Abstract
Background & Aims
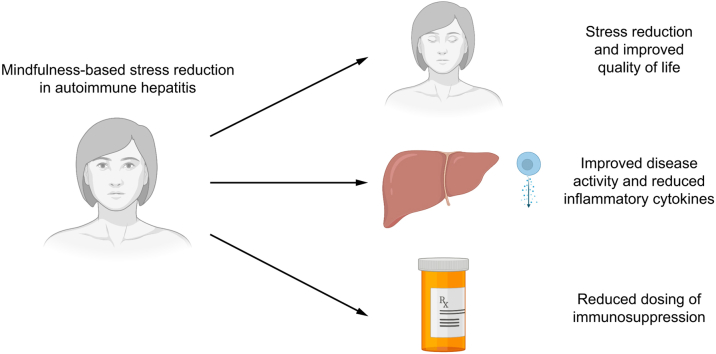
Psychological and life stressors may impact autoimmune hepatitis (AIH) disease activity and increase relapse risk. Mindfulness-based stress reduction (MBSR) is a validated course that reduces stress reactivity, and improves stress and emotion regulation. This single-arm exploratory pilot study of adult patients with AIH aimed to define the impact of an 8-week MBSR program on quality of life, disease activity, and cytokine mediators.
Methods
The perceived stress survey-10 (PSS) and the brief self-control scale (BSCS) measured subjective distress and self-control. Serum alanine aminotransferase (ALT) and cytokine levels were measured, and immunosuppressant doses recorded.
Results
Seventeen patients completed the MBSR program. Post-MBSR, 71% (n = 12) showed PSS score improvement at 8 weeks vs. baseline (median 15 vs. 21, p = 0.02). At 12 months, PSS improvement persisted vs. baseline (median 15 vs. 21, p = 0.02). Post-MBSR, 71% (n = 12) showed BSCS score improvement at 8 weeks vs. baseline (median 4.1 vs. 3.8, p = 0.03). At 12 months, the median BSCS score remained significant (3.9 vs. 3.8, p = 0.03). After the 8-week MBSR, the 35% of patients with ALT >34 U/L had a median ALT reduction (44.5 vs. 71.5 U/L, p = 0.06), whereas the 71% of patients on prednisone had significant dose reductions (5.75 vs. 10 mg, p = 0.02) which persisted at 12 months vs. baseline (3.75 vs. 10 mg, p = 0.02) without a compensatory increase in steroid-sparing dosing. Significant improvement was noted in peripheral blood cytokine levels (IL-6, IL-8, IL-10, IL-17, IL-23, and sCD74/MIF ratio) from baseline to 8 weeks.
Conclusions
MBSR significantly improved perceived stress and self-control scores while decreasing ALT levels, steroid requirements, and inflammatory cytokine levels in this pilot study in adult AIH. Stress modification may impact quality of life and disease activity, and should be further evaluated as an intervention in AIH.
Clinical Trials registration
This study is registered at ClinicalTrials.gov (NCT02950077).
Lay summary
Autoimmune hepatitis can reduce quality of life and mental health, while stress may impact autoimmune hepatitis itself. We piloted mindfulness-based stress reduction as a strategy to reduce stress in adult patients with autoimmune hepatitis and found that the intervention reduced perceived stress and may have also impacted the disease by improving inflammation and medication needs. Stress reduction should be further studied to improve quality of life and possibly to impact disease activity in autoimmune hepatitis.
Keywords: Autoimmune hepatitis, Psychological stress, Stress reduction, MBSR, Cytokines
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; BSCS, brief self-control scale; MBSR, mindfulness-based stress reduction; MIF, macrophage migratory inhibitor factor; PSS, perceived stress scale; REDCap, research electronic data capture; SRRS, social readjustment rating scale; ULN, upper limits of normal
Graphical abstract
Highlights
-
•
Autoimmune hepatitis (AIH) reduces quality of life and mental health; psychological stress may also impact AIH activity.
-
•
Mindfulness-based stress reduction (MBSR) is a strategy to reduce stress and improve quality of life.
-
•
A pilot study of MBSR in adult patients with AIH evaluated its effect on stress, disease activity, and cytokines.
-
•
MBSR reduced perceived stress, which persisted after the study; ALT, medication doses, and cytokines also improved.
-
•
Stress reduction should be further studied in AIH to improve quality of life and possibly to impact disease activity.
Introduction
Autoimmune hepatitis (AIH) is a chronic liver disease that is more common in women with heterogeneous presentation and outcomes, and an unknown trigger.1 Survival rates improve with immunosuppression, yet very high relapse rates result in the need for long-term therapy which imposes a significant, long-term burden on health and lifestyle. As with many chronic conditions, the health and quality of life burdens carried by patients with AIH are underestimated. As previously reported by Schramm et al.,2 patients with AIH were 5 times as likely to develop a major depressive disorder compared with the general population and also had significant increase in anxiety.
Increasing evidence indicates that psychological and life stressors can, in turn, exacerbate AIH disease activity. Previously, our group performed a retrospective study of AIH patients followed for at least 7 years. In this cohort, with a 16-year mean follow-up, 36 of 42 patients experienced a relapse, with a mean of 1.78 relapses/patient (range 0–8) following steroid withdrawal.3 Surprisingly, the study revealed situational stress in 30 of 36 patients (83%) who relapsed. Specifically, relapses occurred surrounding stressful life situations including work issues and deaths in the family as 2 examples. To further analyse this finding, Srivastava et al.4 performed a case-control study to quantitatively evaluate correlations of psychological stress and AIH activity. Higher serum alanine aminotransferase (ALT) levels, higher prednisone use, and also increased psychological stress were all associated with exacerbations in disease activity.4 Patients who relapsed had a higher mean social readjustment rating scale (SRRS) score, a validated patient questionnaire of life stressors,5 compared with patients who did not relapse (239.31 vs. 152.55; p = 0.048). This was the first study to explore the potential relationship of psychological stress to disease relapse in AIH. A follow-up study evaluated 53 patients with AIH by correlating self-reported stressors identified by the SRRS survey with serum ALT. This study found that ALT strongly correlated with SRRS scores in patients who reported any level of stress within the past 4 weeks (r = 0.48) and 6 months (r = 0.39), p = 0.009 and p = 0.015, respectively.6
The critical mediators that link psychological stress and liver inflammation in patients with AIH are currently unknown. However, there is growing interest in studying the impact of neuroendocrine-derived cytokines on peripheral tissue inflammation in autoimmune diseases. Specifically, studies have reported a potential link between stress and serum levels of IL-6, cortisol, and prolactin.7,8 In addition, our group has recently provided evidence that the neuroendocrine mediator macrophage migration inhibitory inhibitor (MIF) is a pituitary-derived cytokine that potentiates lethal endotoxaemia and may be entrained by cortisol.[9], [10], [11] MIF exhibits a pronounced circadian rhythm effect relevant to its role as a glucocorticoid counter-regulator.11 Interestingly, MIF is responsive to psychological stress and is also a key immune-based cytokine and biomarker in AIH.12,13 In our previous study where AIH patients self-reported stress, MIF levels positively correlated with ALT levels (r = 0.32, p = 0.034).6 Thus, MIF is proposed as an additional candidate cytokine potentially linking inflammation in AIH and neuroendocrine activity in stress.
Given that AIH patients have a lifelong disease, there is a clear unmet need to identify therapeutic modalities to reduce stress and improve quality of life as well as reduce disease activity. Mindfulness-based stress reduction (MBSR) is an increasingly studied approach for effective long-term psychological stress reduction. This formal, validated psychoeducational program facilitates cognitive, emotional, and behavioural changes known to reduce stress by focusing on a systematic, patient-centred, experiential introduction to mindfulness.14 MBSR has been shown to have effective results in those with high anxiety, pain, and chronic stress.15,16 A standard MBSR course should be taught by an experienced and trained MBSR instructor. The sessions are delivered in a small group setting once weekly for 2–2.5 h over an 8-week period in addition to a 6–7-h retreat. Intriguingly, implementation of mindfulness-based interventions has also been shown to impact various inflammatory cytokines.[17], [18], [19]
To evaluate the possible effect of a stress-reduction program in patients with AIH, we performed a single-arm exploratory pilot study aiming to define the impact of MBSR training on quality of life, disease activity, and cytokine mediators.
Patients and methods
Patients
Adults 18–80 years with type I AIH as defined by the International Autoimmune Hepatitis Group20 who were receiving routine clinical care at the Yale Liver Center were approached during clinic visits for possible enrolment. Patients were excluded if hospitalised in the past 30 days, if new immunosuppression was started <6 weeks before the study, if they had concurrent viral hepatitis and/or alcoholic liver disease, decompensated cirrhosis (defined as ascites, encephalopathy, or variceal haemorrhage), concurrent diagnosis of hepatocellular carcinoma or were post-liver transplantation. Other exclusions included the presence of a psychotic disorder, attitudinal disinterest, and non-proficiency in English. Patients meeting any of the above exclusion criteria on chart review were not approached for screening or were excluded following screening at a clinic.
Cases consisted of adult patients with AIH who were either in biochemical remission or had active disease as a result of relapse or recrudescence. Biochemical remission was defined as the absence of clinical symptoms and normalisation of ALT and IgG, on or off maintenance dose immunosuppression. Relapse was defined once immunosuppression has been tapered off either as: (1) an increase in the ALT ≥2× upper limits of normal (ULN) or (2) an increase in ALT ≥2× the prior level of ALT on routine labs at the start of the clinical study. Recrudescence was defined as relapse while on maintenance or suppression therapy. Changes in clinical status from baseline and immunosuppression doses were evaluated over the course of the study.
Study design
This was designed as a single-arm, open exploratory pilot study. Patient enrolment and participation in the MBSR protocol was completed over a 1-year period. Study visits occurred at enrolment (orientation), and weekly during weeks 1–8 for the MBSR intervention. In addition, patients completed post-MBSR follow-up visits at 8 months (6 months from completion of the 8-week MBSR protocol) and at 1 year.
The study protocol was approved by the Yale University Human Investigation Committee/Institutional Review Board (#200020043). Written informed consent was obtained from all individuals at enrolment and before participation in study-related procedures. The study (ClinicalTrials.gov Identifier: NCT02950077) was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Medication monitoring
Immunosuppression medications were recorded by patients weekly throughout the MBSR course, at follow-up visits and reviewed during the data analysis stage. Routine medical management of AIH by the patient’s hepatology provider continued independently and uninterrupted during and after MBSR. Individual medication adjustment decisions were determined by the provider as part of usual clinical care and were not according to planned interventions.
MBSR intervention
The MBSR intervention was conducted by a certified instructor for 3 consecutive small cohorts of 6–8 patients and consisted of an 8-week standardised course involving didactic teaching, mindfulness practices, and class discussion. The course includes psychoeducation on the psychophysiology of stress, methods of coping, and communication. The structure of the course consisted of a 1-h orientation session, followed by group classes for 8 weeks that met weekly for 2 h. A 6-h silent retreat was also part of the course, occurring between Weeks 6–8. All 3 cohort groups were taught by a single trained and certified MBSR instructor at the Yale Stress Center in New Haven, CT, USA. An outline of the course is as follows. Week 1: Introduction to stress and the practice of mindfulness; body scan; theory and evidence for mind–body medicine. Weeks 2–5: Cultivation of mindfulness including teaching of present-focused awareness, non-judgement, the role of perception in stress, non-reactivity to experience, and teaching of mindfulness practices including seated meditation, gentle hatha yoga, and mindful walking. Weeks 6–7: Increasing awareness of emotional and behavioural reactivity, teaching resilience, transformational coping strategies, learning the fundamentals of interpersonal mindfulness, and learning ways that mindfulness can be integrated into daily life. Week 8: Focus on continued practice, validating skills, and identifying support systems available to help individuals to integrate, learn, and grow.21 Approximately half of each weekly session was devoted to the practice of formal mindfulness practices. The other half of each session involved didactic and group dialogue. There was approximately 1 h of homework each day involving both formal and informal mindfulness practices.
Measurement of psychological stress
Surveys were conducted at the start of the study and at study completion to determine baseline stress, emotion regulation, and self-control measures. The perceived stress scale-10 (PSS)22 and the brief self-control scale (BSCS)23 measured subjective distress and self-control as measures of quality of life (Tables S1 and S2). The PSS survey was designed to evaluate how unpredictable and uncontrollable respondents find their lives. The PSS survey assesses the degree to which individuals appraise situations in their lives in the past month as stressful and is used to assess subjective interpretation of stress. PSS is not a diagnostic instrument, therefore, score results are compared between people in a sample. Higher PSS scores (maximum 40) indicate increased perceived stress. For a comparative measure, in a large population national survey, the mean PSS score in men was reported as 15.52 and as 16.14 in women.24 The PSS has been utilised in clinical research to assess the impact of perceived stress on various medical co-morbidities such as coronary artery disease.25 By contrast, the BSCS measures self-reported self-control and impulsivity. The survey asks 10 questions that individuals are asked to rate on a scale from 1 to 5 based on what was most representative of themselves. Higher BSCS scores (maximum 5) indicate better self-control and lower scores (minimum 1) indicate poor self-control. BSCS scores in adults have been associated with better grades, good judgment, and interpersonal success.23 The BSCS has also been studied to assess the link between self-control and cumulative stress.26
Data collection
Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at Yale University. REDCap is a secure, web-based software platform designed to support data capture for research studies. Additional correlates were retrieved from electronic medical records, including patient demographics (age, sex, ethnicity), clinical history, laboratory data, and disease characteristics (AIH type, length of time since diagnosis, disease stage). Medication doses were correlated with patient responses for all enrolled patients. Study data points were collected at baseline, 8 weeks (post-MBSR), 8 months, and at 1 year.
Plasma samples
To evaluate the biochemical effect of MBSR on AIH disease activity, serum ALT and IgG were measured at baseline, at the conclusion of MBSR (week 8), and at 8- and 12-month follow-up time points. Following blood collection, ALT and IgG levels were submitted and immediately processed at the Yale New Haven Hospital clinical laboratory. Normal values for Yale New Haven Hospital standards were ALT (10–34 U/L) and IgG (700–1,600 mg/dl). Whole blood was collected from each patient in EDTA tubes. Cytokines were measured and analysed at baseline and 8 weeks by sandwich ELISA for MIF and CD74 as previously described,13 and a LEGENDplex multi-analyte flow assay (Biolegend, San Diego, CA, USA) was performed for IL-6, IL-8, IL-10, IL-17, and IL-23 using a Stratedigm-13 Loader flow cytometer (Stratedigm, San Jose, CA, USA). Please see supplementary CTAT table for details on clones and reagents.
Statistical data analysis
Quantitative variables are summarised using mean or median and range and categorical variables are summarised using frequency and percentage. The non-parametric Wilcoxon signed rank test was performed to compare 8-week (post-MBSR) measurements with baseline measurements. The 8-month and 1-year measurements were similarly compared with the baseline. All analyses were conducted using GraphPad Prism version 7.00 for Mac OS X, GraphPad Software (La Jolla, CA, USA). A p-value ≤0.05 was considered statistically significant.
Results
Patient population
Twenty-one patients were enrolled in the study with 17 individuals completing the MBSR programme. Sixteen additional patients were approached for possible enrolment but were not able to participate in the study for varied reasons (10 uninterested, 3 because of driving distance, 4 because of date/time conflict). Of the 21 enrolled patients, 3 patients withdrew before completion of the study as a result of medical illness unrelated to liver disease or job/living relocation. One individual withdrew who attributed the commitment to attend the weekly study sessions as causing increased stress. None of the participants had completed an MBSR course previously or identified any mindfulness practice regularly. Two patients had a history of depression and 1 of these was taking a serotonin norepinephrine reuptake inhibitor at the time of the study (desvenlafaxine). This same patient, and 1 additional patient, had a diagnosis of anxiety and took alprazolam as needed.
The 17 individuals who completed the MBSR program and follow-up visits were included in the analysis. The cohort included 15 women and 2 men with a mean age at enrolment of 53 years (34–74). Patient characteristics are shown in Table 1. At enrolment, 65% (n = 11) were in biochemical remission, defined as disappearance of clinical symptoms and complete normalisation of ALT and IgG, on or off maintenance dose immunosuppression. Thirty-five percent (n = 6) of participants had active disease with ALT that was greater than the ULN. There was no difference between patients diagnosed with AIH within a year and those with longer duration of disease regarding baseline PSS and BSCS scores (18 vs. 21, p = n.s. and 4.9 vs. 3.7, p = n.s., respectively).
Table 1.
Patient demographics.
| Demographics | Cases (n = 17) |
|---|---|
| Sex, n (%) female | 15 (88) |
| Mean age (range) years | 53 (34–74) |
| Race, n (%) white | 14 (83) |
| Mean disease duration (range) years | 7.4 (0.4–38) |
| Cirrhosis, n (%)∗ | 3 (18) |
| Other autoimmune diseases, n (%)† | 8 (47) |
Cirrhosis was defined either on liver biopsy specimen or magnetic resonance enterography imaging.
Other autoimmune diseases that were diagnosed include thyroid disease (n = 7) and Crohn’s disease (n = 1).
MBSR requires participant commitment and a time commitment for most success. In our study, all 17 patients who participated in the study completed the orientation and follow-up study dates at 8 weeks, 8 months (6 months after completion of MBSR) and 1 year. With regards to attendance at the weekly MBSR course, nearly all 17 patients attended all 8 sessions with all completing 6 or more sessions. At the follow-up evaluation at 8 months and 1 year, some patients reported incorporating some aspect of their learned methods from the MBSR into their lifestyle, although this was not further quantified.
Effect of MBSR on quality of life measures and stress reduction
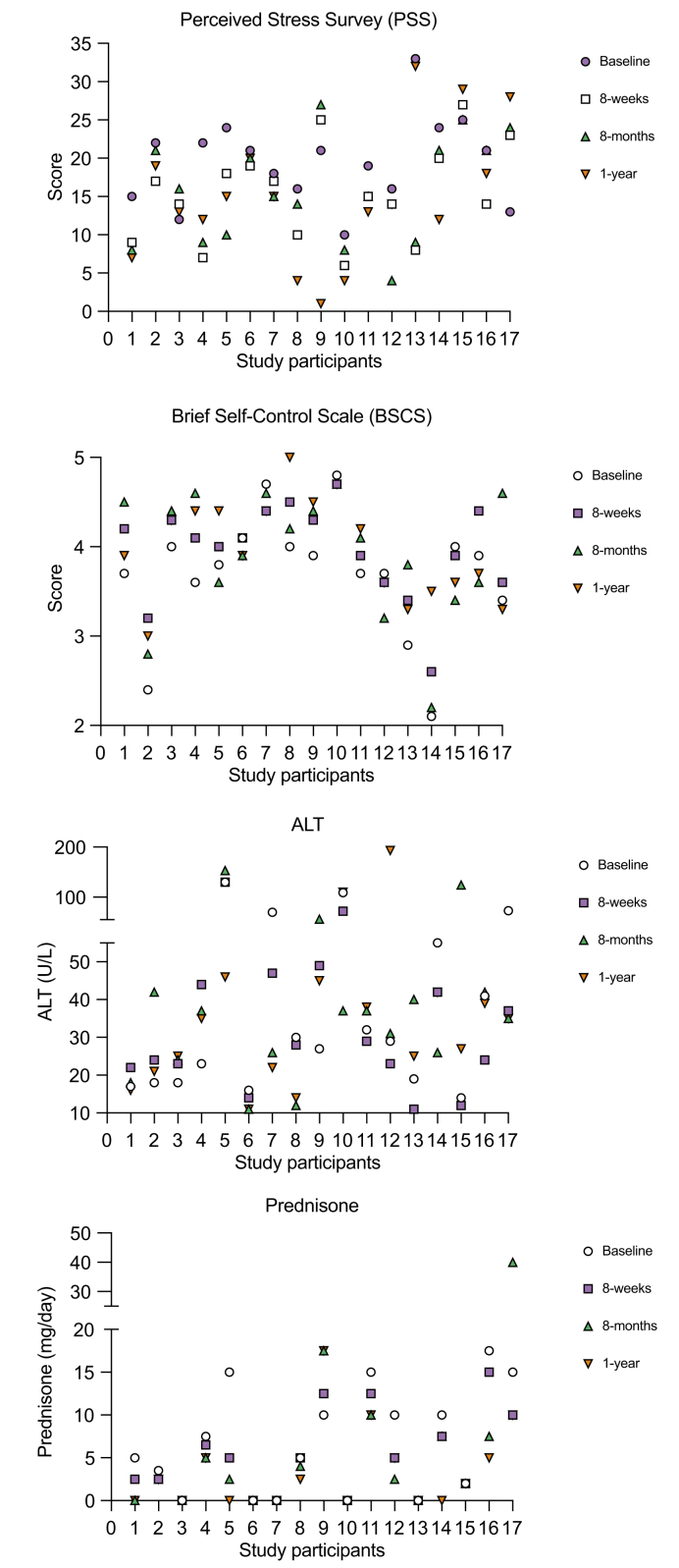
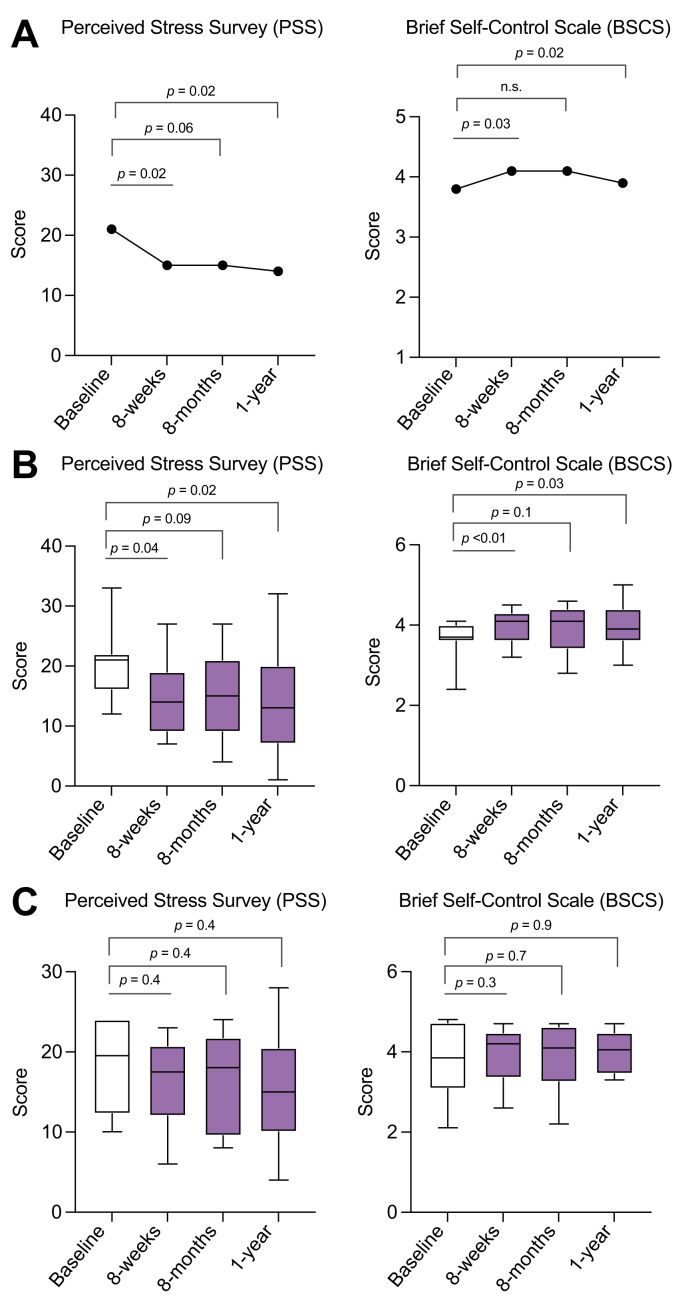
Individual patient scores for PSS and BSCS, ALT levels, and prednisone doses at specified time points throughout the study are shown in Fig. 1. After MBSR, 71% (n = 12) of patients showed improvement in the PSS score with a median reduction at 8 weeks to 15 vs. 21 at baseline for all patients, p = 0.02 (Fig. 2A). Importantly, at 8 months and 12 months, the PSS improvement persisted vs. baseline (median 15 and 14 vs. 21, p = 0.065 and p = 0.02, respectively), suggesting a durable response well beyond the 8-week intervention period. After MBSR, 71% (n = 12) also showed BSCS score improvement at 8 weeks vs. baseline with a median increase to 4.1 vs. 3.8, p = 0.03. At 8 months, the BSCS score was stable and increased from baseline but was not a statistically significant increase (4.1 vs. 3.8, p = n.s.). At 12 months, the median BSCS score had fallen but did remain above baseline and was clinically significant (3.9 vs. 3.8, p = 0.03) (Fig. 2A). Data segregated by status of biochemical remission vs. active disease at the time of enrolment shows that patients in remission at baseline (n = 11) demonstrated significant improvement in PSS and BSCS scores (Fig. 2B) while there was not a significant improvement in the patients with active disease at baseline (n = 6) (Fig. 2C).
Fig. 1.
Individual participant data throughout the study.
Perceived stress survey and brief self-control scale scores, alanine aminotransferase (ALT), and prednisone doses by study participants at baseline, completion of mindfulness-based stress reduction (8 weeks), and follow-up at 8 months and 1 year.
Fig. 2.
Impact of mindfulness-based stress reduction on quality of life measures.
(A) Median perceived stress survey (PSS) and brief self-control scale (BSCS) scores at given time intervals. (B) PSS and BSCS scores for study participants in biochemical remission at baseline. (C) PSS and BSCS scores for study participants with active autoimmune hepatitis at baseline. Non-parametric Wilcoxon signed rank test.
Impact of MBSR on AIH disease activity and immunosuppression requirements
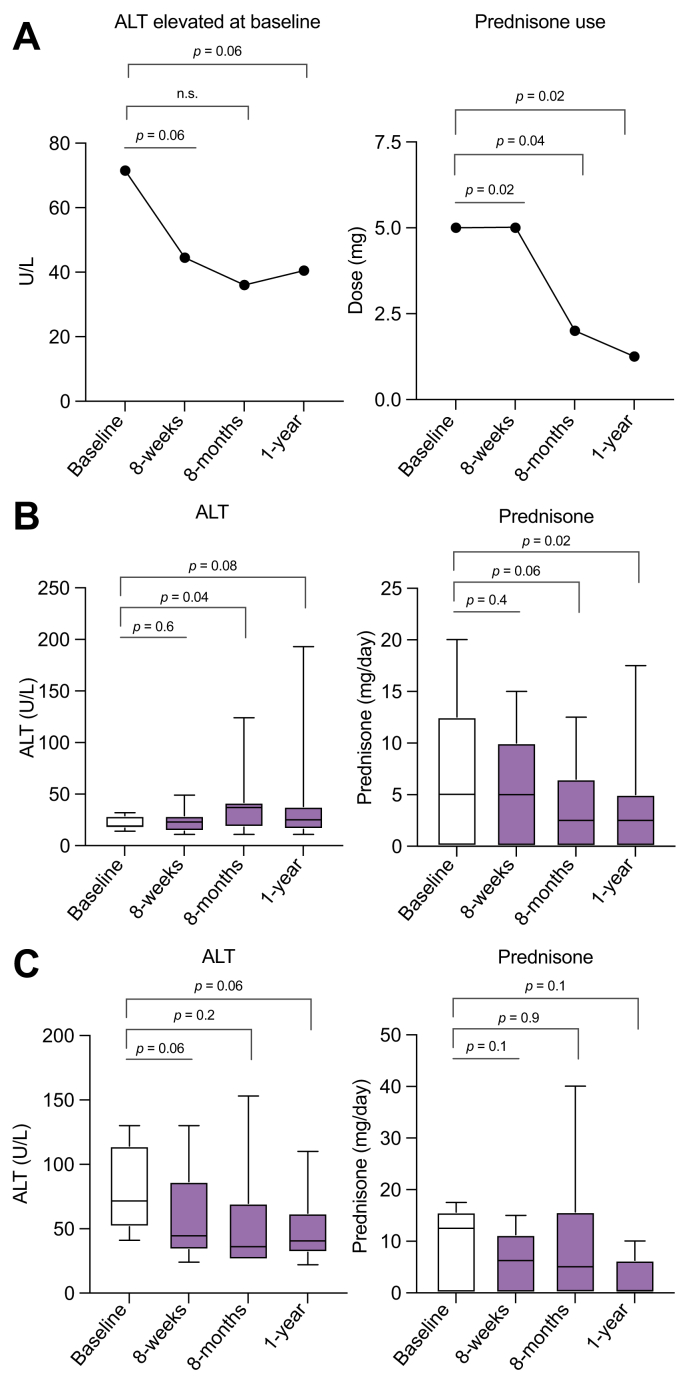
After MBSR, 65% (n = 11) showed a significant reduction in ALT at 8 weeks. Of the patients (n = 6) who had an elevated ALT (>34 U/L) at baseline, ALT reductions were noted post-MBSR at 8-weeks vs. baseline (median 44.5 vs. 71.5 U/L, p = 0.06) (Fig. 3A). Only 12% (n = 2) of the study cohort had a baseline IgG > ULN (>1,600 mg/dl). There was no significant change at 8 weeks from baseline in the IgG level.
Fig. 3.
Impact of mindfulness-based stress reduction on disease activity.
(A) Median alanine aminotransferase (ALT) change at given time points for study participants with elevated ALT at baseline, and median prednisone use change at given time points. (B) ALT and prednisone changes in study participants in biochemical remission at baseline. (C) ALT and prednisone changes for study participants with active autoimmune hepatitis at baseline. Non-parametric Wilcoxon signed rank test.
At the time of enrolment, 71% (n = 12) of the patients were receiving prednisone for treatment of AIH. Prednisone doses ranged from 2 to 17.5 mg daily. After MBSR, 75% (n = 9) of the cohort had a reduction in steroid dose, 16.7% (n = 2) had an unchanged dose and 1 patient had an increase in dose noted. The median dose of prednisone was significantly reduced (5.75 vs. 10 mg, p = 0.02) at 8 weeks vs. baseline (Fig. 3A). Other immunosuppressant medications that patients were taking included budesonide (n = 3), azathioprine (n = 10), mycophenolic acid (n = 2), and tacrolimus (n = 1). One patient was not on any medications at the time of enrolment or post-MBSR intervention. In the data analysis post-MBSR at 8 weeks, there were no significant changes in the median doses of azathioprine or other second-line agents (p = n.s.). For the 6 patients who were not in biochemical remission at the time of enrolment, there was a trend toward improvement in the median ALT at 8 months and 1 year from baseline (36 and 40.5 vs. 71.5 U/L, p = 0.16 and p = 0.06, respectively) (Fig. 3A). Similarly, the 12 patients who were on prednisone at the time of enrolment had a decrease in their prednisone requirements both at 8 months (n = 7) and at 12 months (n = 5). Dose reductions were found to be significant at 8 months and 12 months vs. baseline (4.5 and 3.75 vs. 10 mg, p = 0.04 and p = 0.02, respectively) (Fig. 3A). There were no compensatory increases in the median dosing of non-prednisone immunosuppressant medications at 8 months or 1 year. Data segregated by status of biochemical remission (Fig. 3B) vs. active disease at the time of enrolment (Fig. 3C) shows a similar trend for each subgroup. Patients who were in biochemical remission did not experience a further reduction in ALT or prednisone dosing as a result of MBSR by week 8 of the intervention, although at 8 months and 1 year there were more significant reductions in each variable (Fig. 3B). Individual patient doses of immunosuppressive medications at each timepoint are provided in Table S3.
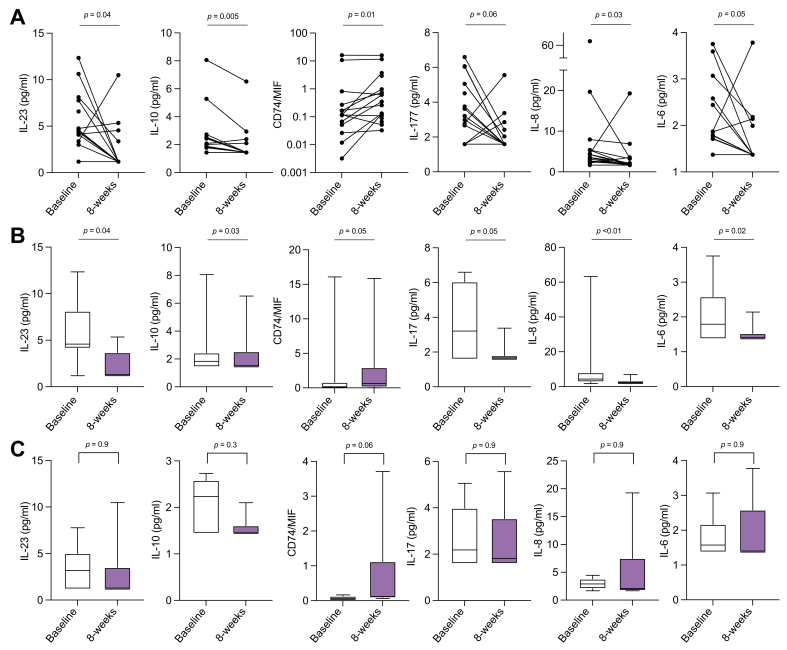
Impact on cytokine mediators
After MBSR, there were significant improvements in the levels of various peripheral blood inflammatory cytokine levels (IL-6, IL-8, IL-10, IL-17, IL-23), at 8 weeks compared with baseline (Table 2 and Fig. 4A). The soluble form of the MIF receptor, sCD74, which neutralises MIF activity, circulates in elevated levels in AIH and the measured sCD74/MIF ratio correlates with ALT in patients with AIH.13 A change in the sCD74/MIF ratio also correlated with MBSR intervention. Peripheral levels of prolactin and cortisol remained unchanged from baseline at 8 weeks. Persistent reductions in cytokine levels were not noted during the follow-up periods of 8 months and 1 year (data not shown), suggesting that the change in cytokines may have been temporally related to the active participation in MBSR. Data segregated by status of biochemical remission (Fig. 4B) vs. active disease at baseline (Fig. 4C) shows that, overall, patients in biochemical remission experienced a more significant change in cytokine levels compared with patients with active disease.
Table 2.
Change in levels of peripheral blood cytokine levels at baseline and at the conclusion of mindfulness-based stress reduction (8 weeks).
| Peripheral cytokines | Baseline, pg/ml Median (range) |
8 weeks, pg/ml Median (range) |
p value |
|---|---|---|---|
| Ratio of CD74/MIF | 0.11 (0–16.07) | 0.35 (0.03-15.86) | 0.01 |
| IL-10 | 1.88 (1.44–8.06) | 1.44 (1.44–6.52) | 0.005 |
| IL-8 | 3.55 (1.68–19.69) | 1.93 (1.68–19.26) | 0.03 |
| IL-23 | 4.37 (1.18–12.34) | 1.18 (1.18–10.48) | 0.04 |
| IL-6 | 1.78 (1.37–3.75) | 1.37 (1.37–3.78) | 0.05 |
| IL-17 | 2.99 (1.59–6.60) | 1.59 (1.59–5.56) | 0.06 |
Fig. 4.
Impact of mindfulness-based stress reduction (MBSR) on peripheral cytokine levels.
(A) Peripheral blood levels of cytokines IL-23, IL-17, IL-10, IL-8, IL-6, CD74 and MIF (represented as the CD74/MIF ratio) measured at baseline and post-MBSR (8 weeks). (B) Cytokine levels for study participants in biochemical remission at baseline. (C) Cytokine levels for study participants with active autoimmune hepatitis at baseline. Non-parametric Wilcoxon signed rank test.
Discussion
To our knowledge, this is the first study to evaluate the use of MBSR as a non-pharmacological adjunct modality in patients with AIH. MBSR was initially designed to complement standard clinical care. Mindfulness has been shown to be impactful in individuals with addiction27 and psychiatric diseases relating to pain, anxiety, and depression as well as for patients with various chronic diseases.[28], [29], [30], [31], [32] Our results in this exploratory pilot study of MBSR in adults with AIH suggest that mindfulness training may improve perceived stress and self-control scores while decreasing ALT levels and also glucocorticoid requirements. Measures that focused on subjective stress and patient self-control significantly improved suggesting that implementation of such interventions as an additive intervention in the management of AIH could improve patient health quality of life. The durable effect seen in our patients at follow-up is also noteworthy as there is the added prospect of long-term efficacy by applying MBSR techniques in a patient’s life to impact long-term outcomes.
The ALT reduction in 65% of the cohort following MBSR is significant in the context of AIH. Furthermore, the continued downtrend of ALT during follow-up suggests that application of stress reduction measures can potentially lead to lasting improvement of clinical disease markers activity, in addition to psychological benefits. Furthermore, our data showed a reduction in median prednisone doses in post-MBSR without a compensatory increase in the doses of other immunosuppressant medications. Therefore, the overall improvement in ALT without increase in immunosuppression suggests the possibility that the changes in disease activity in these adults with AIH could be related to overall stress reduction through MBSR. The possibility of meaningfully reducing immunosuppression requirements in this patient population group is especially appealing given their known associated side effects and need for life-long therapy in most cases. Therefore, the application of mindfulness to reduce psychological stress in AIH should be further studied to determine its effect on disease activity.
Given the need for a better understanding and development of predictive immune biomarkers in AIH patients, investigating the role of serum cytokines in relationship to MBSR and disease activity is also meaningful. In our study, a significant decrease in numerous peripheral cytokine levels (IL-6, IL-8, IL-10, IL-17, and IL-23) was detected at the conclusion of the 8-week MBSR course compared with baseline. These reductions are potentially interesting given the association of IL-10, and IL-6 and IL-23/IL-17, with disease pathophysiology in AIH through promotion of pathogenic B-cell and effector T-cell responses, respectively.1,33,34 A better understanding of the relationship between these peripheral cytokine levels and AIH disease activity in the context of stress reduction through MBSR remains to be determined through future studies.
Another cytokine of interest, MIF, is increasingly regarded as a key pathogenic mediator in various inflammatory and autoimmune diseases.35 Studies also show that MIF levels become significantly elevated in the setting of psychological stressors.8,9 Our previous work describing MIF, and its neutralising soluble receptor CD74, shows that MIF levels are elevated in AIH and that the ratio of cytokine to neutralising receptor (MIF/sCD74) in the circulation correlates with serum ALT in AIH patients with active disease.13 The potential role of this and similar cytokines as links between psychological stress and hepatic inflammation in the context of AIH warrants further investigation.
This exploratory, single-arm pilot study aimed to evaluate the addition of an MBSR intervention to the ongoing management of patients receiving clinical care for AIH. As such, there are several inherent limitations to this study including a small sample-size, non-randomisation, lack of a control non-mindfulness condition and confounding factors. Patients who agreed to enrol in the MBSR intervention could potentially be more open to stress reduction than patients who declined to participate. Patients were not excluded based on previous mindfulness practice, although none of the participants had prior experience with MBSR or similar techniques. Patients were not excluded based on their baseline level of perceived stress or based on the presence of a psychiatric disorder. However, in this study only 2 patients had a diagnosis of depression, only 1 of those patients was taking an antidepressant, and that same patient along with only 1 additional patient had intermittent anxiety. Therefore, we did not find that this pilot study included a significant number of patients with depression or anxiety beyond the general population. To gain a better understanding of the role of MBSR in AIH, and the potential impact of mindfulness on disease activity and cytokine mediators of inflammation, it is clear that larger, randomised studies will be needed and this should be performed utilising a controlled immunosuppression adjustment protocol. Larger studies must also include analysis for potential relationships between medication adherence, disease activity, and stress in the context of a MBSR intervention. In addition, clinical characteristics regarding baseline levels of disease activity were heterogeneous including patients in biochemical remission and those with active disease, which limits the interpretation of the study outside of this feasibility pilot context. However, analysis of stress scores, ALT, immunosuppression doses, and cytokines in the subgroup with biochemical remission revealed an improvement equal to and often greater than that in the smaller subset with active disease, suggesting that the MBSR approach could be useful in the long-term management of stress for AIH patients in remission. Finally, this initial pilot was limited to adults with AIH, however there is a great need to explore for non-pharmacologic, stress-reducing approaches in children and adolescents with AIH. The testing and potential incorporation of stress reduction for this important demographic may be beneficial and thus should be considered.
In summary, this single-arm exploratory pilot study showed that an 8-week stress intervention in the form of a standardised MBSR course was well tolerated and feasible for adult patients with AIH. Participant responses demonstrated lasting beneficial effects on their quality of life as well as suggested improvement in disease activity, medication requirements, and inflammatory cytokines. It is therefore reasonable to further study non-pharmacological stress reduction as a new tool for the management of patients with AIH.
Financial support
No funding was received. No honorarium grant or other form of payment was given to anyone to produce the manuscript.
Authors’ contributions
Designed the study: LSA, RS, JLB, DNA. Recruited patients for the study and obtained informed consent: LSA, LC, MGS, JLB, DNA, LC. Administered the MBSR intervention: AD. Performed laboratory cytokine assays: SJR. Performed data analysis: LSA, JLB, DNA. Performed statistical analysis: YD, MC. Wrote the manuscript: LSA. Critically reviewed the manuscript: RB, RS, JLB, DNA.
Data availability statement
Because of the sensitive nature of this study measuring stress among a small number of individual patients with autoimmune hepatitis treated at a single centre, and the individual patient-level clinical information already contained in this pilot study, raw individual-level data should remain confidential.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the patients as well as the study-site personnel. Daniel Grossman and Chloe Larkin of the Yale Stress Center in New Haven, CT provided assistance with study visits and data entry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100450.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Mack C.L., Adams D., Assis D.N., Kerkar N., Manns M.P., Mayo M.J., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 2.Schramm C., Wahl I., Weiler-Normann C., Voigt K., Wiegard C., Glaubke C., et al. Health-related quality of life, depression, and anxiety in patients with autoimmune hepatitis. J Hepatol. 2014;60:618–624. doi: 10.1016/j.jhep.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Seela S., Sheela H., Boyer J.L. Autoimmune hepatitis type 1: safety and efficacy of prolonged medical therapy. Liver Int. 2005;25:734–739. doi: 10.1111/j.1478-3231.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava S., Boyer J.L. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver Int. 2010;30:1439–1447. doi: 10.1111/j.1478-3231.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes T.H., Rahe R.H. The social readjustment rating scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 6.Assis D.N., Bucala R., Boyer J.L. Macrophage migration inhibitor factor (MIF) may mediate the deleterious effect of psychological stress in autoimmune hepatitis. Hepatology. 2015;62(S1):A298. [Google Scholar]
- 7.Elenkov I.J. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13:177–190. doi: 10.2174/1871528113666140522104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang I., Bucala R. The immunobiology of MIF: function, genetics and prospects for precision medicine. Nat Rev Rheumatol. 2019;15:427–437. doi: 10.1038/s41584-019-0238-2. [DOI] [PubMed] [Google Scholar]
- 10.Bernhagen J., Calandra T., Mitchell R.A., Martin S.B., Tracey K.J., Voelter W., et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 11.Petrovsky N., Socha L., Silva D., Grossman A.B., Metz C., Bucala R. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol. 2003;81:137–143. doi: 10.1046/j.0818-9641.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 12.Katsuura S., Kamezaki Y., Tominaga K., Masuda K., Nishida K., Yamamoto Y., et al. High-throughput screening of brief naturalistic stress-responsive cytokines in university students taking examinations. Int J Psychophysiol. 2010;77:135–140. doi: 10.1016/j.ijpsycho.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Assis D.N., Leng L., Du X., Zhang C.K., Grieb G., Merk M., et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2014;59:580–591. doi: 10.1002/hep.26664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psych Sci Pract. 2003;10:144–156. [Google Scholar]
- 15.Chiesa A., Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 16.Holzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. How Does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 17.Memon A.A., Sundquist K., Ahmad A., Wang X., Hedelius A., Sundquist J. Role of IL-8, CRP and epidermal growth factor in depression and anxiety patients treated with mindfulness-based therapy or cognitive behavioral therapy in primary health care. Psychiatry Res. 2017;254:311–316. doi: 10.1016/j.psychres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Walsh E., Eisenlohr-Moul T., Baer R. Brief mindfulness training reduces salivary IL-6 and TNF-alpha in young women with depressive symptomatology. J Consult Clin Psychol. 2016;84:887–897. doi: 10.1037/ccp0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Sundquist K., Palmer K., Hedelius A., Memon A.A., Sundquist J. Macrophage migration inhibitory factor and microRNA-451a in response to mindfulness-based therapy or treatment as usual in patients with depression, anxiety, or stress and adjustment disorders. Int J Neuropsychopharmacol. 2018;21:513–521. doi: 10.1093/ijnp/pyy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez F., Berg P.A., Bianchi F.B., Bianchi L., Burroughs A.K., Cancado E.L., et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 21.Center for Mindfulness in Medicine, HealthCare and Society. UMass Medical School; 2017. https://www.ummhealth.org/center-mindfulness MBSR Course Outline. [Google Scholar]
- 22.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 23.Tangney J.P., Baumeister R.F., Boone A.L. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S., Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006 and 2009. J Appl Soc Psychol. 2012;42:1320–1334. [Google Scholar]
- 25.Richardson S., Shaffer J.A., Falzon L., Krupka D., Davidson K.W., Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol. 2012;110:1711–1716. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton K.R., Sinha R., Potenza M.N. Self-reported impulsivity, but not behavioral approach or inhibition, mediates the relationship between stress and self-control. Addict Behav. 2014;39:1557–1564. doi: 10.1016/j.addbeh.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elwafi H.M., Witkiewitz K., Mallik S., Thornhill TAt, Brewer J.A. Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use. Drug Alcohol Depend. 2013;130:222–229. doi: 10.1016/j.drugalcdep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speca M., Carlson L.E., Goodey E., Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62:613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Gross C.R., Kreitzer M.J., Russas V., Treesak C., Frazier P.A., Hertz M.I. Mindfulness meditation to reduce symptoms after organ transplant: a pilot study. Altern Ther Health Med. 2004;10:58–66. [PubMed] [Google Scholar]
- 30.Grossman P., Tiefenthaler-Gilmer U., Raysz A., Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother Psychosom. 2007;76:226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 31.Rosenzweig S., Greeson J.M., Reibel D.K., Green J.S., Jasser S.A., Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Ali A., Weiss T.R., Dutton A., McKee D., Jones K.D., Kashikar-Zuck S., et al. Mindfulness-based stress reduction for adolescents with functional somatic syndromes: a pilot cohort study. J Pediatr. 2017;183:184–190. doi: 10.1016/j.jpeds.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floreani A., Restrepo-Jiménez P., Secchi M.F., De Martin S., Leung P.S.C., Krawitt E., et al. Etiopathogenesis of autoimmune hepatitis. J Autoimmun. 2018;95:133–143. doi: 10.1016/j.jaut.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Assis D.N. Immunopathogenesis of autoimmune hepatitis. Clin Liver Dis. 2020;15:129–132. doi: 10.1002/cld.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calandra T., Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the sensitive nature of this study measuring stress among a small number of individual patients with autoimmune hepatitis treated at a single centre, and the individual patient-level clinical information already contained in this pilot study, raw individual-level data should remain confidential.