Abstract
Background
Chemoradiotherapy with durvalumab consolidation has yielded excellent results in stage III non-small-cell lung cancer (NSCLC). Therefore, it is essential to identify patients who might benefit from a surgical approach.
Material and methods
Data from 437 patients with operable stage III NSCLC enrolled in four consecutive Swiss Group for Clinical Cancer Research (SAKK) trials (16/96, 16/00, 16/01, 16/08) were pooled and outcomes were analyzed in 431 eligible patients. All patients were treated with three cycles of induction chemotherapy (cisplatin/docetaxel), followed in some patients by neoadjuvant radiotherapy (44 Gy, 22 fractions) (16/00, 16/01, 16/08) and cetuximab (16/08).
Results
With a median follow-up time of 9.3 years (range 8.5-10.3 years), 5- and 10-year overall survival (OS) rates were 37% and 25%, respectively. Overall, 342 patients (79%) underwent tumor resection, with a complete resection (R0) rate of 80%. Patients (n = 272, 63%) with R0 had significantly longer OS compared to patients who had surgery but incomplete resection (64.8 versus 19.2 months, P < 0.001). OS for patients who achieved pathological complete remission (pCR) (n = 66, 15%) was significantly better compared to resected patients without pCR (86.5 versus 37.0 months, P = 0.003). For patients with pCR, the 5- and 10-year event-free survival and OS rates were 45.7% [95% confidence interval (CI) 32.8% to 57.7%] and 28.1% (95% CI 15.2% to 42.6%), and 58.2% (95% CI 45.2% to 69.2%) and 45.0% (95% CI 31.5% to 57.6%), respectively.
Conclusion
We report favorable long-term outcomes in patients with operable stage III NSCLC treated with neoadjuvant chemotherapy with cisplatin and docetaxel ± neoadjuvant sequential radiotherapy from four prospective SAKK trials. Almost two-third of the patients underwent complete resection after neoadjuvant therapy. We confirm R0 resection and pCR as important predictors of outcome.
Key words: operable stage III non-small-cell lung cancer, neoadjuvant therapy, surgery, long-term outcomes, prognostic factors
Highlights
-
•
Combined modality treatment in operable stage III NSCLC results in 5- and 10-year survival rates of 37% and 25%.
-
•
Long-term survival for patients with incomplete resection is poor.
-
•
Complete resection and pCR are important predictors for outcome.
Introduction
Five-year overall survival (OS) rates of stage III non-small-cell lung cancer (NSCLC) range from 13% (IIIC) to 36% (IIIA).1 Treatment approaches vary considerably depending on tumor size, invasion of local structures, and extent of ipsilateral mediastinal lymph node involvement. While in patients with N3 disease definitive concurrent chemoradiation remains the standard of care, controversy exists in the treatment of stage IIIA disease, particularly for patients with T1-2 and non-invasive T3 N2. In these patients, either multimodal approaches consisting of neoadjuvant chemotherapy ± radiotherapy followed by surgery (bi- or trimodal concepts) or definitive concurrent chemoradiation are valid treatment options. The key question at multidisciplinary boards for patients with stage III N2 NSCLC is resectability for which currently no standardized definition exists. For patients with unresectable stage III NSCLC, concurrent chemoradiation followed by consolidation immunotherapy with the programmed death-ligand 1 (PD-L1) inhibitor durvalumab has emerged as the new standard of care, based on the results from the PACIFIC trial.2 The 4-year OS rates with durvalumab and placebo were 50% versus 36% [hazard ratio (HR) 0.71].3
For operable stage III NSCLC, the Swiss Group for Clinical Cancer Research (SAKK) has been investigating the optimal treatment management since 1996 in five consecutive studies including one phase III trial (SAKK 16/00).4, 5, 6, 7, 8 In all studies, patients received three cycles of identical neoadjuvant chemotherapy with cisplatin and docetaxel. In arm A of SAKK 16/00, in SAKK 16/01, and SAKK 16/08, patients received immediately after completion of chemotherapy a course of preoperative accelerated radiotherapy to 44 Gy in 22 fractions over 3 weeks. Furthermore, SAKK 16/08 investigated the addition of cetuximab to preoperative chemoradiotherapy. All studies recruited only patients who were considered potentially resectable at the time of diagnosis as assessed by a multidisciplinary board. Importantly, many patients enrolled in these trials may be considered non-resectable today by various treating teams due to multi-level N2, bulky mediastinal lymph nodes, or even N3 involvement.
While the advent of immune checkpoint inhibitors (ICIs) in treating cancers has changed the therapeutic strategy in unresectable stage III NSCLC,2 for operable NSCLC, data from phase II trials and one phase III trial suggest that incorporation of ICIs before surgery is safe and promising.9, 10, 11, 12, 13 Several randomized phase III trials investigating the use of ICIs in the neoadjuvant or adjuvant setting are currently ongoing or have been published. Furthermore, the results of treatment with adjuvant atezolizumab in resected stage IB-IIIA NSCLC have been published recently demonstrating an improved disease-free survival in the PD-L1-positive population, including stage IIIA patients.14 Results from all these trials in operable stage III patients including ICIs, however, are relatively recent and no long-term survival outcomes have been reported. Similarly, outcomes from the PACIFIC trial in inoperable stage III patients beyond 5 years are also unknown.
A previous pooled analysis by our group including data from the SAKK 16/96, 16/00, and 16/01 studies reported favorable 10-year survival rates of 29% in stage IIIA and 27% in stage IIIB.15 Comparable long-term results have been achieved with a trimodal treatment concept in the German–French trial CISTAXOL (10-year survival of 26%, ranging from 37% in IIIA N2 to 18% in IIIB)16 also in the pre-immunotherapy era.
Herein we report an updated long-term efficacy analysis from the pooled SAKK 16/96, 16/00, and 16/01 studies, including patients from the recent SAKK 16/08 study. In addition, prognostic factors associated with improved outcomes were explored.
Material and methods
Study design and treatment
The detailed study designs, inclusion and exclusion criteria, and methods of the SAKK 16/96, 16/00, 16/01, and 16/08 studies have been previously published.4, 5, 6, 7 In brief, these studies were designed for operable stage III NSCLC, both stage IIIA N2 and IIIB. Patients were treated with three cycles of neoadjuvant chemotherapy (cisplatin 100 mg/m2 and docetaxel 85 mg/m2, given once every 3 weeks) followed by surgery (bimodal therapy group), or neoadjuvant chemotherapy followed by radiotherapy (44 Gy in 22 fractions in 3 weeks with accelerated concomitant boost radiotherapy) (trimodal therapy group). Patients in 16/08 received concomitant cetuximab (loading dose with 400 mg/m2, followed by 250 mg/m2 weekly) during both chemo- and radiotherapy. Surgery in all studies included an anatomical tumor resection with mediastinal lymph node dissection as described by Martini et al.17 All four studies were done in accordance with the Declaration of Helsinki and the guidelines on Good Clinical Practice. The protocols were approved by local ethics committees. Written informed consent was obtained for every patient.
Patient population
Overall, 437 patients were enrolled in these four trials: 90 patients in 16/96, 232 in 16/00, 46 in 16/01, and 69 in 16/08. The trials were conducted consecutively. The first patient was included in April 1997, the last, in January 2016. Patients were pathologically staged with mediastinoscopy or (once available) with endobronchial ultrasound. Only 62% of patients had staging with 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT), as introduction in clinical practice was only in the early 2000s. The details on staging procedures have been described in the respective studies. SAKK 16/01 and 16/08 enrolled patients with T4 N0-3 or T1-4 N3 (excluding malignant pleural or pericardial effusion, invasion of the aorta, esophagus, myocardium, and supraclavicular, scalene N3 nodes, or with satellite lesions in the same lobe), whereas SAKK 16/96 and 16/00 included T1-3 N2 patients only. The then used fifth (16/96) and sixth tumor–node–metastasis (TNM) staging edition (16/00, 16/01, and 16/08) were transferred for the current analysis to the seventh version to make the results more comparable with the recent literature. Tumor stages of patients who were initially T2 were transferred to T2∗-T3∗ according to their baseline tumor size measurements (T2∗: 3 cm < size ≤ 7 cm, T3∗: size > 7 cm). Patients who had separate tumor nodules in the same lobe (satellite lesions within the same lobe) were shifted from T4 to T3∗. For the present analysis, six patients were excluded: two patients were retrospectively detected to have stage IV disease at diagnosis and the others had stage IIB disease with the adapted seventh TNM staging edition.
Outcomes
Response and follow-up assessments for each of the four SAKK studies were carried out according to the specific study protocol. All patients were followed for OS and event-free survival (EFS). For the present analysis, survival data have been updated. Data cut-off was 8 February 2021.
Endpoints of the pooled analysis were OS and EFS at defined timepoints (1, 2, 3, 4, 5, and 10 years) as well as median OS and median EFS, in the overall population, in patients who had tumor resection, in patients with stage IIIA and IIIB, and in patients with resected stage IIIA N2 (T1-3 N2) disease. OS was the interval from the date of registration or randomization until the date of death from any cause. For comparison between groups, survival curves were evaluated from the date of surgery. EFS was defined as the time from registration or randomization to objective tumor progression or relapse, secondary tumor (only in 16/00), or death due to any cause, whichever occurred first. Patients who did not experience an event were censored at the time of last known survival if they were alive. The definition of pathological complete remission (pCR) was transferred from each trial: pCR was defined as ≥95% necrosis or fibrosis in the SAKK 16/96 study and absence of tumor cells (0%) in the other studies.
Additionally, we analyzed potentially prognostic factors for complete resection (R0) and pCR using univariable logistic regression. This analysis included age (continuous variable, and with a cut-off of <70 versus ≥70 years), sex (male versus female), smoking status (never versus current and former smokers; never smokers: < 100 cigarettes per lifetime, current smokers: active smokers, former smokers: ex-smokers; all at study inclusion), Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1 and 2), histology (squamous cell versus non-squamous cell carcinoma), stage IIIA versus IIIB, T-stage (T1 and T2 versus T3 and T4), treatment modality (bimodal versus trimodal), and advanced versus non-advanced stage III disease characteristics (advanced was defined as N3- or multiple nodal involvement or presence of a mediastinal nodal bulk of ≥5 cm). Furthermore, we analyzed whether there was an association of dynamic standardized FDG uptake value (SUV) changes from the initial to the preoperative PET/CT. Overall, PET-SUV were retrievable and digitally assessable from 128 patients, including 61 patients with matched pretreatment (= timepoint 1) and pre-surgery (= timepoint 2) PET/CTs. SUV was analyzed in the primary tumor. Analyzed SUV included SUV maximum, SUV minimum, SUV median, and SUV mean. For patients with paired PET/CTs, the following values were then calculated: the difference between SUV at timepoint 2 and timepoint 1 for SUV maximum (Delta SUV maximum), SUV median (Delta SUV median), and SUV mean (Delta SUV median), as well as the percentage of the residual SUV maximum (% SUV remaining = SUV maximum timepoint 2/SUV maximum timepoint 1).
Statistical analyses
Patients’ characteristics were summarized by median and range for continuous variables and by frequency and proportion for categorical variables. Time-to-event endpoints were analyzed using the Kaplan–Meier method along with its corresponding 95% confidence interval (CI) based on the log–log approach. Between-group survival curves and rates were compared using the log-rank test and the Kaplan–Meier method at a specific time point, respectively. Univariable logistic regression was used to investigate the association between binary outcomes and factors. Statistical significance was set at P < 0.05. All statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R v4.1.0.
Results
Patient population and treatment
A total of 431 patients were included in our pooled analysis. Patient demographics and disease characteristics are summarized in Table 1. Median age was 59.8 years (range 28-76 years). Most patients were male (71%), current or former smokers (92%), and had an ECOG performances status of 0 (66%). Tumors were classified as adenocarcinoma (39%), squamous cell carcinoma (37%), large cell carcinoma (8%), and 17% were defined at the time as poorly differentiated NSCLC. Overall, 354 patients (82%) had stage IIIA and 77 (18%) had stage IIIB disease. Most patients were diagnosed with N2 disease (81%), whereas 11% had N3 disease. Overall, 79 (18%) patients had advanced stage III characteristics according to the aforementioned definition. Overall, 202 patients (47%) were assigned to the bimodal therapy group, and 229 patients (53%) to a trimodal therapy group. Of the 431 patients, 342 (79%) had surgery with tumor resection, of whom 80% (272 patients) had complete surgical resection. Among all resected patients, pCR was achieved in 66 patients (19%). Reasons for not having surgery (n = 89) were tumor progression (n = 38, 43%), death (n = 5, 6%), complications or toxicities leading to study drop-out (n = 5, 6%), withdrawal of study consent (n = 6, 6%), and other reasons (n = 35, 39%). Among the 342 patients who had tumor resection, 33% had pneumonectomy and the rest had lesser-extent surgeries. The R0 resection rate of each SAKK trial is presented in Supplementary Table S1 (available at https://doi.org/10.1016/j.esmoop.2022.100455). For patients with stage IIIA, complete resection was achieved in 80%. For the subset of patients with stage IIIB, 58 out of 77 patients (75%) underwent tumor resection, with R0 resection in 45 patients (78%).
Table 1.
Patient’s demographics and disease characteristics
| Overall (N = 431) | |
|---|---|
| Trial, n (%) | |
|
88 (20.4) |
|
231 (53.6) |
|
43 (10.0) |
|
69 (16.0) |
| Therapy group, n (%) | |
|
202 (46.9) |
|
229 (53.1) |
|
|
|
59.8 (28.0-76.0) |
| Age group, n (%) | |
|
38 (8.8) |
|
393 (91.2) |
| Sex, n (%) | |
|
126 (29.2) |
|
305 (70.8) |
| Smoking status, n (%) | |
|
149 (34.6) |
|
249 (57.8) |
|
32 (7.4) |
|
1 (0.2) |
| Smoking burden (pack-years)a | |
|
45 (3-182) |
| Histological subtype, n (%) | |
|
|
|
|
|
|
|
|
|
|
| ECOG performance status, n (%) | |
|
285 (66.1) |
|
145 (33.6) |
|
1 (0.2) |
| Weight lossb, n (%) | |
|
198 (45.9) |
|
72 (16.7) |
|
161 (37.4) |
| Nodal status (TNM seventh edition), n (%) | |
|
32 (7.4) |
|
4 (0.9) |
|
347 (80.5) |
|
48 (11.1) |
| T-stage (TNM seventh edition), n (%) | |
|
51 (11.8) |
|
170 (39.4) |
|
144 (33.4) |
|
66 (15.3) |
| UICC stage (TNM seventh edition), n (%) | |
|
354 (82.1) |
|
77 (17.9) |
ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small-cell lung cancer; SAKK, Swiss Group for Clinical Cancer Research; TNM, tumor–node–metastasis; UICC, International Union Against Cancer (Union Internationale Contre le Cancer).
Data collected for 389 patients.
Data collected only for patients from SAKK 16/00 and SAKK 16/01 trials.
Efficacy
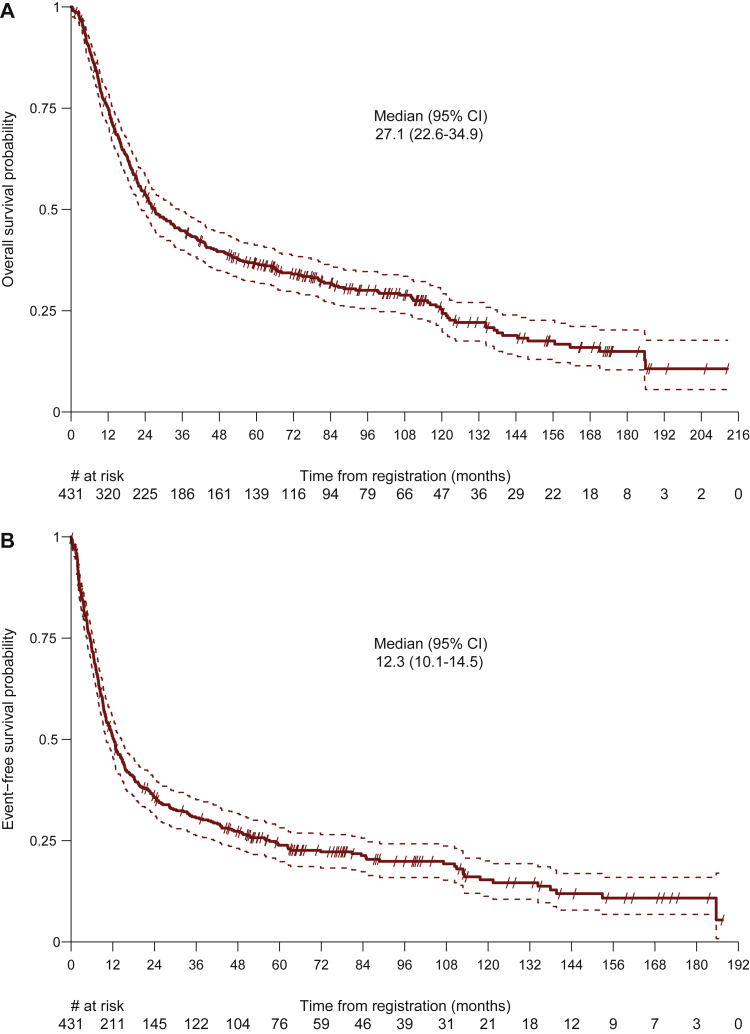
At the data cut-off for this analysis (8 February 2021), the median duration of follow-up was 9.3 years (95% CI 8.5-10.3 years), 317 of 431 patients (74%) had died, and 92 patients (21%) remained free from recurrence. Twenty-two patients (5%) were lost to follow-up. Death was due to tumor progression in 233 patients (74%), secondary malignancies in 12 patients (4%), and not tumor-related in 51 patients (16%). The median OS in the overall population was 27.1 months (95% CI 22.6-34.9 months) (Figure 1 and Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100455). The 1-, 2-, 5-, and 10-year OS rates were 75% (95% CI 71% to 79%), 54% (95% CI 49% to 58%), 37% (95% CI 32% to 41%), and 25% (95% CI 21% to 30%), respectively. At the time of data cut-off, 339 EFS events (79%) had occurred and the median EFS was 12.3 months (95% CI 10.1-14.5 months) (Figure 1). EFS at 1, 2, 5, and 10 years were 50% (95% CI 46% to 55%), 36% (95% CI 31% to 40%), 24% (95% CI 20% to 28%), and 15% (95% CI 11% to 20%), respectively. Among the 280 patients with tumor relapse, 163 patients (58%) had distant relapse, 82 had locoregional relapse (29%), and 35 patients (13%) presented with both distant and locoregional relapse.
Figure 1.
Overall survival with median overall survival (months) (A) and event-free survival with median event-free survival (months) in the overall population (B).
CI, confidence interval.
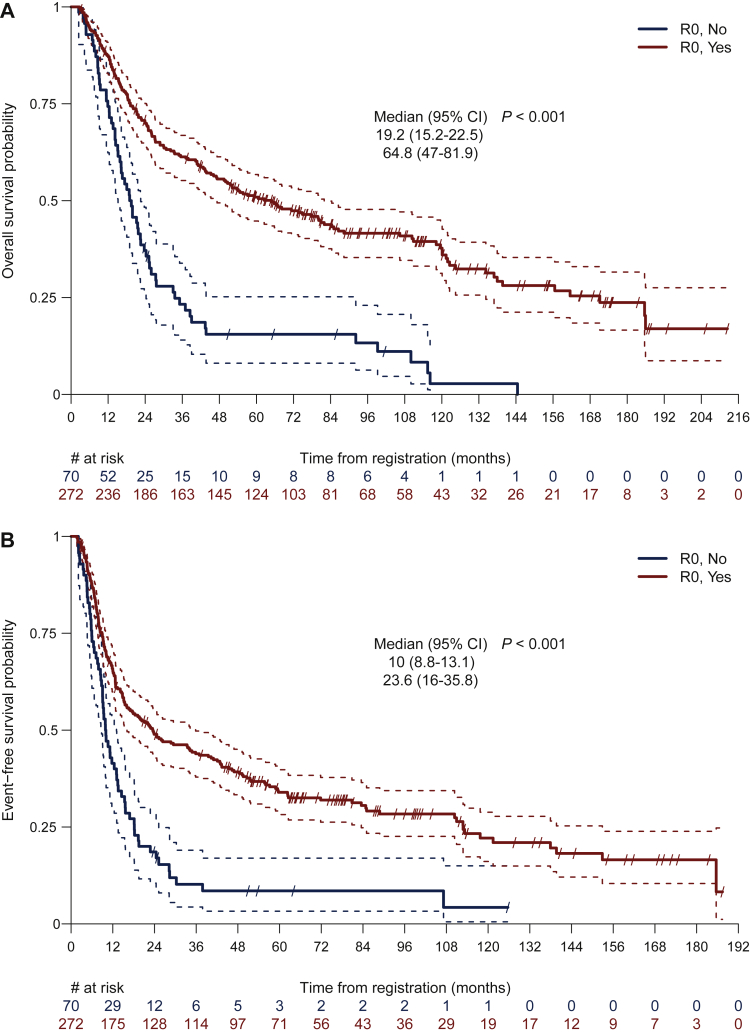
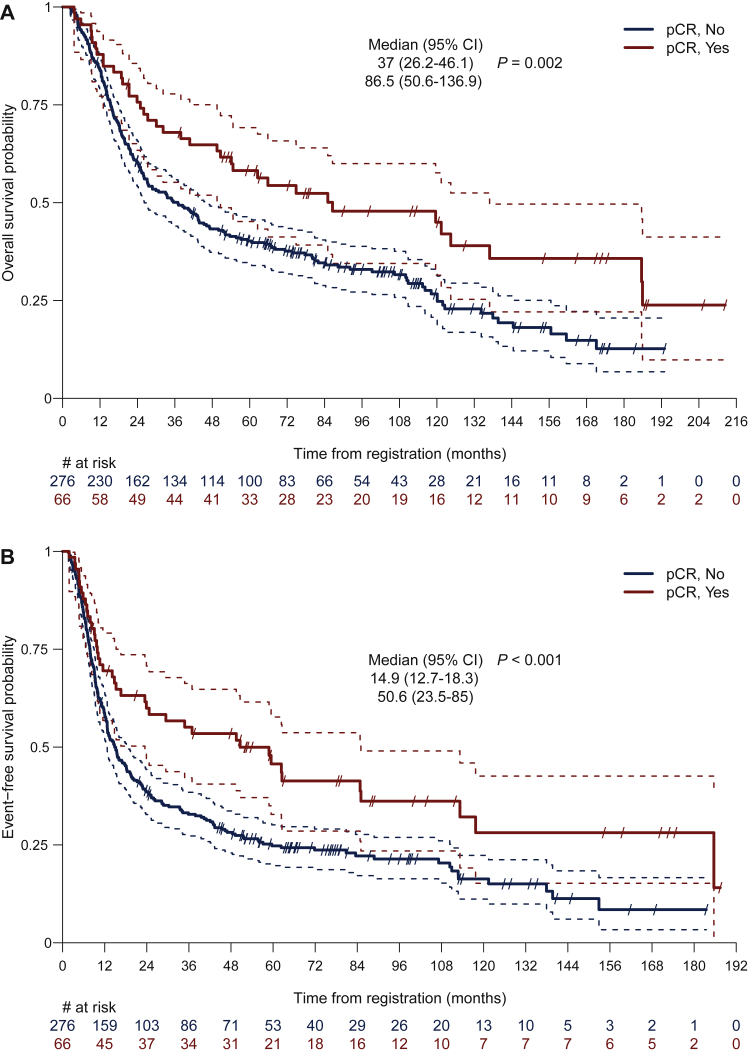
Outcomes were significantly improved if patients had R0 resection (P < 0.001) or if pCR was achieved (P = 0.002). Among patients who had R0 resection, the median OS was 64.8 months (95% CI 47.0-81.9 months), which was significantly higher than that for patients without complete resection (19.2 months, 95% CI 15.2-22.5 months) (Figure 2 and Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100455). Likewise, EFS was longer for patients with R0 resection (median EFS, 23.6 versus 10.0 months; P < 0.001) compared with patients who were incompletely resected (Figure 2 and Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100455). Five- and 10-year OS rates for patients with complete resection were 50.9% and 37.7%, respectively, while 15.5% and 2.8% for patients without R0 resection. Of the 66 resected patients who had pCR, the median EFS was 50.6 months (95% CI 23.5-85.0 months), compared to 14.9 months (95% CI 12.7-18.3 months) in patients with residual tumor (P = 0.002) (Figure 3). The median OS was significantly improved for patients with pCR (86.5 versus 37.0 months; P = 0.003) (Figure 3). In these patients, the 5- and 10-year EFS rates were 45.7% (95% CI 32.8% to 57.7%) and 28.1% (95% CI 15.2% to 42.6%), respectively. Five- and 10-year OS rates were 58.2% (95% CI 45.2% to 69.2%) and 45.0% (95% CI 31.5% to 57.6%), respectively.
Figure 2.
Overall survival with median overall survival (months) (A) and event-free survival with median event-free survival (months) (B) for patients with complete resection (R0, Yes) and incomplete resection (R0, No).
CI, confidence interval; R0, complete surgical resection.
Figure 3.
Overall survival with median overall survival (months) (A) and event-free survival with median event-free survival (months) (B) for patients who achieved complete pathological remission (pCR, Yes) and those without (pCR, No).
CI, confidence interval; pCR, pathological complete remission.
Both for OS (median OS, 27.4 versus 22.5 months, P = 0.952) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100455) and for EFS (median EFS, 12.4 versus 12.0 months, P = 0.454) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100455) no significant difference was observed between patients with stage IIIA and IIIB disease.
Patients with stage IIIA N2 disease
In the overall population, 318 patients had stage IIIA N2 disease. Among those patients, 259 (82%) had surgery and tumor resection. Median EFS was 12.3 months (95% CI 9.9-14.8 months). Five- and 10-year EFS rates in stage IIIA N2 patients were 22.8% (95% CI 18.3% to 27.7%) and 15.3% (95% CI 10.8% to 20.6%), respectively, and 5- and 10-year OS rates were 36.8% (95% CI 31.4% to 42.1%) and 25.5% (95% CI 20.2% to 31.0%), respectively. Among the 259 patients who had tumor resection, 193 (75%) had disease recurrence and 178 (69%) have died. Median EFS was 18.4 months (95% CI 13.4-24.5 months) and median OS was 43.7 months (95% CI 33.5-59.3 months) (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100455). We could not detect any significant difference in comparison of EFS and OS for stage IIIA N2 patients from the bimodal therapy group compared with the trimodal therapy group. Median EFS was 11.6 months for the bimodal therapy approach and 12.8 months for the trimodal concept containing radiotherapy (P = 0.677). The corresponding median OS was 26.2 and 37.2 months (P = 0.859), respectively.
Prognostic factors for R0 resection and pCR
Patients without the presence of advanced disease characteristics were more likely to have pCR [odds ratio (OR) 1.96, 95% CI 1.03-3.72] compared to patients with at least one of the advanced stage factors (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100455). Patients with bimodal treatment approach were more likely to have pCR (OR 1.80, 95% CI 1.04-3.11) compared to trimodal treatment. Patients with stage IIIA were less likely to achieve pCR (OR 0.37, 95% CI 0.18-0.76) than stage IIIB. Decrease of SUV median (OR 0.62, 95% CI 0.42-0.93) and of SUV mean (OR 0.64, 95% CI 0.43-0.94) from baseline PET/CT to preoperative PET/CT was prognostic for pCR. Among all tested factors for R0 resection, bimodal therapy was associated with a decreased likelihood of achieving a complete resection (OR 0.61, 95% CI 0.41-0.90) compared to trimodal (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100455).
In an exploratory analysis, decrease of SUV maximum of >50% from pre- to post-induction therapy PET/CT was associated with an improved survival (HR 0.28, 95% CI 0.13-0.62) (data not shown).
Discussion
In the present analysis we report an updated follow-up from now four pooled SAKK studies including a total of 431 patients with a multimodal and interdisciplinary treatment approach in operable stage III NSCLC including induction chemotherapy ± radiotherapy followed by surgery. With a median follow-up of 9.3 years and the inclusion of the SAKK 16/08 study, we present favorable long-term outcomes with 5- and 10-year survival rates of 37% and 25%, respectively, indicating a long-time cure in one out of four patients. Comparison of our pooled analysis with contemporaneous trials in a pre-immunotherapy era is presented in Table 2. It is important to note that long-term outcomes of patients with stage III NSCLC treated with regimens incorporating ICIs either as consolidation after chemoradiotherapy or pre- or post-operatively are still pending, and it will be crucial that long-term follow-up beyond 5 years of these trials will be reported similarly to our analysis to definitively determine the effect of ICIs on cure rates.
Table 2.
Randomized phase III trials with a neoadjuvant surgical treatment approach in stage III NSCLC before the introduction of immune checkpoint inhibitors
| Trial Recruitment Number of enrolled/surgery/resected patients |
Phase | Stage TNM | Treatment arms | R0a | pCRa | OSb (number of patients included in the OS analysis per arm) | OSc |
|---|---|---|---|---|---|---|---|
|
SAKK 16 trials 1997-2016 n = 431/342 |
II-III | IIIA-N2 IIIB TNM seventh |
RCT > S CT > S |
272/342 (80%) | 66/342 (19%) | 5-year OS: 37% mOS: 27.1 months | |
|
Rosell et al.22 1989-1991 n = 60/50/50 |
III | IIIA-N2 Mountain 1986 |
S CT > S |
27/30 (90%) 23/27 (85%) |
0/30 (0%) 1/27 (4%) |
5-year OS: 0% versus 17% mOS: 10 versus 22 months | |
|
RTOG 89-0123 1990-1994 n = 73/26/23 |
III | IIIA-N2 Mountain 1986 |
CT > S > CT CT > RT > CT |
19/26 (73%) | 4-year OS: 22% versus 22% mOS: 19.4 versus 17.4 months (N = 29/N = 32) | ||
|
Stephens et al.24 1995-1999 n = 48/7/4 |
III | IIIA IIIA-N2 Mountain 1986 |
RT CT > S |
2-year OS: 16% versus 15% mOS: 11.2 versus 13.8 months | |||
|
Intergroup 013925 1994-2001 n = 396/164/155 |
III | IIIA-N2 Mountain 1986 |
RCT > S RCT |
144/164 (88%) | 29/164 (18%) | 5-year OS: 27% versus 20% mOS: 23.6 versus 22.2 months | |
|
EORTC 0894126 1994-2002 n = 579/154/131 |
III | IIIA-N2 TNM fifth |
CT > RT CT > S |
77/154 (50%) | 8/154 (5%) | 5-year OS: 14% versus 16% mOS: 17.5 versus 16.4 months (N = 165/N = 167) | mOS: 15.4 months |
|
GLCCG27 1995-2003 n = 524/296/272 |
III | IIIA-N2 IIIB Mountain 1986 |
CT > RCT > S CT > S > RT |
182/296 (62%) | 76/296 (26%)d | 5-year OS: 21% versus 18% mOS: 15.7 versus 17.6 months | |
|
WJTOG990318 2000-2005 n = 60/51/49 |
III | IIIA-N2 TNM sixth |
RCT > S CT > S |
3/51 (6%) | 3-year OS: 52% versus 39% mOS: 39.6 versus 29.9 months | ||
|
ESPATUE20 2004-2013 n = 246/70/70 |
III | IIIA-N2 IIIB TNM sixth |
CT > RCT > RCT CT > RCT > S |
66/70 (94%) | 27/70 (39%) | 5-year OS: 40% versus 44% (N = 80/N = 81) | 5-year OS: 34% |
Comparison with pooled analysis of SAKK 16 trials.
CT, chemotherapy; mOS, median overall survival; NSCLC, non-small-cell lung cancer; OS, overall survival; pCR, pathological complete remission; R0, complete surgical resection; RCT, chemoradiation; RT, radiotherapy; S, surgery; SAKK, Swiss Group for Clinical Cancer Research; TNM, tumor–node–metastasis.
Proportion of patients who had surgery.
Patients who were randomized.
Intention-to-treat population.
>90% regression.
We were able to corroborate previous observations by others and our own group demonstrating significantly improved survival in patients whose tumors were completely resected (median OS, 64.8 versus 19.2 months) and in those who achieved complete pathological remission (median OS, 86.5 versus 37.0 months).4,18,19 Patients with complete surgical resection had excellent long-term survival rates (5- and 10-year OS rates of 50.9% and 37.7%, respectively). However, complete surgical resection was possible only in 63% of the enrolled patient population. Twenty-one percent of patients did not proceed to surgery, mostly due to tumor progression under induction treatment, and in 16% of patients, surgical resection was incomplete. Patients with incomplete resection had poor long-term outcomes (10-year OS rate of 2.8%). This observation emphasizes the importance of optimizing multimodal treatment strategies especially for the 37% of patients not achieving complete resection after induction therapy: these include more effective induction treatment regimens including ICIs, accurate preoperative patient selection to avoid incomplete resection, or upfront selection of a non-surgical strategy of definitive radiochemotherapy followed by ICIs.
With univariate logistic regression models, we then aimed at identifying factors associated with R0 resection. The only factor that resulted prognostic for complete surgical resection in our model was trimodal compared to bimodal treatment. This observation is likely driven by the results of the SAKK 16/00 trial, where chemoradiotherapy led to a 10% increase of the R0 resection rate over chemotherapy alone (91% versus 81%). Consistent with our results, patients in the ESPATUE trial who were randomized after completion of chemoradiotherapy had a high R0 resection rate of 81%.20 However, it is important to point out that the higher R0 resection rate in the chemoradiotherapy group of SAKK 16/00 did not translate into a prolonged OS. Further research is needed to identify the reasons why the improved R0 resection rate after trimodal compared to bimodal treatment did not translate into improved OS. Incidental irradiation of the heart has recently been associated with increased cardiac toxicity and increased non-cancer death rates.21 Radiation-induced fibrosis might increase the difficulties to differentiate post-treatment normal tissue effects from locoregional in-field tumor recurrence in radiological follow-up imaging. Advanced technologies such as conformal heart-avoiding radiotherapy planning and FDG-PET/CT for follow-up might address these issues in the future.
We were not able to identify other prognostic factors for complete surgical resection. A significant number of patients in the SAKK 16 trials, although considered operable by local trial surgeons, had rather advanced disease including bulky nodal disease, multistation N2 lymph involvement, or even stage IIIB N3 disease. While there was a tendency of lower rates of R0 resections in patients with advanced stage III disease, the difference was not significant. Particularly for stage IIIA versus IIIB, the rate of complete resection was nearly identical (80% versus 78%) suggesting that disease stage may not primarily be the best indicator to identify appropriate surgical candidates. Furthermore, FDG-PET response to neoadjuvant treatment was also not prognostic for R0 resection. Therefore, its preoperative value for selecting appropriate surgical candidates remains unclear. This finding must be interpreted with caution as only a minority of patients in the SAKK trials had both baseline and preoperative FDG-PET/CT, and FDG-PET/CT imaging protocols were not standardized across institutions.
As the SAKK 16 trials were conducted over a period of almost 20 years and during an era of considerable improvement and innovation in diagnostic and surgical procedures, we were able to compare the rate of complete resection from the earlier to the later SAKK trials. While complete resection was achieved in 55% of the patients undergoing surgery in SAKK 16/96, the R0 resection rate increased to 81% in the chemotherapy-alone arm of the more recent SAKK 16/00.4,6 However, it remains unclear whether the improvement can be attributed to better diagnostic staging procedures (e.g. introduction of FDG-PET/CT) or potential learning curve and better surgical techniques over time.
The incorporation of ICIs into the multimodal treatment of operable stage III NSCLC is likely to change future treatment standards (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2022.100455). SAKK 16/148 and the NADIM trial,12 both phase II studies with a neoadjuvant immunotherapy strategy including treatment with an ICI, had excellent rates of complete surgical resection (93% and 100%, respectively) and high rates in long-term outcome. It remains to be seen whether these excellent results are attributed to neoadjuvant immunotherapy, preoperative patient selection, and/or improved surgical expertise. Further studies will also show whether additional treatment approaches are able to further improve the efficacy of neoadjuvant therapy. The combination of a neoadjuvant immune checkpoint inhibitor with immunomodulatory radiotherapy is currently investigated by our group (SAKK 16/18, NCT04245514) as well as multiple phase III randomized trials.
In our pooled analysis, 19% of patients achieved pCR and we were able to confirm previous findings that pCR was associated with significantly improved outcomes.4,18,19 In our univariate logistic regression model, patients with the presence of advanced stage III disease characteristics, such as N3-involvement, multiple nodal involvement, or bulky nodal disease, were less likely to achieve a pCR. Surprisingly, the probability of achieving pCR was higher for stage IIIB than stage IIIA disease, indicating again, that stage per se may not be a relevant selection criterion for a multimodal approach including surgery. This counterintuitive finding could be explained by the high rate of pCR of 29% in the SAKK 16/08 trial that included mainly patients with TNM seventh stage IIIB (66% of study patients with T3 or T4 tumors). For all calculated dynamic FDG-PET-SUV, a decrease in SUV median and a decrease in SUV mean from pre- to post-induction therapy PET/CT were prognostic for pCR, which appears biologically plausible. Interestingly, a decrease in SUV maximum of >50% from pre- to post-induction therapy PET/CT was associated with improved OS. However, these findings must be interpreted with caution due to the relatively low number of patients with FDG-PET/CTs available for our logistic regression analysis.
Our study has several strengths and limitations. This pooled analysis combines a large, homogenous study population of patients with operable stage III NSCLC treated in a multicentric setting over approximately two decades using the same neoadjuvant chemotherapy backbone. We demonstrated that intense neoadjuvant chemotherapy with cisplatin and docetaxel ± radiotherapy is safe in fit patients and effective resulting in a pCR rate of 19%. Although for IIIA N2 patients we observed similar survival for patients who underwent trimodal versus bimodal treatment (median OS 37.1 versus 26.2 months; P = 0.859), it is noteworthy that the approach of the SAKK 16 trials included the application of sequential preoperative radiotherapy instead of the more commonly used concurrent chemoradiotherapy approach. We previously showed that sequential hyperfractionated administration of preoperative radiotherapy was not associated with increased mortality rates.15
Conclusions
In summary, combined modality treatment including induction chemotherapy ± radiotherapy followed by surgery results in long-term disease control in 25% of patients after 10 years. Our analysis underscores the importance of interdisciplinary evaluation and determination of treatment strategy to identify the patients who benefit from a surgical approach. In the absence of sufficiently defined objective pre-therapeutic prognostic factors, the evaluation by an experienced multidisciplinary treating team is crucial. Pathological complete response and complete surgical resection are essential prognostic factors for outcome. Our results emphasize the importance of implementing highly active preoperative treatment regimen resulting in high pCR rates in future studies and underpin the need for identification of pre-treatment factors associated with R0 resection as well as identification of factors favoring a non-surgical treatment strategy.
Acknowledgements
We thank the patients and their families for their participation in the studies, and the investigators and staff for their contributions.
Funding
This work was supported by an unrestricted grant from AstraZeneca (no grant number), as well as research agreements with the following institutions: Swiss State Secretary for Education (no grant number), Research and Innovation (SERI) (no grant number), Swiss Cancer Research Foundation (SCS), and Swiss Cancer League (SCL) (no grant numeber).
Disclosure
DK: consulting or advisory role: AstraZeneca (AZ) (fee to institution). IO: grants (all fees to institution): Roche, Medtronic; honoraria (all fees to speakers bureau): AZ, Roche; advisory role: AZ, Merck, Sharp, and Dohme (MSD). WW: honoraria, consulting or advisory role, travel/accommodations: AZ, Medtronic. SIR: honoraria, consulting or advisory role (all fees to institution): AbbVie, Amgen, AZ, Boehringer Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Eisai, Janssen, MSD, Merck Serono, Novartis, PharmaMar, Pfizer, Roche, Takeda; travel/accommodations: Boehringer Ingelheim, BMS, Roche; Research support: AZ, Roche. AA: consulting or advisory role: AZ, Astella, BMS, MSD, Novartis, Roche; honoraria: AZ, Eli Lilly, Novartis. MM: consulting or advisory role: AZ (institution), BMS, Janssen, MSD, Roche. SP (all fees to institution): consulting or advisory role: AbbVie, Amgen, AZ, Bayer, Beigene, Biocartis, Boehringer Ingelheim, BMS, Clovis, Daiichi Sankyo, Debiopharm, ecancer, Eli Lilly, Elsevier, Fishawack, Foundation Medicine, Illumina, Imedex, IQVIA, Incyte, Janssen, Medscape, MSD, Merck Serono, Merrimack, Novartis, OncologyEducation, PharmaMar, Phosplatin Therapeutics, PER, Pfizer, PRIME, Regeneron, RMEI, Roche/Genentech, RTP, Sanofi, Seattle Genetics, Takeda, Talk: AZ, Boehringer Ingelheim, BMS, ecancer, Eli Lilly, Illumina, Imedex, Medscape, MSD, Novartis, PER, Pfizer, Prime, Roche/Genentech, RTP, Sanofi, Takeda; Grants/research support: Amgen, AZ, Biodesix, Boehringer Ingelheim, BMS, Clovis, GSK, Illumina, Lilly, MSD, Merck Serono, Mirati, Novartis, and Pfizer, Phosplatin Therapeutics, Roche/Genentech. MP: honoraria: Bayer, Janssen, Nestle; consulting or advisory role: AbbVie, Amgen, AZ, Bayer, Boehringer Ingelheim, BMS, Eisei, Janssen, MSD, Merck Serono, Nestle, Novartis, Pfizer, Roche, Sanofi, Takeda; travel/accommodations: AZ, Boehringer Ingelheim, BMS, Roche, Takeda, Vifor. MF: grants (all to institution): AZ, BMS; consulting or advisory role: AZ, Boehringer Ingelheim, BMS, MSD, Pfizer, Roche, Takeda; travel/accommodations: Merck Serono. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Brierley J., Gospodarowicz M.K., Wittekind C. Eighth ed. John Wiley & Sons, Inc.; Chichester, West Sussex, UK; Hoboken, NJ: 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 2.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 3.Faivre-Finn C., Vicente D., Kurata T., et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC trial. J Thorac Oncol. 2021;16:860–867. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Betticher D.C., Hsu Schmitz S.F., Totsch M., et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21(9):1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R., Mayer M., Kann R., et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol. 2009;10(8):785–793. doi: 10.1016/S1470-2045(09)70172-X. [DOI] [PubMed] [Google Scholar]
- 6.Pless M., Stupp R., Ris H.B., et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 7.Curioni-Fontecedro A., Perentes J.Y., Gelpke H., et al. Preoperative chemotherapy and radiotherapy concomitant to cetuximab in resectable stage IIIB NSCLC: a multicentre phase 2 trial (SAKK 16/08) Br J Cancer. 2019;120(10):968–974. doi: 10.1038/s41416-019-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothschild S.I., Zippelius A., Eboulet E.I., et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol. 2021:JCO2100276. doi: 10.1200/JCO.21.00276. [DOI] [PubMed] [Google Scholar]
- 9.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu C.A., Gainor J.F., Awad M.M., et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–795. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 11.Rothschild S., Zippelius A., Eboulet E.I., et al. SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)—a multicenter single-arm phase II trial. J Clin Oncol. 2020;38(suppl 15):9016. doi: 10.1200/JCO.21.00276. [DOI] [PubMed] [Google Scholar]
- 12.Provencio M., Nadal E., Insa A., et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21 doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 13.Spicer J., Wang C., Tanaka F., et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39(suppl 15):8503. [Google Scholar]
- 14.Wakelee H.A., Altorki N.K., Zhou C., et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39(suppl 15):8500. [Google Scholar]
- 15.Fruh M., Betticher D.C., Stupp R., et al. Multimodal treatment in operable stage III NSCLC: a pooled analysis on long-term results of three SAKK trials (SAKK 16/96, 16/00, and 16/01) J Thorac Oncol. 2019;14(1):115–123. doi: 10.1016/j.jtho.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt W.E., Gauler T.C., Lepechoux C., et al. 10-year long-term survival (LTS) of induction chemotherapy with three cycles cisplatin/paclitaxel followed by concurrent chemoradiation cisplatin/etoposide/45 Gy (1.5 Gy bid) plus surgery in locally advanced non-small-cell lung cancer (NSCLC)-a multicenter phase-II trial (CISTAXOL) Lung Cancer. 2013;82(1):83–89. doi: 10.1016/j.lungcan.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am. 1995;5(2):189–203. [PubMed] [Google Scholar]
- 18.Katakami N., Tada H., Mitsudomi T., et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903) Cancer. 2012;118(24):6126–6135. doi: 10.1002/cncr.26689. [DOI] [PubMed] [Google Scholar]
- 19.Pottgen C., Stuschke M., Graupner B., et al. Prognostic model for long-term survival of locally advanced non-small-cell lung cancer patients after neoadjuvant radiochemotherapy and resection integrating clinical and histopathologic factors. BMC Cancer. 2015;15:363. doi: 10.1186/s12885-015-1389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberhardt W.E., Pottgen C., Gauler T.C., et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE) J Clin Oncol. 2015;33(35):4194–4201. doi: 10.1200/JCO.2015.62.6812. [DOI] [PubMed] [Google Scholar]
- 21.Bradley J.D., Hu C., Komaki R.R., et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell R., Gomez-Codina J., Camps C., et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330(3):153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone D.W., Byhardt R.W., Ettinger D., Scott C.B. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54(2):365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 24.Stephens R.J., Girling D.J., Hopwood P., Thatcher N. Medical Research Council Lung Cancer Working Party. A randomised controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer. 2005;49(3):395–400. doi: 10.1016/j.lungcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Albain K.S., Swann R.S., Rusch V.W., et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Meerbeeck J.P., Kramer G.W.P.M., Van Schil P.E.Y., et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M., Rube C., Hoffknecht P., et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9(7):636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.