of the film strips was attained as following:
| (10) |
3. Results and discussion
2.1. Film morphology
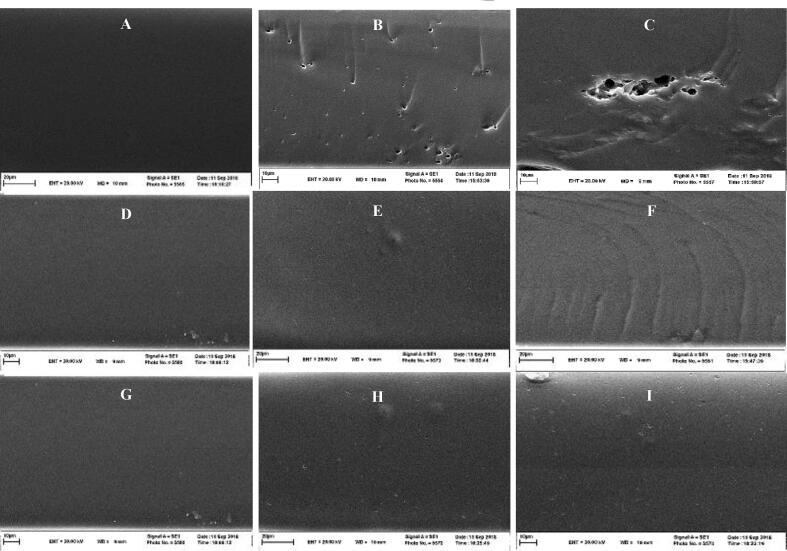
The SEM evaluations of bilayer films provide important information associated with the microstructure distribution of components in the film fabricating matrix and also present a practical understanding of a film’s characteristics. SEM images of film samples are provided in Fig. 1. Prepared films with a higher portion of HO displayed remarkable changes in terms of surface microstructure, that is, rough surface compared to the pure control film, which enjoys coherent, homogenous, and relatively smooth dispersion of the components due to the cohesion and compatibility of G/F film matrix network. As it is clear in Fig. 1, the film microstructure tends to be more heterogeneous by increasing the HO concentration. It could be attributed to the distribution of HO particles in G/F polymer, causing roughness and porousness in the microstructure of the herein biopolymer film. The results are in line with a report of Ghasemlou et al., 2013 (Ghasemlou et al., 2013).
Fig. 1.
SEM micrographs of the microstructures of the films. (A) control, (B) 0.75%HO, (C) 1.5% HO, (D) 1%AA, (E) 1%AA + 0.75%HO, (F) 1%AA + 1.5%HO, (G) 2%AA, (H) 2%AA + 0.75%HO, (I) 2%AA + 1.5%HO.
Besides, evaporating the essential oils during the drying stage of the biopolymer film might lead to the presence of micropores and cavities in the film surface. According to the SEM images, films containing AA (i.e., D and G) are much smoother than those with AA and HO (i.e., F and I). The homogeneity of the film containing AA and HO is not as much as the ascorbic acid-loaded films. Ma et al., 2018 stated that by the integration of citric acid to the edible soybean film, the surface was likely to be smoother, and the average gap size was decreased compared to the controls. Having said that, the highest tensile strength of the control and samples incorporated just with AA might be ascribed to the compact and dense microstructure in films which is clear in Fig. 1 A, D, G. Even though the pores and cracks in samples incorporated with HO affect the tensile strength conversely, make them present significant lower tensile strength (Ma et al., 2018, Zareie et al., 2020).
2.2. Mechanical properties and thickness
Mechanical properties were evaluated in terms of Tensile Strength (TS) and Elongation at Break (EB) (%). Generally, TS is described as the amount of maximum stress of a prepared film against applied tensile stress. Also, EB is recognized as a capability of a film to stretch. According to Fig. 2, the value of TS in control was higher, as well as the samples just incorporated with AA. However, the tensile strength decreased significantly with the addition of HO into the film matrix (p < 0.05). The highest tensile strength of the control and samples incorporated just with AA might be ascribed to the compact and dense microstructure in films which is shown in Fig. 1 A, D, G. It seems that the presence of the essential oil in the film matrix causes a structural disruption, and decreased the resistance of the films incorporated with HO. Also, the micropores and cracks in the SEM images of the films might be a strong reason to reduce the TS of HO-based films. Additionally, partial replacement of strong polymer–polymer bonds by weak oil-polymer bonds in essential oil weaken the network structure and, consequently, the emulsified films' tensile strength. Similar results were reported in the study of Rodriguez et al. (2019)(Rodríguez et al., 2020). Maizura et al. (2007) reported that growing the concentration of lemongrass oil in the partially hydrolyzed sago starch-alginate edible film reduced the tensile strength due to lipids' effect on starch chains (Maizura et al., 2007).
Fig. 2.
Tensile strength (A), elongation at break (B) of the gelatin/frankincense bilayer films containing different concentrations of Hyssop oil (HO) and ascorbic acid (AA).
On the other hand, herein films incorporated with 1%AA + 1.5% HO, 1.5% HO, and 2% AA + 1.5% HO presented the highest EB% compared to the other counterparts. Although EB% shows an upward trend over incorporating HO essential oil, EB% of the samples fabricated with just AA and control film were significantly lower (p < 0.05). It is noteworthy to remind that elongation at break and tensile strength are associated with the fabricated films' network microstructure and intermolecular forces. Therefore, the improvement in EB% may be described by the cross-linking and networks, which might be generated as a result of the Millard reaction between amino acids and reducing sugars in the stage of film elaboration. Besides, the improvement in cohesive and extensibility properties of the films might be ascribed to the interactions between phenolic content of the frankincense and other constituents of the film matrix. Ashwar et al. (2015) indicated that the AA might change the network of starch-based films, shrink the movement of the macromolecules in the film environment, resulting in %EB reduction (Ashwar et al., 2015).
The thickness of G/F bilayer films is shown in Table 1. Although the thickness of the bilayer films underwent, a remarkable went up, from 0.152 mm to 0.187 mm, as a function of HO essential oil (p < 0.05), incorporation of ascorbic acid in film samples had no considerable impact on the thickness of prepared films (p > 0.05). Lei et al. (2019) reported that the thickness variation with the increasing essential oils concentration was mainly related to the much more solid content in film-forming solutions. It means that the increase in thickness can be attributed to the entrapment and accumulation of essential oil droplets in the bilayer film (Lei et al., 2019). This could be due to the differences in the two constituents' structure, chemical composition, hydrophobicity, and properties. Additionally, a compact and dense network that is clear in the SEM image of a film based on 2%AA + 1.5%HO might be formed due to the Millard reaction. The results are in line with Rodriguez et al. (2019), who claimed that the thickness of papaya-based edible film incorporated with AA had no significant difference compared to the control (Rodríguez et al., 2020).
Table 1.
Physical properties of gelatin/frankincense (Ge/F) bilayer films incorporated with different Hyssopus oil (HO) and ascorbic acid (AA) concentrations*.
| AA concentration (%v/v) | HO concentration (%v/v) | Thickness (mm) | Moisture content (%) | Water contact angle (˚) | WVP (×10-11 g/s m Pa) |
Water Solubility % |
|---|---|---|---|---|---|---|
| 0 | 0.152 ± 0.003a | 15.85 ± 0.04a | 54.44 ± 0.8 a | 16.95 ± 0.27a | 20/0 ± 46/63 | |
| 0 | 0.75 | 0.175 ± 0.002b | 15.62 ± 0.25b | 59.05 ± 0.26b | 14.81 ± 0.17b | 18 ± 45/52b |
| 1.5 | 0.187 ± 0.001c | 15.20 ± 0.26c | 64.81 ± 0.3c | 12.21 ± 0.33c | 15/0 ± 15/53c | |
| 0 | 0.155 ± 0.003a | 15.91 ± 0.04a | 52.78 ± 0.26d | 16.84 ± 0.14a | 21/0 ± 05/24a | |
| 1 | 0.75 | 0.175 ± 0.002b | 15.7 ± 0.03b | 56.53 ± 0.33e | 15.10 ± 0.56b | 18/0 ± 03/66b |
| 1.5 | 0.188 ± 0.003c | 15.32 ± 0.12c | 58.54 ± 0.24b | 12.52 ± 0.53c | 16/0 ± 62/65c | |
| 0 | 0.154 ± 0.004a | 15.95 ± 0.11a | 49.92 ± 0.15f | 17.05 ± 0.65a | 21/0 ± 25/63a | |
| 2 | 0.75 | 0.178 ± 0.002b | 15.66 ± 0.13b | 54.38 ± 0.77a | 14.95 ± 0.27b | 18/0 ± 78/45b |
| 1.5 | 0.190 ± 0.003c | 15.28 ± 0.52c | 56.54 ± 0.32e | 12.03 ± 0.63c | 16/0 ± 02/36c |
* Reported values correspond to the mean ± standard deviation. Different letters in the same column indicate significant differences (p < 0.05).
2.3. Water vapor Permeability, water Solubility, and water contact angle
High water vapor permeability (WCP) is known as one of the major practical limitations regarding edible films is, mostly found in protein-based films in the packaging industry. One of the ways forward to tackle this problem is the addition of some functional compounds to the biopolymer film matrices. The WVP values of the G/F films as a function of the HO are shown in Table 1. It can be seen that; the addition of AA caused a slight decrease in water vapor permeability (p > 0.05), while WVP of the bilayer films saw a considerable fall with increasing the concentration of HO essential oil in the film matrix (p < 0.05). It might be imputed to the oily and hydrophobic character of the HO essential oil, which can interfere with the film networks lead to a decrease in the transfer of water molecules and absorption rate. Similarly, HO essential oil has reduced the solubility coefficient of the film components; thus, the WVP was dropped. Same results were also reported by other researchers who indicated a reduction in the WVP of films by the incorporation of essential oil (Maizura et al., 2007). Nonetheless, Kowalczyk et al., 2018 showed that increasing AA concentration (0–25-50 Mm) in oxidized potato starch films did not affect water vapor barrier property (Kowalczyk et al., 2018).
Regarding water solubility (WS), as it is shown in Table.1, WS% decreased significantly (p < 0.05) as a function of HO concentration. Solubility is considered a crucial parameter in biodegradable packaging films has a significant role in the application of packaging, particularly in packaged foods, including meat products, fruits, and vegetables, by affecting water permeability. Moreover, it can affect the release rate of antioxidant and antimicrobial components of a film matrix. The maximum WS% was seen in film incorporated with AA without any portion of HO.
Nonetheless, it was not detected significant (p > 0.05). It might be ascribed to the presence of AA which attracts water molecules in the film matrix due to its chemical structure. Incorporating HO in 0.75 and 1.5 % leads to a significant fall in WS%. It might be arising from the creation of cross-linking between the functional group of Gelatin and Frankincense with HO essential oil (Ojagh et al., 2010). The created network in film matrix reduces the tendency of film component toward solubility in water. Besides, the hydrophobic nature of the essential oil also causes a reduction in WS%, in film samples with higher percent of HO.
In respect of Water Contact Angle (WCA), it should be noted that evaluation of surface-tension characterization through WCA (named θ), which is currently used as an indicator for hydrophilicity, hydrophobicity, and wettability of the film surface properties, is considered a key factor in packaging materials. This assay applies to measuring water's evaporation and penetration in the film matrix (Abdelhedi et al., 2018). According to the results, increasing the HO essential oil concentration increased the films' initial distilled water contact angle (p < 0.05), from 54.44˚ to 64.81˚. While increasing ascorbic acid concentration reduced the water contact angle remarkably, from 54.44˚ to 49.92˚ (p < 0.05) (Table 1). It was proven that water contact angle would increase with raising surface hydrophobicity; thereby, AA increased the tendency of water droplets to diffuse to the film surface and reduced the water contact angle. The increased WCA as a function of incorporation of HO ascribed to the hydrophobic nature of the HO.
2.4. Moisture content
Although the moisture content of bilayer films went down significantly (p < 0.05) during the addition of HO essential oil, the effects of AA addition were detected insignificant, the related data is presented in Table.1.
Indeed, the repulsive effect of non-polar compounds of essential oil on water molecules accelerated the passage of moisture through the films and led to a reduction in the moisture content of the film samples.
On the other hand, the incorporation of AA in the film matrices causes the collapse of the film network. Thereby, the moisture content will be increased. In this case, the number of water molecules among the polymer chains increased with increasing hydrogen bonds. The main reason for the difference in moisture content with the addition of HO essential oil and AA is the difference in the portion of dry solids (Jouki et al., 2014). Additionally, it might be attributed to the hygroscopic activity of film incorporated with AA. The chemical composition of AA include four hydrogen bonds as donor sites, making it a potent component for water molecules absorption (Kowalczyk et al., 2018).
2.5. Color and transparency measurement
Color is considered one of the determinant attributes of packaging, which directly affects the look and consumer satisfaction. The color parameters of bilayer film are presented in Table.2. Generally, all of the color indicators changed significantly upon the incorporation of HO and AA. The films exhibited a significant decrease in T%, L*(lightness), a*(redness), and WI (Whiteness Index) and an increase in b* (yellowness) as the HO content rose from 0.75 to 1.5. The increase in b* causes a yellowish color in films fabricated with HO. Occurrence of whiteness, lightness, and transparency decrement might be ascribed to the presence of compounds produced due to the Millard reactions, as a crosslinking reaction between reducing sugars and protein in the heating stage decreased the lightness and whiteness of the samples with a higher portion of HO. Furthermore, the phenolic compounds present in HO which absorb light at low wavelength might affect the color significantly(Shojaee-Aliabadi et al., 2013). The study of Mohsenabadi et al. 2018 on the Starch/Carboxy methyl cellulose-based films containing rosemary essential oil indicated the similar results (Mohsenabadi et al., 2018).Conversely, there was no significant effect on color indices upon the enhancing AA. They reported that after adding citric acid to soybean EAEP3 residue edible film was studied by Ma et al.2018(Ma et al., 2018). They reported that after the addition of citric acid, the L* indice of films increased compared with blank film and there was no significant trend.
Table 2.
Effect of various concentrations of HO and AA on color values including L, a, and b, total color difference (ΔE) and whiteness index (WI) of Ge/F bilayer films a.
| AA concentration (%v/v) | HO concentration (%v/v) | L* | a* | b* | ΔE | WI |
|---|---|---|---|---|---|---|
| 0 | 86.47 ± 0.44a | 1.26 ± 0.04a | 8.46 ± 0.12a | 13.49 ± 0.14a | 83.91 ± 0.02a | |
| 0 | 0.75 | 81.04 ± 0.16b | 0.52 ± 0.04b | 13 ± 0.11b | 19.21 ± 0.22b | 76.78 ± 0.03b |
| 1.5 | 75.76 ± 0.57c | 0.15 ± 0.14c | 15.57 ± 0.11c | 24.29 ± 0.14c | 70.59 ± 0.03c | |
| 0 | 85.67 ± 0.29 a | 0.56 ± 0.03b | 6.66 ± 0.16d | 11.63 ± 0.09d | 84.33 ± 0.09a | |
| 1 | 0.75 | 83.49 ± 0.33d | −0.35 ± 0.05d | 9.22 ± 0.08a | 14.63 ± 0.1e | 81.39 ± 0.28d |
| 1.5 | 78.81 ± 0.25e | −0.7 ± 0.64e | 17.40 ± 0.13d | 24.05 ± 0.17c | 72.64 ± 0.19e | |
| 0 | 86.31 ± 0.28 a | 0.54 ± 0.14b | 8.31 ± 0.1a | 13.33 ± 0.13a | 84.44 ± 0.16a | |
| 2 | 0.75 | 83.38 ± 0.28d | −0.36 ± 0.03d | 13.65 ± 0.09b | 19.29 ± 0.13b | 78.28 ± 0.18f |
| 1.5 | 80.60 ± 0.39b | −0.55 ± 0.12f | 15.53 ± 0.09c | 21.55 ± 0.21f | 75.33 ± 0.18b |
Presented value correspond to the mean ± standard deviation. Different letters in the same column show significant differences (p < 0.05).
2.6. Total phenolic content and antioxidant activity
Various degradation effects, including the growth of microorganisms, enzymatic browning, and lipid oxidation, are dependent on the presence of oxygen. Instead of tackling oxygen-related drawbacks by the incorporation of antioxidant compounds directly into the food matrix, it could be performed by antioxidant agents into packaging materials (Arias et al., 2018, Rodríguez et al., 2020).
Oxidation includes a chain reaction where free radicals are produced and is a major reason for the loss of quality in the food industry in several ways. For instance, the oxidation of proteins may affect the texture of meat products by increasing toughness. Lipid oxidation, known as a major food deterioration in foods with high lipid content, mostly causes changes in nutritional and sensorial attributes in nuts, meats, and fish products and trigger the health-related disease. Although lipid hydroperoxides (which is one of the most important primary products of lipid oxidation) is odor-less and taste-less, they can lead to the off-flavor through decomposition in the existence of metal ions, light, or heat (Priyadarshi et al., 2018, Reddy and Lai, 2021).
However, one of the easy-apply schemes to manage the oxidation is the direct addition of antioxidants agents to the food components. It is not considered the best strategy because as soon as the oxidation reactions consume the antioxidants, the extension of food protection through the antioxidants will be shrunk, followed by a remarkable drop in food quality. Another way is to apply the high barrier packaging compounds with vacuum or modified-atmosphere food packaging (Lai, 2022, Obireddy and Lai, 2021). Extending the functionality of antioxidant film packaging materials is complicated because of the optimization and determination of antioxidants distribution in packaging films. The efficiency of active antioxidant packaging might be hindered via inconsistent and suboptimal distribution of antioxidants in materials. Besides, another key thing to consider is that the packaging film should be designed according to the oxidation rate of food components to match the kinetic of the release rate of antioxidants via kinetic of food components oxidation. (Falah et al., 2021, Lai, 2022, Lei et al., 2019, Reddy and Lai, 2021). The antioxidant activity of biologically active ingredients (antioxidants) might be hampered when they do not easily react with oxidation radicals under oxidative stress. Dealing with this issue involves coating the film layer with an active layer that contains antioxidant ingredients that have been used recently in many studies (Barreto et al., 2008, Priyadarshi et al., 2018).
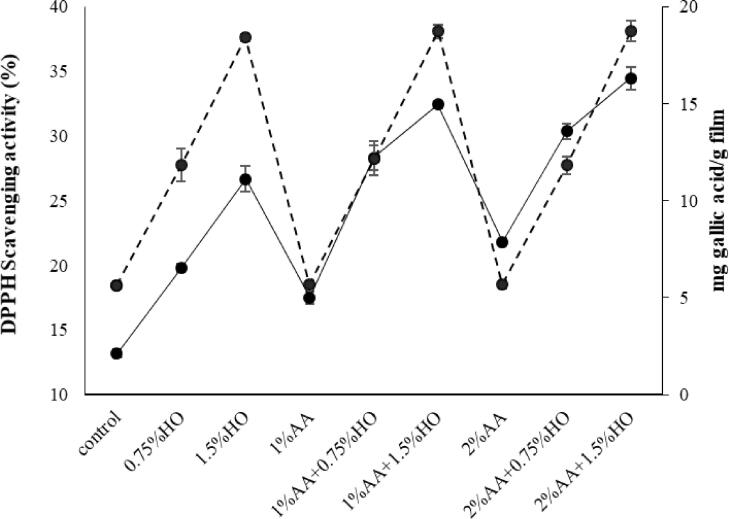
Fig. 3 provides TPC and DPPH radical-scavenging activity of G/F bilayer films containing different concentrations of HO (0, 0.75%, 1.5%) and AA (0, 1%, 2%). It can be perceived that the TPC of the control film was 5.64 mg gallic acid /g film. Subsequently, the TPC and antioxidant activity of films incorporated with HO went up significantly (p < 0.05) as the levels of HO rose from 0.75 to 1.5.
Fig. 3.
DPPH radical scavenging activity (continuous line) and total phenolic content (interrupted line) of Ge/F bilayer films containing various concentrations of ascorbic acid (AA) and Hyssop oil (HO).
Many biological features of l-ascorbic acid (AA) are hydrogen donation capabilities. Acid ascorbic can scavenge the free radicals by transferring the electrons. Moreover, AA represents as an oxygen scavenger and chelator of pro-oxidative metal ions. Professional antioxidant capacity and cheap mass production make AA an appropriate ingredient in active packaging. The maximum TPC and antioxidant activity were detected for films with HO and AA. Although films incorporated with AA exhibited low antioxidant activity, it was higher than the control sample. Different concentrations of acid ascorbic had no noteworthy impact on total phenol content (p > 0.05). The higher DPPH radical scavenging activity is mostly ascribed to the functional groups and higher phenolic content of HO. However, the presence of HO and AA might have a synergistic effect in herein films with 1.5% HO + 2%AA and cause the highest antioxidant activity in comparison with other counterparts.
In line with the study, similar results were reported for k-carrageenan films incorporated with S.hortensis essential oil (Shojaee-Aliabadi et al., 2013) and quince seed mucilage films formulated with thyme essential oil Results indicated a linear correlation between radical scavenging activity and total phenol content, which was also mentioned by Jouki et al. (2014) and Shojaee -Aliabadi et al. (2012)(Jouki et al., 2014, Shojaee-Aliabadi et al., 2013).
4. Conclusion
Over the last few decades, there has been an increasing trend in the popularity of active ecofriendly in science and industries due to the serious environmental concerns arising from traditional packaging components. In this study a novel bilayer coating film were fabricated based on gelatin and frankincense gum. Antioxidant, morphological, mechanical, and barrier properties of films were screened upon adding different concentrations of AA (0, 1, 2%) into the gelatin layer and HO essential oil (0, 0.75, 1.5%) into the frankincense layer. Thickness, antioxidant activity, phenolic content, lightness, transparency, yellowness, water contact angle, and elongation at break increased significantly as a function of HO concentration. The herein films exhibited promising attributes for application in the active food packaging industry.
Ethical Statements.
This study was approved by the Institutional Review Board of the Ferdowsi University of Mashhad.
CRediT authorship contribution statement
Motahare Pirnia: Conceptualization, Methodology, Resources, Investigation, Formal analysis, Data curation. Khatereh Shirani: Investigation, Methodology, Conceptualization, Writing – original draft, Writing – review & editing. Farideh Tabatabaee Yazdi: Funding acquisition, Project administration, Supervision. Seyed Ali Moratazavi: Supervision, Funding acquisition. Mohebbat Mohebbi: Data curation, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to sincerely thank the Research Deputy of Ferdowsi University of Mashhad for funding this project with the code of 47102.
Footnotes
It is also known as Boswellia Carteri in some references.
This research was published in Persian.
Enzyme-assisted aqueous extraction processing.
Contributor Information
Farideh Tabatabaee Yazdi, Email: tabatabai@um.ac.ir.
Seyed Ali Moratazavi, Email: morteza@um.ac.ir, noorbakhsh.hamid@gmail.com.
References
- Abdelhedi O., Nasri R., Jridi M., Kchaou H., Nasreddine B., Karbowiak T.…Nasri M. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocolloids. 2018;74:176–186. [Google Scholar]
- Arias E., Oria R., López-Buesa P. Determination of acceptability and shelf life of fresh-cut pear by digital image analysis. Journal of Food Measurement and Characterization. 2018;12(4):2916–2926. [Google Scholar]
- Ashwar B.A., Shah A., Gani A., Shah U., Gani A., Wani I.A.…Masoodi F.A. Rice starch active packaging films loaded with antioxidants—development and characterization. Starch-Stärke. 2015;67(3–4):294–302. [Google Scholar]
- Baron, R. D., Pérez, L. L., Salcedo, J. M., Córdoba, L. P., & do Amaral Sobral, P. J. (2017). Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. International journal of biological macromolecules, 98, 676-683. [DOI] [PubMed]
- Barreto J.C., Trevisan M.T., Hull W.E., Erben G., De Brito E.S., Pfundstein B.…Owen R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.) Journal of agricultural and food chemistry. 2008;56(14):5599–5610. doi: 10.1021/jf800738r. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Oria A., Rodríguez-Gutiérrez G., Vioque B., Rubio-Senent F., Fernández-Bolaños J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3, 4-dihydroxyphenylglycol. Carbohydrate polymers. 2017;178:368–377. doi: 10.1016/j.carbpol.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Džamić A.M., Soković M.D., Novaković M., Jadranin M., Ristić M.S., Tešević V., Marin P.D. Composition, antifungal and antioxidant properties of Hyssopus officinalis L. subsp. pilifer (Pant.) Murb. essential oil and deodorized extracts. Industrial Crops and Products. 2013;51:401–407. [Google Scholar]
- Espitia, P. J. P., Du, W.-X., de Jesús Avena-Bustillos, R., Soares, N. d. F. F., & McHugh, T. H. (2014). Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food hydrocolloids, 35, 287-296.
- Falah F., Shirani K., Vasiee A., Tabatabaee Yazdi F., Alizadeh Behbahani B. In vitro screening of phytochemicals, antioxidant, antimicrobial, and cytotoxic activity of Echinops setifer extract. Biocatalysis and Agricultural Biotechnology. 2021;35 doi: 10.1016/j.bcab.2021.102102. In press. [DOI] [Google Scholar]
- Gennadios A. CRC Press; 2002. Protein-based films and coatings. [Google Scholar]
- Ghasemlou M., Aliheidari N., Fahmi R., Shojaee-Aliabadi S., Keshavarz B., Cran M.J., Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydrate polymers. 2013;98(1):1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Hosseini M., Razavi S., Mousavi M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. Journal of food processing and preservation. 2009;33(6):727–743. [Google Scholar]
- Jouki M., Yazdi F.T., Mortazavi S.A., Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocolloids. 2014;36:9–19. [Google Scholar]
- KIZIL, S., HAŞİMİ, N., Tolan, V., Kilinc, E., & KARATAŞ, H. (2010). Chemical composition, antimicrobial and antioxidant activities of hyssop (Hyssopus officinalis L.) essential oil. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38(3), 99-103.
- Kowalczyk D. Biopolymer/candelilla wax emulsion films as carriers of ascorbic acid–A comparative study. Food Hydrocolloids. 2016;52:543–553. [Google Scholar]
- Kowalczyk D., Kazimierczak W., Zięba E., Mężyńska M., Basiura-Cembala M., Lisiecki S.…Baraniak B. Ascorbic acid-and sodium ascorbate-loaded oxidized potato starch films: Comparative evaluation of physicochemical and antioxidant properties. Carbohydrate polymers. 2018;181:317–326. doi: 10.1016/j.carbpol.2017.10.063. [DOI] [PubMed] [Google Scholar]
- Lai W.-F. Design of Polymeric Films for Antioxidant Active Food Packaging. International Journal of Molecular Sciences. 2022;23(1):12. doi: 10.3390/ijms23010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Wu H., Jiao C., Jiang Y., Liu R., Xiao D.…Li S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocolloids. 2019;94:128–135. doi: 10.1016/j.foodhyd.2019.03.011. [DOI] [Google Scholar]
- Ma, W., Rokayya, S., Xu, L., Sui, X., Jiang, L., & Li, Y. (2018). Physical-chemical properties of edible film made from soybean residue and citric acid. Journal of Chemistry, 2018.
- Maizura M., Fazilah A., Norziah M., Karim A. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch–alginate edible film containing lemongrass oil. Journal of Food Science. 2007;72(6):C324–C330. doi: 10.1111/j.1750-3841.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- Mohamed S.A., El-Sakhawy M., Nashy E.-S.-H., Othman A.M. Novel natural composite films as packaging materials with enhanced properties. International journal of biological macromolecules. 2019;136:774–784. doi: 10.1016/j.ijbiomac.2019.06.130. [DOI] [PubMed] [Google Scholar]
- Mohsenabadi N., Rajaei A., Tabatabaei M., Mohsenifar A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. International journal of biological macromolecules. 2018;112:148–155. doi: 10.1016/j.ijbiomac.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Obireddy S.R., Lai W.-F. Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics. 2021;13(3):313. doi: 10.3390/pharmaceutics13030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojagh S.M., Rezaei M., Razavi S.H., Hosseini S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chemistry. 2010;122(1):161–166. [Google Scholar]
- Pereda M., Ponce A.G., Marcovich N.E., Ruseckaite R.A., Martucci J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocolloids. 2011;25(5):1372–1381. doi: 10.1016/j.foodhyd.2011.01.001. [DOI] [Google Scholar]
- Priyadarshi, R., Sauraj, Kumar, B., & Negi, Y. S. (2018). Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydrate polymers, 195, 329-338. https://doi.org/https://doi.org/10.1016/j.carbpol.2018.04.089. [DOI] [PubMed]
- Reddy O.S., Lai W.-F. Development of a composite film fabricated from carboxymethyl chitosan and magnetite nanoparticles for p H-responsive bioactive agent release. Biointerphases. 2021;16(2) doi: 10.1116/6.0000726. [DOI] [PubMed] [Google Scholar]
- Rijkers T., Ogbazghi W., Wessel M., Bongers F. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. Journal of applied ecology. 2006;43(6):1188–1195. [Google Scholar]
- Rivero S., Garcia M.A., Pinotti A. Composite and bi-layer films based on gelatin and chitosan. Journal of food engineering. 2009;90(4):531–539. [Google Scholar]
- Rodríguez G.M., Sibaja J.C., Espitia P.J., Otoni C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food hydrocolloids. 2020;103 [Google Scholar]
- Said-Al Ahl H.A., Abbas Z.K., Sabra A.S., Tkachenko K.G. Essential oil composition of Hyssopus officinalis L. cultivated in Egypt. International Journal of Plant Science and Ecology. 2015;1(2):49–53. [Google Scholar]
- Sani M.A., Azizi-Lalabadi M., Tavassoli M., Mohammadi K., McClements D.J. Recent advances in the development of smart and active biodegradable packaging materials. Nanomaterials. 2021;11(5):1331. doi: 10.3390/nano11051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Chhikara S., Ghodekar S., Bhatia S., Kharya M., Gajbhiye V.…Mahadik K. Phytochemical and pharmacological investigations on Boswellia serrata. Pharmacognosy Reviews. 2009;3(5):206. [Google Scholar]
- Shirani Khatereh, et al. K. Shirani, F. Shahidi and S. A. Mortazavi Investigation of decontamination effect of argon cold plasma on physicochemical and sensory properties of almond slices. International Journal of Food Microbiology. 2020 doi: 10.1016/j.ijfoodmicro.2020.108892. In press. [DOI] [PubMed] [Google Scholar]
- Shojaee-Aliabadi S., Hosseini H., Mohammadifar M.A., Mohammadi A., Ghasemlou M., Ojagh S.M.…Khaksar R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. International journal of biological macromolecules. 2013;52:116–124. doi: 10.1016/j.ijbiomac.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U., Harte B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloids. 2010;24(8):770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- Soleimani H., Barzegar M., Sahari M., Naghdi Badi H. An investigation on the antioxidant activities of Hyssopus officinalis L. and Echinacea purpurea L. plant extracts in oil model system. Journal of Medicinal Plants. 2011;10(37):61–72. [Google Scholar]
- Vejdan A., Ojagh S.M., Adeli A., Abdollahi M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT-Food Science and Technology. 2016;71:88–95. [Google Scholar]
- Yakimets I., Wellner N., Smith A.C., Wilson R.H., Farhat I., Mitchell J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer. 2005;46(26):12577–12585. [Google Scholar]
- Zareie Z., Yazdi F.T., Mortazavi S.A. Development and characterization of antioxidant and antimicrobial edible films based on chitosan and gamma-aminobutyric acid-rich fermented soy protein. Carbohydrate Polymers. 2020;244 doi: 10.1016/j.carbpol.2020.116491. [DOI] [PubMed] [Google Scholar]
- Zhang S.J., Hu T.T., Chen Y.Y., Wang S., Kang Y.F. Analysis of the polysaccharide fractions isolated from pea (Pisum sativum L.) at different levels of purification. Journal of Food Biochemistry. 2020;44(8) doi: 10.1111/jfbc.13248. [DOI] [PubMed] [Google Scholar]