Abstract
Parkinson's Disease remains a diagnostic challenge. Misdiagnosis during life is approximately 25%. Diseases that resemble PD clinically, such as the Parkinsonianplus disorders usually have a poorer prognosis. A diagnostic biomarker is needed to differentiate PD from PPS. Geographical differences in PD prevalence, genetics and environmental factors may suggest a different pathogenesis of PD in Africa which may affect metabolic changes seen on 18F-FDG-PET. We investigated the utility of 18FFDG-PET in differentiating PD from PPS in a real-life clinical setting. The study was conducted at the Movement Disorder Clinic, South Africa. 81 patients with Parkinsonism had fluorine-18-labelled-fluorodeoxyglucose-PET; 53 PD and 28 PPS. Six persons living with HIV and Parkinsonism were included. Of the 22 Black African patients, 21 had PD and only one had a PPS. Image-based diagnosis was made by visual interpretation aided by statistical parametric mapping (SPM) analysis by a Nuclear Medicine Physician blinded to the clinical diagnosis. This was compared to the final clinical diagnosis made by two Movement disorder Neurologists blinded to the 18F-FDG-PET diagnosis. Patients were followed up for a median of 4 years. 18F-FDGPET diagnosis was in agreement with final clinical diagnosis in 91% of all subjects (90% PD, 93% all PPS). Our paper reports the clinically realistic sample of patients seen with Parkinsonism in Africa. The present data shows that 18F-FDG-PET can distinguish PD from PPS with good accuracy. Few Black Africans present with an Atypical Parkinsonian syndrome. The pattern of metabolism in PLH-PD is similar to PD patients without HIV.
Keywords: Parkinson's disease, Parkinsonian plus syndromes, Africa, 18F-FDG-PET, Multiple system atrophy, Progressive supranuclear palsy, Dementia with lewy bodies, Corticobasal degeneration
Highlights
-
•
The diagnostic accuracy of Parkinson's disease is low.
-
•
Parkinsonian-plus syndromes mimic PD but have a poorer prognosis.
-
•
18F-FDG-PET is a supportive biomarker for the differential diagnosis of Parkinsonism.
-
•
Heterogeneity of PD is driven by geographical and genetic differences in Parkinsonism in Africa.
-
•
In real-life clinical setting,18F-FDG-PET differentiates PD from the Parkinsonian-plus syndromes with an accuracy of 91%.
1. Introduction
Parkinsonism is a clinical diagnosis characterised by bradykinesia in combination with either rest tremor or rigidity [1]. The commonest cause of Parkinsonism is Parkinson's disease (PD) [2]. The diagnostic accuracy of diagnosing PD by general neurologist is 75% [3,4]. This diagnostic error is caused by the heterogeneous clinical presentation of PD [5]. The common conditions that mimic PD are the parkinsonian-plus syndromes (PPS): multiple systems atrophy (MSA), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB) and corticobasal degeneration (CBD) [4]. PD responds to medical and surgical therapy, whereas the PPS are less likely to do so [6]. A clear diagnosis is important to patient treatment, prognosis, and enrolment into clinical trials [7]. There are no biological markers for the antemortem differential diagnosis of Parkinsonism [7,8]. Imaging of dopamine function with SPECT or PET is widely used in the diagnosis of PD to differentiate PD from healthy controls and non-degenerative causes of Parkinsonism, but dopamine imaging cannot distinguish PD from PPS [9,10]. The PET tracer 18F-flourodeoxyglucose (18F-FDG) accurately measures cerebral glucose metabolism thereby reflecting neuronal and synaptic activity [11]. In neurodegenerative disorders specific regions degenerate and patterns of altered cerebral glucose metabolism develop [11]. Disease specific metabolic patterns have been identified for PD, MSA, PSP, DLB and CBD [[12], [13], [14]]. 18F-FDG is included in diagnostic criteria as a supportive biomarker for the diagnosis of MSA, PSP and DLB [[15], [16], [17], [18]].
The aetiology of PD is not known [5]. Research suggests there is a complex interaction between genetic and environmental factors [5,19]. Heterogeneity of PD presentation is determined by age at onset, ethnicity, co-morbidities such as HIV, genetic factors, and geographical location [20,21]. Black Africans in Sub-Saharan Africa have a lower prevalence of PD compared to Black Africans in the USA and a lower frequency of known PD mutations [22,23]. The lower PD prevalence may be due to lower case ascertainment in Africa due to resource limitations of health care resources and cultural perceptions; and a lower life expectancy. Genetic mutations are known to contribute to the heterogeneity of PD, for example, PD patients with LRRK2-related mutations lack the pathological hallmark Lewy bodies and 18F-FDG-PET in these patients show a pattern of posterior cortical hypometabolism which is less severe [24]. These factors - geographical location and low frequency known PD mutations may suggest that the pathogenesis and cause of Parkinsonism in Africa may be more diverse than Europe and USA [20]. This may affect metabolic changes on 18F-FDG-PET in Parkinsonism in an African population [25,26]. No studies have been published from Africa for the role of 18F-FDG-PET in Parkinsonism. South Africa has a population of 60mil persons, 81% of whom are Black African [26]. The estimated HIV prevalence rate is approximately 13.0% among the South African population [27]. We, therefore undertook this study to determine the use of 18F-FDG-PET in Parkinsonism in Africa in an ethnic and immunologically diverse population. Validation using established criteria is necessary before considering recommendation of application across centres in a specific region [28]. Several countries have investigated the role of 18F-FDG-PET in Parkinsonism for (possibly) similar reasons - from 1998 to 2020 there were reports from USA, India, Germany, Serbia, Korea, Slovenia, Netherlands, Italy and Spain [9,12,28,[30], [31], [32]].
The Movement Disorder Society's gold standard of PD diagnosis in life is clinical expert opinion; this is based on post-mortem studies demonstrating low diagnostic errors among experts [1,3]. This case ascertainment method has been validated in the aforementioned studies of 18F-FDG-PET and Parkinsonism.
The aim of this study was to determine the value of 18F-FDG PET in differentiating PD from PPS in a South African cohort which has not been previously described. We used clinical diagnosis of PD by Neurologists experienced in Movement Disorders as the gold standard. The clinical features which predicted PPS are reported here.
2. Methods
2.1. Patients
The study recruited patients with Parkinsonism from 2011 to 2019 from the Movement Disorder Clinic of Inkosi Albert Luthuli Hospital, a tertiary hospital in Durban, South Africa. Patients with secondary causes of Parkinsonism that were identified from history or MRI were excluded as 18FDG-PET would not have added any more value to the diagnosis (such as drug induced Parkinsonism or normal pressure hydrocephalus) [18]. The gold standard of diagnosis was the final clinical diagnosis made by two neurologists experienced in the field of Movement Disorders (Dr FA and Prof AIB). The Movement Disorder specialists were blinded to the 18F-FDG-PET diagnosis. At the end of follow up the final clinical diagnosis was compared to the 18F-FDG-PET diagnosis. Clinical characteristics of patients in the two groups were compared to determine which features predicted a diagnosis of PD versus PPS. All participants gave informed consent for the study. Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BF319/16).
2.2. 18F-FDG-PET imaging
The patients were instructed to fast for 4–6 h prior to the scan. Antiparkinsonian medication, including levodopa, was stopped 12 h prior to scan [32]. The blood glucose prior to injection was checked and 18F-FDG administered if the blood glucose was under 8.9 mmol/l as per available guidelines. Prior to injection patients were kept calm in a dim lit, quiet room for 30 min. 18F-FDG PET [Fluorine-18 fluorodeoxyglucose (FDG)] was administered with activity ranging between 296 and 370 MBq followed by limited field brain imaging at 30–60 min post injection with the patient lying supine. A Siemens Biograph 16 PET/CT scanner applying low dose non-enhanced CT parameters for attenuation correction and localization followed by the PET brain scan was acquired over 10 min with the patient in the same position. The imaging was done craniocaudally. Three dimensional attenuation-corrected images were reconstructed and filtered using iterative reconstruction. The axial scan length of the PET was 14.5 cm covering the whole brain. PET data was generated from the trimmed sinogram to ensure a smaller FOV appropriate for brain imaging. Corrected, uncorrected and fused PET and CT images were displayed. Visual interpretation as well as SPM reading using the Siemens syngo.via software database comparison (Siemens Healthineers, Germany) was used to evaluate the images and reported by an experienced Nuclear Medicine Physician (Dr NN). Visual analysis comprised of a subjective visual evaluation to assess areas of decreased tracer accumulation relative to the whole brain. Initial evaluation was done in grey scale. Fusion to normal dataset software was also used to assist in confirming the findings. Further, a voxel-based analysis using standard parametric mapping software (SPM) was done. Images were normalized to whole brain, with proportion to the brightest voxel set to 25%. This software is selected automatically upon statistical analysis of the images, using the 18F-FDG PET from FBP reconstructions of FDG 1A ECAT HR+ scanner age-matched database. The normal database for brain 18F-FDG PET on the syngo-via software is based on 30 normal subjects with age range from 54 to 72 years (Siemens Healthineers · brochure S4 portrait · Template (scrvt.com) [33]. The SSP 3D volume images were viewed to determine the variation of uptake in the brain of the patient. Regional standard deviations assessed using the predefined regions of interest automatically generated by the software. These are created by calculating ROI statistics, the population mean and standard deviation values for a particular ROI, by first calculating the mean value in that ROI for each of the normal subjects, thereafter computing the mean and standard deviation of those mean values. Areas of glucose metabolism were reported as increased or decreased if above and below the mean +/− 2 standard deviation of normal data respectively. We used prespecified criteria of patterns of glucose metabolism to classify the 18F-FDG PET images. Table 1 summarizes the image-based diagnostic criteria [9,12,14,30].
Table 1.
Patterns of glucose metabolism for the image-based diagnosis of individual patients.
| PD |
|
| MSA |
|
| PSP |
|
| DLB |
|
| CBD |
|
2.3. Statistical methods
Chi Square test or Fisher's exact test was used to compare demographic and clinical characteristics between the groups and p values as well as odds ratios reported for categorical data. Shapiro Wilks test was used to assess the frequency distributions of continuous variables. Means and standard deviations are reported for those satisfying the condition and t-tests used to compare groups. Medians and interquartile are reported for data with significantly skewed distributions and comparisons made using the two-sample Wilcoxon rank-sum (Mann-Whitney) test. Standard measures of sensitivity, specificity, positive and negative predictive values and 95% confidence limits are reported as measures of the diagnostic efficacy of the overall results of 18F-FDG PET where the final clinical diagnosis was considered as the gold standard. Stata V13.1 statistical software was used in the analysis.
3. Results
3.1. Patients
Eighty-one patients (mean age 66 ± 9 years) with Parkinsonism were included in the study, 53 PD and 28 PPS patients after a median duration of follow up of 4 [[2], [3], [4], [5]] years; range 6–185 months. Twenty four patients were excluded; their aetiologies were – 7 drug induced Parkinsonism, 3 Essential tremor, 6 vascular Parkinsonism, 2 normal pressure hydrocephalus, 1 acquired hepatolenticular degeneration, 1 frontotemporal dementia with Parkinsonism, 1 depression and 3 patients did not have a final diagnosis due to lack of follow up. There were 7 MSA, 11 PSP, 8 DLB and 2 CBD patients. Seventy seven patients had a minimum follow up of 2 years. The four patients who were followed up for less than 2 years had a clinical features of an atypical parkinsonian disorder and met the criteria for DLB (2 patients), PSP (1 patient) and MSA (2 patients) [[15], [16], [17]]. Patient characteristics are summarised in Table 2. There were no differences in age and gender between the two groups. There was a statistically significant difference in Ethnicity (p < 0.001); majority of patients in the PD group were Black African but there was only one patient of Black African ethnicity who had a PPS. There were fewer representations from White and Mixed ancestry populations. PPS patients presented more frequently with Hoehn and Yahr ≥3 (82% compared to 17% in PD) and were on a lower dose of LEDD compared to PD patients. PD patients had a longer disease duration at last follow up, but fewer PD patients had severe disease (30% with H&Y ≥ 3) compared to the PPS group (89% H&Y ≥ 3). The clinical phenotype strongly associated with PPS was the akinetic rigid subtype (54%) and only 3% had a tremor dominant phenotype. This was significantly different from the clinical phenotype of PD patients', where tremor dominant and mixed phenotype accounted for 94% of the clinical phenotype. Therefore, lack of tremor was more likely to be associated with PPS than PD. PPS patients reported sudden onset of symptoms more frequently (29% compared to PD =9%). Rapid progression of Parkinsonism is defined as reaching H&Y stage 3 by 3 years of symptom onset [34]. There were 22 (79%) PPS patients and 2 (4%) PD patients who had rapidly progressive Parkinsonism. There were 6 patients with PD who were HIV positive – these patients were previously described as a part of a larger cohort of Persons Living with HIV (PLH) and PD [21]. PLH-PD were all on antiretroviral therapy and had an earlier age at onset for PD compared to controls. Mini-Mental State Exam was available for all 6 PLH-PD, scores were within normal limits, range = 25–30. For the one patient PLH-PD who had a HAND metabolic pattern on 18F-FDG-PET imaging, we also performed the International HIV Dementia Scale = 10.5. This was within normal limits.
Table 2.
Demographic and clinical features of the 81 patients with Parkinsonism.
| PD = 53 | PPS = 28 | P value | |
|---|---|---|---|
| Age at onset, years ± SD | 58 ± 11 | 61 ± 8 | 0.19 |
| Male: n (%) | 29 (55) | 15 (54) | 0.94 |
| Ethnicity: | <0.001 | ||
| Black | 21 (40) | 1 (4) | |
| Asian | 19 (36) | 23 (82) | |
| White | 11 (20) | 3 (10) | |
| Mixed ancestry | 2 (4) | 1 (4) | |
| Hoehn & Yahr ≥3 first visit | 9 (17) | 23 (82) | <0.001 |
| Hoehn & Yahr ≥3 last visit | 16 (30) | 25 (89) | <0.001 |
| UPDRS 3 | 42 ± 15 | 51 ± 9 | 0.01 |
| Disease duration at last visit, median (IQR) months | 84 (56–114) | 56 (36–72) | <0.001 |
| Disease duration at PET, months | 64 ± 44 | 37 ± 25 | 0.004 |
| LEDD⁎, mg | 643 ± 276 | 368 ± 336 | <0.001 |
| Phenotype: n < 0.001 | |||
| Akinetic rigid | 3 (6) | 15 (54) | |
| Mixed | 32 (60) | 12 (43) | |
| Tremor dominant | 18 (34) | 1 (3) | |
| Sudden symptom onset: n | 5 (9) | 8 (29) | 0.03 |
Continuous variables are presented as (mean ± SD) unless indicated.
LEDD: Levodopa equivalent dose.
3.2. 18F-FDG-PET results
Table 3, Table 4 summarise the diagnostic sensitivity and specificity of 18F-FDG-PET interpretation. 18F-FDG-PET demonstrated a sensitivity of 91% (79–97) and a specificity of 89% (72–98) for the diagnosis of PD. For all PPS combined, FDG-PET classification showed a sensitivity of 89% (72–98) and specificity of 94% (84–99). 18F-FDG-PET metabolic patterns in 48 of the 53 PD patients were in keeping with an imaging diagnosis of PD: normal in 19 patients, 18 had both hypermetabolism of the basal ganglia and parietotemporal hypometabolism and 10 PD patients had parietotemporal hypometabolism only. In the remaining 5 PD patients, the 18F-FDG-PET diagnosis was: 2 MSA, 1 DLB, 1 HIV associated neurocognitive disorder (HAND) and 1 frontotemporal dementia. The metabolic patterns of metabolism for the 6 PLH-PD patients were normal in 2, 3 demonstrated hypometabolism of the parietotemporal regions and 1 patient had global hypometabolism suggestive of HAND. This patient had normal cognition. Hypometabolism of the midline frontal regions was found in all 11 PSP patients, in addition 1 patient had midbrain hypometabolism and 1 patient had hypometabolism of the basal ganglia. Sensitivity of 18F-FDG-PET diagnosis in PSP was 100% (72–100). In the 7 MSA patients 18F-FDG-PET was normal in 3 patients, 2 had cerebellar hypometabolism, 1 had hypometabolism of the pons and the 18F-FDG-PET in 1 patients demonstrated both basal ganglia and cerebellar hypometabolism. The sensitivity of 18F–18F-FDG-PET for the diagnosis of MSA was 57% (18–90). Diagnostic sensitivity for DLB was 100% (63–100) and for CBD 100% (15–100). The 18F-FDG-PET in all 11 DLB patients demonstrated occipital hypometabolism. In the 2 CBD patients 18F-FDG-PET showed asymmetric hypometabolism of the basal ganglia and frontoparietal cortices contralateral to the clinically affected side. 18F-FDG-PET diagnostic sensitivity for PD was 91%, 57% for MSA, 100% for PSP, 100% for DLB and 100% for CBD. Overall concordance of 18F-FDG-PET diagnosis with clinical diagnosis was 91%. Fig. 1 shows representative patterns of cerebral glucose metabolism in Parkinsonism from patients in this cohort.
Table 3.
Diagnostic values of 18F-FDG-PET using final clinical diagnosis as the gold standard % (95% CI).
| PD | PSP | MSA | DLB | CBD | PPS | |
|---|---|---|---|---|---|---|
| Sensitivity | 91% (79–97) | 100% (72–100) | 57% (18–90) | 100% (63–100) | 100% (15–100) | 89% (72–98) |
| Specificity | 89% (72–98) | 100% (95–100) | 97% (91–100) | 99% (93–100) | 100% (95–100) | 94%(84–99) |
| PPV | 94% (84–99) | 100% | 67% (31–90) | 89% (53–98) | 100% | 89%(73–96) |
| NPV | 83% (65–94) | 100% | 96% (91–98) | 100% | 100% | 94%(85–98) |
| Agreement | 90% (82–96) | 100% (96–100) | 94% (86–98) | 99% | 100% (96–100) | 93%(85–97) |
Table 4.
Diagnostic classification of 18F-FDG-PET.
| Clinical classification (n) | PD | PSP | MSA | DLB | CDB | Other |
|---|---|---|---|---|---|---|
| PD (53) | 48 (91%) | 0 | 2 | 1 | 0 | 2⁎ |
| PSP (11) | 0 | 11 (100%) | 0 | 0 | 0 | 0 |
| MSA (7) | 3 | 0 | 4 (57%) | 0 | 0 | 0 |
| DLB (8) | 1 | 0 | 0 | 8 (100%) | 0 | 0 |
| CDB (2) | 0 | 0 | 0 | 0 | 2 (100%) | 0 |
One PD patient was reported to have HIV associated neurocognitive Disorder on 18F-FDG-PET
One patient was reported to have a diagnosis of Fronto-temporal Dementia on 18F-FDG-PET
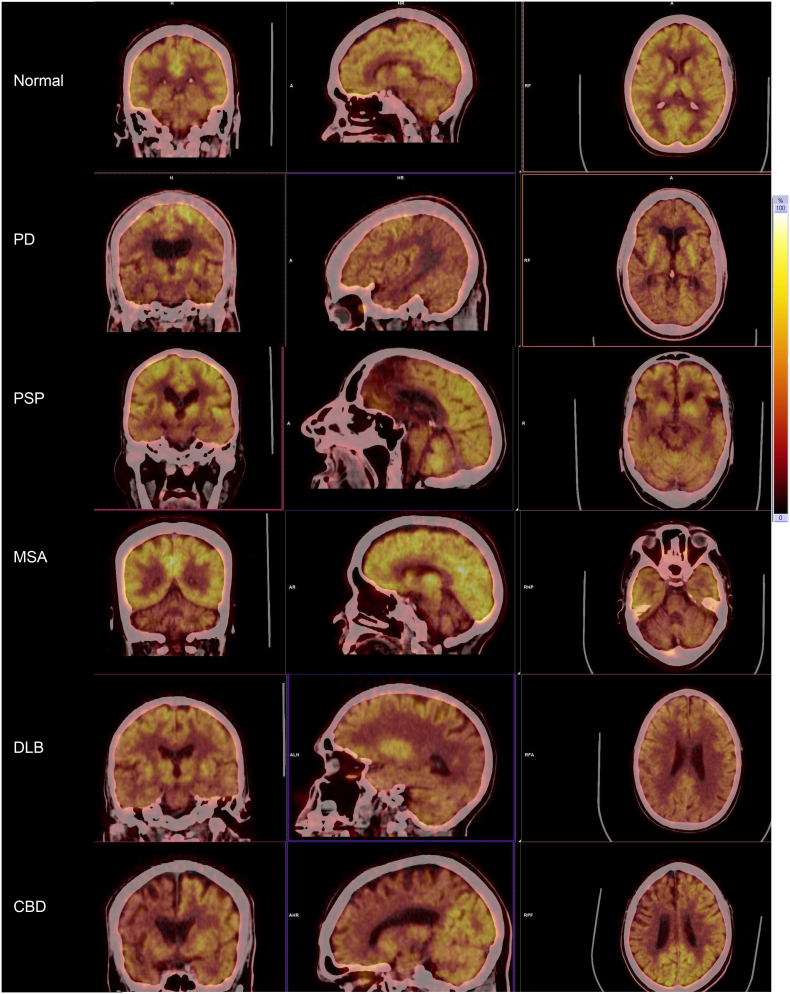
Fig. 1.
Patterns of Cerebral glucose metabolism in Parkinsonism.
Shown are the images of regional 18F-FDG-PET cerebral metabolic patterns in Parkinsonism (hot body colour scale).
Normal cerebral uptake of glucose.
PD: relative hypermetabolism in the basal ganglia and hypometabolism in bilateral parietotemporal regions.
PSP: hypometabolism of the midline frontal lobes and midbrain.
MSA: hypometabolism of both cerebellar hemispheres.
DLB: hypometabolism in both occipital lobes.
CBD: hypometabolism of the right parietal, temporal and striatal regions.
4. Discussion
PD remains a diagnostic challenge. Ideally, an observer independent biomarker that can improve clinical accuracy and that is reproducible across populations is needed [8]. Imaging tests, structural and functional, are already part of routine protocols in many clinical centres. There is American Academy Level 1 evidence for the use of 18F-FDG-PET in Parkinsonism [9]. The diagnostic accuracy of parkinsonian syndromes increases from 75% with clinical evaluation by a Neurologist to 90% when combined with an 18F-FDG-PET [[35], [36], [37]]. The methods of image analysis included visual assessment, SPM and scaled subprofile model with principal component analysis [11,36]. In a previously published study from the same hospital, we found that 25% of patients were incorrectly classified as PD [38]. This is the first study in an African population validating the utility of 18F-FDG-PET in Parkinsonism. We used observer dependant visual interpretation aided by SPM for the 18F-FDG-PET diagnosis. Overall diagnostic accuracy of 18F-FDG-PET diagnosis was 91% which is in keeping with other studies [9,12,30,31]. A metaanalysis of eight studies investigating the use of 18F-FDG-PET in Parkinsonism was published by Meyer et al. in 2017, there was an overall sensitivity of 91.4% for 18F-FDG-PET visual assessment supported by voxel-based statistical analysis. This metaanalysis included cases from South Korea, United States of America (2 studies), Germany, Belgium, India and Serbia [36]. Only one study included patients with DLB (Hellwig et al. 2012), exclusion of patients with DLB did not change the diagnostic accuracy of 18F-FDG-PET. Three studies did not include patients with CBD. We included all patients who were referred to the Movement Disorder Clinic who fulfilled the inclusion criteria; inclusion of DLB and CBD therefore reflects our referral pathway in a real life clinical setting. The overall diagnostic accuracy of our study was in agreement with other studies, however there two unusual observations. One is the high sensitivity and specificity for PSP and CBD of 100%, for CDB it may be due to the small numbers. PSP criteria include many phenotypes, not all of which have been investigated with 18F-FDG-PET, on review of PSP charts we included only the PSP-Richardson variant; this may account for the patterns of metabolism that were present in all patients. Secondly, the sensitivity of 18F-FDG-PET diagnosis of MSA patients was lower than reported [36]. The specificity of image-based classification of our MSA patients was 97%; the higher specificity is more clinically relevant as FDG –PET is used as a confirmatory test. The lower sensitivity may be due to interpretation pitfalls or the small number of patients. For patients with disease duration <2 years, we found good correlation similar to Tang et al. [35]. The clinical predictors of PPS in our cohort were: severe disease stage at first visit (H&Y ≥ 3), lack a tremor, less likely to respond to levodopa, rapid progression of Parkinsonism and sudden onset of symptoms. These features are red flags for the diagnosis of PD and their presence supports a diagnosis of an atypical Parkinsonian syndrome [1,39]. We have therefore provided evidence for the use of 18F-FDG-PET in the differential diagnosis of Parkinsonism in an African population. The distribution of Parkinsonism (PD and Atypical Parkinsonism) in our different ethnic groups requires further exploration. For all Parkinsonism, there were 22 patients of African ancestry – 21 PD and one Atypical Parkinsonism. This low frequency of atypical Parkinsonism in persons of African ancestry may represent a true lower prevalence or a referral bias. Incidence and prevalence of Parkinsonism in persons of African ancestry is not well investigated to provide further reasons [40]. There was a higher frequency of PPS in the Indian group. This may be due to demographics as our region has the largest number of Indians in South Africa or this may be a true higher prevalence as was reported by Chaudhari et al.: there was a fourfold increase in atypical Parkinsonism in persons of Indian origin in the UK compared to white subjects [41]. 18F-FDG-PET cannot differentiate PD from Essential Tremor or drug induced Parkinsonism, Dopamine imaging is more suitable for this differentiation [9,42,43].
4.1. Limitations
The Movement Disorder Clinic at IALCH is a tertiary referral centre where atypical and unusual presentations of PD are seen, thus we have a bias of a higher frequency of PPS. There are smaller number of individual PPS patients, for this reason we would like to further investigate sub classes of PPS in future studies. We do not have a healthy control group from this population. A normal 18F-FDG-PET with visual reading is supportive of PD [9,30]. Therefore, 18F-FDG-PET visual reading diagnosis cannot be used as a screening tool for Parkinsonism. The modality is suited to patients who have clinical Parkinsonism (as defined by MDS Criteria) and there is doubt regarding PD or an atypical Parkinsonian syndrome. MRI of the brain is suggested as a first line modality to exclude secondary causes such as structural lesions, vascular pathology, normal pressure hydrocephalus and abnormal brain mineralization [43]. If a functional cause of Parkinsonism is suspected, then dopamine imaging is more suitable as FDG PET with visual reading cannot differentiate PD from normal persons [44]. The presence of posterior cortical dysfunction on 18F-FDG-PET in PD patients is a marker for the development of PD dementia, we did not do neuropsychological tests to test for mild cognitive impairment.
5. Conclusion
Our paper reports the clinically realistic sample of patients seen with Parkinsonism in Africa. The present data shows that 18F-FDG-PET can distinguish PD from PPS with good accuracy. Few Black Africans present with an Atypical Parkinsonian syndrome. The pattern of metabolism in PLH-PD is similar to PD patients without HIV.
Conflicts of interest
The authors declare that there are no conflicts of interest related to this research. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Conceptulization: FA, NN. Data curation: FA, NN. Formal analysis: FA, NN. Funding acquisition: FA. Investigation: FA, AIB, NN. Methodology: FA, AIB, NN. Project administration: FA, AIB. Resources: FA, AIB, NN. Software: NN. Supervision: AIB, NN. Validation: FA, NN, AIB Visualization: FA, NN. Writing - original draft: FA, NN Writing - review editing: AIB, NN, FA
Acknowledgements
We would like to acknowledge Ms. Cathy Connolly for providing the statistical support. We acknowledge the Professor Pruim's contribution to posing questions to contextualize the research. We would like to acknowledge Dr. Veneson Pillay from the Department of Nuclear Medicine for assisting with image downloading.
References
- 1.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 2.Weiner W.J. A differential diagnosis of parkinsonism. Rev. Neurol. Dis. 2005;2(3):124–131. [PubMed] [Google Scholar]
- 3.Hughes A.J., Daniel S.E., Ben-Shlomo Y., Lees A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 4.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 6.Stamelou M., Bhatia K.P. Atypical parkinsonism: diagnosis and treatment. Neurol. Clin. 2015;33(1):39–56. doi: 10.1016/j.ncl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Litvan I., Bhatia K.P., Burn D.J., Goetz C.G., Lang A.E., McKeith I., et al. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov. Disord. 2003;18(5):467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 8.Poston K.L., Eidelberg D. FDG PET in the evaluation of Parkinson’s disease. PET Clin. 2010;5(1):55–64. doi: 10.1016/j.cpet.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellwig S., Amtage F., Kreft A., Buchert R., Winz O.H., Vach W., et al. [(1)(8)F]FDG-PET is superior to [(1)(2)(3)I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology. 2012;79(13):1314–1322. doi: 10.1212/WNL.0b013e31826c1b0a. [DOI] [PubMed] [Google Scholar]
- 10.Thobois S., Prange S., Scheiber C., Broussolle E. What a neurologist should know about PET and SPECT functional imaging for parkinsonism: a practical perspective. Parkinsonism Relat. Disord. 2019;59:93–100. doi: 10.1016/j.parkreldis.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Meles S.K., Teune L.K., de Jong B.M., Dierckx R.A., Leenders K.L. Metabolic imaging in Parkinson disease. J. Nucl. Med. 2017;58(1):23–28. doi: 10.2967/jnumed.116.183152. [DOI] [PubMed] [Google Scholar]
- 12.Eckert T., Barnes A., Dhawan V., Frucht S., Gordon M.F., Feigin A.S., et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26(3):912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Eidelberg D., Moeller J.R., Dhawan V., Spetsieris P., Takikawa S., Ishikawa T., et al. The metabolic topography of parkinsonism. J. Cereb. Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 14.Brown R.K., Bohnen N.I., Wong K.K., Minoshima S., Frey K.A. Brain PET in suspected dementia: patterns of altered FDG metabolism. Radiographics. 2014;34(3):684–701. doi: 10.1148/rg.343135065. [DOI] [PubMed] [Google Scholar]
- 15.Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D., et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berardelli A., Wenning G.K., Antonini A., Berg D., Bloem B.R., Bonifati V., et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur. J. Neurol. 2013;20(1):16–34. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- 19.Latourelle J.C., Beste M.T., Hadzi T.C., Miller R.E., Oppenheim J.N., Valko M.P., et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson’s disease: a longitudinal cohort study and validation. Lancet Neurol. 2017;16(11):908–916. doi: 10.1016/S1474-4422(17)30328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Joseph A., Marshall C.R., Lees A.J., Noyce A.J. Ethnic variation in the manifestation of Parkinson’s disease: a narrative review. J. Parkinsons Dis. 2020;10(1):31–45. doi: 10.3233/JPD-191763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amod F.H., Bhigjee A.I., Moodley A. Does antiretroviral therapy alter the course of Parkinson’s disease in people living with HIV? J. Neuro-Oncol. 2021;27(4):595–600. doi: 10.1007/s13365-021-00999-5. [DOI] [PubMed] [Google Scholar]
- 22.Dotchin C., Msuya O., Kissima J., Massawe J., Mhina A., Moshy A., et al. The prevalence of Parkinson’s disease in rural Tanzania. Mov. Disord. 2008;23(11):1567–1672. doi: 10.1002/mds.21898. [DOI] [PubMed] [Google Scholar]
- 23.Blanckenberg J., Bardien S., Glanzmann B., Okubadejo N.U., Carr J.A. The prevalence and genetics of Parkinson’s disease in sub-Saharan Africans. J. Neurol. Sci. 2013;335(1–2):22–25. doi: 10.1016/j.jns.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 24.De Rosa A., Peluso S., De Lucia N., Russo P., Annarumma I., Esposito M., et al. Cognitive profile and 18F-fluorodeoxyglucose PET study in LRRK2-related Parkinson’s disease. Parkinsonism Relat. Disord. 2018;47:80–83. doi: 10.1016/j.parkreldis.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Williams U., Bandmann O., Walker R. Parkinson’s disease in Sub-Saharan Africa: a review of epidemiology, genetics and access to care. J. Mov. Disord. 2018;11(2):53–64. doi: 10.14802/jmd.17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics SA Mid-Year Population Estimates. 2020. http://www.statssa.gov.za/publications/P0302/P03022020.pdf [Available from.
- 27.Avert HIV and AIDS in South Africa. 2020. https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/south-africa [updated April 15, 2020. Available from.
- 28.Meles S.K., Renken R.J., Pagani M., Teune L.K., Arnaldi D., Morbelli S., et al. Abnormal pattern of brain glucose metabolism in Parkinson’s disease: replication in three European cohorts. Eur. J. Nucl. Med. Mol. Imaging. 2020;47(2):437–450. doi: 10.1007/s00259-019-04570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripathi M., Dhawan V., Peng S., Kushwaha S., Batla A., Jaimini A., et al. Differential diagnosis of parkinsonian syndromes using F-18 fluorodeoxyglucose positron emission tomography. Neuroradiology. 2013;55(4):483–492. doi: 10.1007/s00234-012-1132-7. [DOI] [PubMed] [Google Scholar]
- 31.Brajkovic L., Kostic V., Sobic-Saranovic D., Stefanova E., Jecmenica-Lukic M., Jesic A., et al. The utility of FDG-PET in the differential diagnosis of Parkinsonism. Neurol. Res. 2017;39(8):675–684. doi: 10.1080/01616412.2017.1312211. [DOI] [PubMed] [Google Scholar]
- 32.Juh R., Kim J., Moon D., Choe B., Suh T. Different metabolic patterns analysis of Parkinsonism on the 18F-FDG PET. Eur. J. Radiol. 2004;51(3):223–233. doi: 10.1016/S0720-048X(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 33.Syngo.via Database Comparison in MI Neurology [Internet] Siemens Healthineers USA, Inc Molecular Imaging; 2021. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/81fcaf12c59c7025/a13ee3bc9f72/siemens-healthineers_mi_syngo_via_database_comparison_neurology_white_paper_july-21.pdf [cited 15/02/2022]. Available from. [Google Scholar]
- 34.Ferguson L.W., Rajput M.L., Muhajarine N., Shah S.M., Rajput A. Clinical features at first visit and rapid disease progression in Parkinson’s disease. Parkinsonism Relat. Disord. 2008;14(5):431–435. doi: 10.1016/j.parkreldis.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Tang C.C., Poston K.L., Eckert T., Feigin A., Frucht S., Gudesblatt M., et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9(2):149–158. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer P.T., Frings L., Rucker G., Hellwig S. (18)F-FDG PET in Parkinsonism: differential diagnosis and evaluation of cognitive impairment. J. Nucl. Med. 2017;58(12):1888–1898. doi: 10.2967/jnumed.116.186403. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo G., Copetti M., Arcuti S., Martino D., Fontana A., Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566–576. doi: 10.1212/WNL.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 38.Amod F.H., Bhigjee A.I. Clinical series of Parkinson’s disease in KwaZulu-Natal, South Africa: retrospective chart review. J. Neurol. Sci. 2019;401:62–65. doi: 10.1016/j.jns.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Bhidayasiri R., Sringean J., Reich S.G., Colosimo C. Red flags phenotyping: a systematic review on clinical features in atypical parkinsonian disorders. Parkinsonism Relat. Disord. 2019;59:82–92. doi: 10.1016/j.parkreldis.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Dekker M.C.J., Coulibaly T., Bardien S., Ross O.A., Carr J., Komolafe M. Parkinson’s disease research on the African continent: obstacles and opportunities. Front. Neurol. 2020;11:512. doi: 10.3389/fneur.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhuri K.R., Hu M.T., Brooks D.J. Atypical parkinsonism in Afro-Caribbean and Indian origin immigrants to the UK. Mov. Disord. 2000;15(1):18–23. doi: 10.1002/1531-8257(200001)15:1<18::aid-mds1005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 42.Sood A., Shukla J., Shree R., Vatsa R., Modi M., Mittal B.R. Comparative performance of 99mTc-TRODAT-1 SPECT/CT and 18F-FDOPA PET/CT imaging in patients with Parkinson’s disease, Parkinson-plus syndrome, and essential tremor. Clin. Nucl. Med. 2021;46(2):95–102. doi: 10.1097/RLU.0000000000003409. [DOI] [PubMed] [Google Scholar]
- 43.Stoessl A.J., Martin W.W., McKeown M.J., Sossi V. Advances in imaging in Parkinson’s disease. Lancet Neurol. 2011;10(11):987–1001. doi: 10.1016/S1474-4422(11)70214-9. [DOI] [PubMed] [Google Scholar]
- 44.Pagano G., Niccolini F., Politis M. Imaging in Parkinson’s disease. Clin. Med. (Lond.) 2016;16(4):371–375. doi: 10.7861/clinmedicine.16-4-371. [DOI] [PMC free article] [PubMed] [Google Scholar]