Highlights
-
•
A phase II trial of AKT inhibitor MK-2206 in uterine serous carcinoma was completed.
-
•
A clinical benefit rate of 14.3% was observed. Two of fourteen patients had progression-free survival of 6 months or longer.
-
•
MK-2206 has limited activity in uterine serous carcinoma; further research may identify features associated with response.
Keywords: Uterine serous carcinoma, MK-2206, AKT inhibitor, PI3K/AKT pathway
Abstract
Uterine serous carcinoma (USC) is an uncommon subtype of endometrial cancer with a poor prognosis. USCs have genomic alterations in the PI3K pathway. A prior phase II study of AKT inhibitor MK-2206 (an allosteric AKT inhibitor, primarily affecting AKT1 and AKT2) in endometrial cancers resulted in progression-free survival (PFS) of ≥6 months in five out of seven patients with USC. To further assess the activity of MK-2206 in USC, we designed a phase II, single-stage assessment of MK-2206 in patients with advanced or recurrent high-grade serous endometrial cancer, who had received up to two lines of prior therapy. MK-2206 (135 mg) was administered orally once per week, in continuous 28-day cycles. Fourteen patients received treatment. The most common treatment-related adverse events were diarrhea (36%), acneiform rash (36%), nausea (29%), fatigue (29%), and hyperglycemia (21%); most events were grade 1–2. One confirmed partial response was observed in a patient who was also alive and progression-free at 6 months. One additional patient was alive and progression-free at 6 months. The clinical benefit rate was 14.3% (95% CI: 1.8 to 42.8). Five patients had stable disease (35.7%) and seven had progressive disease (50%); one was unevaluable. Median PFS was 2 months (95% CI: 1.6 to 4.4) and median overall survival was 6.4 months (95% CI: 5.1 to not reached). In summary, MK-2206 had limited activity in USC, although a few patients achieved sustained progression-free intervals in this study and in the previously reported phase II trial of MK-2206. Further investigations are needed to identify features associated with response.
1. Introduction
Uterine cancer is the most common gynecologic malignancy and the fourth most common cancer diagnosed in women in the United States (Lu and Broaddus, 2020). The incidence of uterine cancers has increased over the past two decades (Lu and Broaddus, 2020). Uterine cancers are comprised of several histologic subtypes. Representing 80–90% of endometrial cancers, endometroid endometrial cancer is the most common type (Lu and Broaddus, 2020). Non-endometrioid endometrial cancers, such as serous carcinoma, clear cell carcinoma, and carcinosarcoma, are rapidly increasing and have a higher incidence relative to endometrioid cancers in Black women (Lu and Broaddus, 2020, Clarke et al., 2019). These rare subtypes of endometrial cancer have worse outcomes than endometrioid carcinomas and cause disproportionately more deaths (Lu and Broaddus, 2020).
Uterine serous carcinoma (USC) is the most common of the non-endometrioid endometrial cancers and is associated with a poor prognosis (Lee et al., 2021, Bogani et al., 2021). USCs have a propensity to spread outside the uterus and are associated with higher rates of lymphovascular invasion and lymph node involvement compared to endometrioid endometrial cancers, with more than half of patients with USC initially presenting with advanced stage disease (stage III-IV) (Lee et al., 2021). Patients with USC also have worse outcomes by stage than patients with endometrioid endometrial cancer, including an increased frequency of relapse and a shorter survival (Lee et al., 2021, Bogani et al., 2021). New therapeutic targets are needed to improve treatment of USC.
The Cancer Genome Atlas study of endometrial cancer identified four molecular subtypes: copy-number low (mostly microsatellite stable endometrioid cancers), copy-number high, hypermutated (microsatellite unstable cancers) and ultramutated (cancers with POLE mutations) (Cancer Genome Atlas Research Network et al., 2013). Uterine serous carcinomas, along with a subset of endometrioid endometrial cancers, were classified within the copy-number high subset. Genomic studies focusing on USC showed a high frequency of copy-number aberrations and TP53 mutations (Cancer Genome Atlas Research Network et al., 2013, Kuhn et al., 2012, Zhao et al., 2013). Amplifications of ERBB2 and overexpression of its encoded protein, HER2, are present in a large proportion of USC. HER2 as a target has been exploited therapeutically, with a phase II clinical trial showing benefit to adding trastuzumab (anti-HER2) to chemotherapy in advanced and recurrent USC (Fader et al., 2020). The PI3K pathway is frequently altered in patients with USC, including mutations or amplifications of PIK3CA (30–60%), and mutations in PTEN and PIK3R1 (Cancer Genome Atlas Research Network et al., 2013, Kuhn et al., 2012, Zhao et al., 2013).
The PI3K pathway may represent a therapeutic target in USC and other endometrial cancers. Multiple agents targeting the PI3K pathway have been evaluated in clinical trials in endometrial cancer (Myers, 2013). However, to date, PI3K pathway inhibitors have not been validated in clinical trials for use in clinical practice, except for mTOR inhibitors everolimus (with letrozole) and temsirolimus. Evaluation of new PI3K pathway inhibitors is ongoing, including isoform-selective PI3K inhibitors (Vanhaesebroeck et al., 2021) and inhibitors of other nodes in the pathway including AKT (Song et al., 2019, Hua et al., 2021, Westin et al., 2021).
MK-2206 is an AKT inhibitor that was evaluated in a phase II trial in recurrent endometrial cancer (Myers et al., 2020). MK-2206 is an allosteric inhibitor of AKT with activity against all three AKT isoforms but the most pronounced activity against AKT1 and AKT2 (Hirai et al., 2010). The phase II trial was a two-stage, two-arm, PIK3CA-mutation stratified trial of MK-2206 as a single agent in women with recurrent endometrial cancer (all histologies except carcinosarcoma) previously treated with up to two lines of chemotherapy (Myers et al., 2020). A dose-reduction from 200 mg to 135 mg weekly was required after an initial cohort of patients exhibited toxicity. Subsequently, 36 patients were treated (9 PIK3CA mutant, 27 PIK3CA wild-type (WT)). Common toxicities included rash, fatigue, nausea, and hyperglycemia. One patient with partial response (PR) and a 6-month progression free survival (PFS), and two additional patients with 6-month PFS were observed in the PIK3CA mutant group; one PR and four 6-month PFS were seen in the WT group. Responses were not clearly associated with genomic alterations in the PI3K pathway. Although activity in the overall population was limited, five of the seven patients with USC achieved 6-month PFS.
Based on the promising signal in USC in the phase II trial of AKT inhibitor MK-2206, as well as the high frequency of PI3K pathway alterations in USC, we undertook a focused assessment of the efficacy of MK-2206 in a cohort of USC patients.
2. Patients and methods
The current study was a phase II, single-stage assessment of MK-2206 in patients with advanced or recurrent uterine serous carcinoma. The study was approved by the Dana-Farber Cancer Institute Institutional Review Board and patients provided written informed consent for participation.
2.1. Patient population and key eligibility criteria
Patients were required to have histologically confirmed recurrent or persistent high-grade endometrial carcinoma with a serous component (any proportion of serous histology was allowed), refractory to curative therapy or established treatments. Patients must have had at least one prior chemotherapeutic regimen for treatment of endometrial carcinoma, and patients were allowed to have received up to one additional prior treatment regimen (e.g., chemotherapy or anti-angiogenic agents; hormonal therapy was not considered an additional line of treatment). Prior use of any drugs targeting the PI3K pathway was not allowed. Prior anti-cancer treatments must have been completed at least three weeks prior to starting MK-2206, and toxicities of prior therapy must have resolved to grade 1 or lower. Measurable disease, as defined by RECIST 1.1, was required. Patients were not stratified by PIK3CA mutation status and mutation status is not available for this cohort.
Patients were required to be 18 years of age or older and have an ECOG performance status of < 2, life expectancy of greater than 6 months, and the ability to tolerate oral medications. Laboratory evidence of normal organ and bone marrow function was required, including absolute neutrophil count > 1,500/mcL, hemoglobin > 9.0 g/dL, platelets > 100,000/mcL, adequate liver and renal function, and no evidence of hyperglycemia (HgbA1c < 7.5% and fasting blood glucose less than 130 mg/dL). Additional exclusion criteria included significant intercurrent illnesses; prolonged QTc interval (baseline QTc > 450 msec (male) or 470 msec (female)), bradycardia, or bundle branch block; diabetes, either requiring insulin or poorly controlled on oral agents; brain metastases; and second malignancies (unless disease-free for at least 5 years with low risk for recurrence, or a diagnosis of breast cancer in situ, cervical cancer in situ, or basal or squamous cell carcinoma of skin).
2.2. Interventions and assessments
MK-2206 (135 mg) was administered orally once per week, in continuous 28-day cycles. Toxicity assessments were performed using the National Cancer Institute Common Terminal Criteria for Adverse Events version 4. Dose reduction guidelines were provided for hyperglycemia, diarrhea, rash, hematologic adverse events (absolute neutrophil count < 1000/mcL, platelets < 75,000/mcL, or hemoglobin < 8 mg/dL), any grade ≥ 3 non-hematologic adverse events, and any grade 2 non-hematologic adverse events related to MK-2206 lasting > 7 days despite maximal support. CT scans were performed every two months to evaluate response. Optional pre-treatment and on-treatment biopsies were included. Treatment was continued until disease progression, unacceptable toxicity, or patient withdrawal. Patients were followed for up to 3 years or until death.
2.3. Study design and statistics
The trial design was a single-stage design for an independent analysis of a cohort of USC patients. The primary endpoint of this study was a composite endpoint of complete or partial response by RECIST or progression-free survival of ≥ 6 months. PFS was defined as the time from registration to disease progression (per RECIST 1.1) or death, whichever occurred first, and summarized using the Kaplan-Meier estimator. Responders were defined as those patients with either complete or partial response or progression-free interval of 6 months or longer. The proportion of responders was estimated and reported with a binomial exact 95% confidence interval. Clinical benefit with MK-2206 was evaluated against a null response rate of 10% as indication of inadequate efficacy. With 14 subjects, there was greater than 80% power to reject a null rate of 10% if the true rate of clinical benefit is 36%, when using a binomial exact test with a one-sided alpha < 0.05. If four or more responses were seen among the 14 subjects, the null hypothesis would be rejected, and this would reflect sufficient efficacy for further investigation of MK-2206.
3. Results
3.1. Patients
Fifteen patients were enrolled to the study, but one patient did not receive the study treatment, yielding 14 patients for this analysis. Baseline clinical characteristics of the enrolled patients are shown in Table 1. The stage at diagnosis ranged from IA to IV, with half of patients having advanced stage (III or IV). Seven patients had received 1 prior line of treatment and the other seven patients had received two lines of treatment.
Table 1.
Patient demographics and characteristics.
| Overall | |
|---|---|
| (N = 14) | |
| Age | |
| Mean (SD) | 64.8 (6.57) |
| Median (Min, Max) | 63.9 (56.9, 84.5) |
| Race | |
| White | 12 (85.7%) |
| Black or African American | 1 (7.1%) |
| Other | 1 (7.1%) |
| Ethnicity | |
| Non-Hispanic | 12 (85.7%) |
| Ethnicity Not Known | 2 (14.3%) |
| Stage at Diagnosis | |
| IA | 2 (14.3%) |
| IB | 4 (28.6%) |
| IIB | 1 (7.1%) |
| IIIA | 1 (7.1%) |
| IIIC | 4 (28.6%) |
| IV | 2 (14.3%) |
| ECOG Performance | |
| 00 - Fully active | 9 (64.3%) |
| 01 - Restricted | 5 (35.7%) |
| Number of prior lines | |
| 1 | 7 (50.0%) |
| 2 | 7 (50.0%) |
3.2. Safety
Treatment-related adverse events are shown in Table 2. The most common treatment-related adverse events of all grades were diarrhea (36%), acneiform rash (36%), nausea (29%), fatigue (29%), and hyperglycemia (21%). Other gastrointestinal toxicities such as anorexia, reflux, vomiting, and weight loss were also observed. Three grade 3 adverse events were reported (one each of diarrhea, rash, and anemia). No grade 4 events were observed.
Table 2.
Treatment-related adverse events.
| Grade 1 |
Grade 2 |
Grade 3 |
All Grade |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse Events | N | % | N | % | N | % | N | % |
| Diarrhea | 3 | 21 | 1 | 7 | 1 | 7 | 5 | 36 |
| Rash, acneiform | 2 | 14 | 2 | 14 | 1 | 7 | 5 | 36 |
| Nausea | 4 | 29 | 0 | 0 | 0 | 0 | 4 | 29 |
| Fatigue | 4 | 29 | 0 | 0 | 0 | 0 | 4 | 29 |
| Hyperglycemia | 3 | 21 | 0 | 0 | 0 | 0 | 3 | 21 |
| Anorexia | 2 | 14 | 0 | 0 | 0 | 0 | 2 | 14 |
| Rash, maculo-papular | 2 | 14 | 0 | 0 | 0 | 0 | 2 | 14 |
| Anemia | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 7 |
| Gastroesophageal reflux disease | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 7 |
| Vomiting | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 7 |
| Weight loss | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 7 |
3.3. Clinical activity
The clinical benefit rate was 14.3% (95% CI: 1.8% to 42.8%). One patient had a confirmed partial response, and two patients were alive and progression-free at 6 months (including the patient with the partial response) among the 14 evaluable patients. Five patients had stable disease (for less than 6 months) (35.7%), and seven had progressive disease (50%); one patient was unevaluable. The study did not achieve the benchmark for further investigation based on the primary composite endpoint.
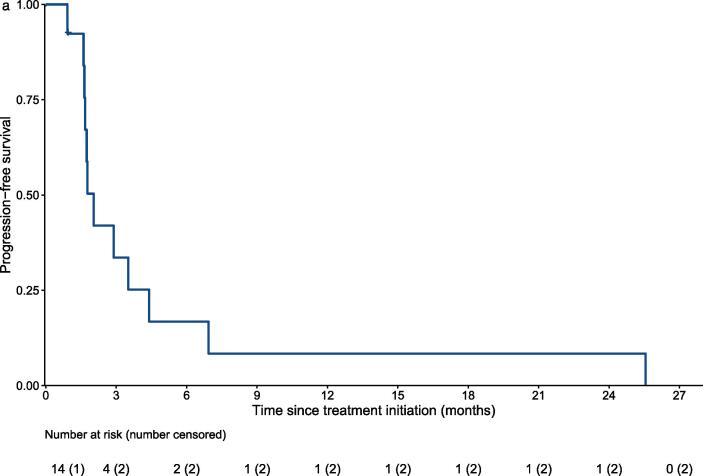
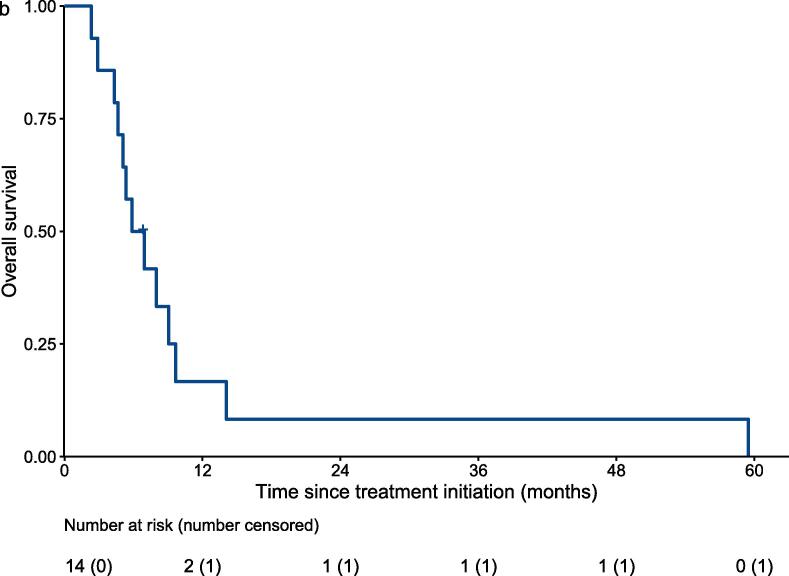
In terms of survival analysis, thirteen patients died and one was lost follow up during the study period. Fig. 1a shows the PFS using RECIST to determine disease progression. Median PFS was 2 months (95% CI: 1.6 to 4.4). Median overall survival (OS) was 6.4 months (95% CI: 5.1 to not reached) (Fig. 1b).
Fig. 1a.
Progression-free survival by Kaplan-Meier analysis. Progression was defined based on imaging using RECIST.
Fig. 1b.
Overall survival by Kaplan-Meier analysis.
4. Discussion
USC is a difficult-to-treat subtype of endometrial cancer with a poor prognosis. The PI3K pathway is an attractive target in USC due to frequent genomic alterations in this pathway, including mutations in PIK3CA and PTEN. A phase II study of MK-2206, an oral allosteric AKT inhibitor, in a cohort of patients with endometrial cancers demonstrated a PFS of 6 months or longer in five of seven patients with USC (Myers et al., 2020). These results motivated the current study, a phase II, single-stage investigation of MK-2206 in patients with USC. Though well-tolerated, MK-2206 showed limited activity in this larger cohort of patients with USC, with only one of fourteen patients having a confirmed partial response (objective response rate 7.1%), and two patients having a PFS of 6 months or longer (including the patient with the partial response) (clinical benefit rate 14.3%).
The clinical and genomic features associated with response or resistance to AKT inhibition in USC remain to be determined. While the prior study was stratified for PIK3CA mutation status, this feature was not clearly associated with responses to MK-2206 (Myers et al., 2020). Within the seven USC patients in that study; six were PIK3CA wild-type and one was PIK3CA mutant. In the current study, patients were not stratified by PIK3CA mutations and genomic data for the patients is not currently available. It is therefore unclear whether genomic features may be one explanation for the differences in response rate to MK-2206 in USC patients in the earlier study compared to this focused USC study. Other factors that could have contributed to the relatively lower efficacy in this study include differences in the study populations, or the lower dose of MK-2206 which may have less inhibition of the signaling pathway; these features should be examined in future studies. Another limitation of this study is the small sample size, which might not have allowed this trial to detect rare individuals with USC who may be sensitive to MK-2206.
In future work, it will be important to further examine the genomic and phenotypic features of the patients with USC in both studies to determine whether alterations in the PI3K pathway or other molecular or clinical characteristics are common to the patients who achieved clinical benefit. Other candidate biomarkers for response to AKT inhibition include mutations in AKT genes, alterations in other PI3K pathway genes such as PTEN or PIK3R1, or other oncogenic changes that impact activity of the PI3K pathway or that bypass the PI3K pathway such as RAS/MAPK pathway activation (Westin et al., 2021). Analysis of biopsy specimens from the study may also enable investigation of immunohistochemical markers of response to AKT inhibition such as phosphorylation of AKT or GSKbeta (Hirai et al., 2010). Finally, numerous other AKT inhibitors have been developed in recent years with different pharmacologic characteristics and mechanisms of resistance (Song et al., 2019), and it is possible that different inhibitors may have more efficacy in the USC context, or different toxicity profiles that allow dosing strategies with stronger or more sustained pathway inhibition. In addition, AKT inhibition may enhance activity of other drug classes including targeted agents (e.g., RAS pathway inhibitors, poly (ADP-ribose) polymerase inhibitors (Westin et al., 2021), and chemotherapy (Hirai et al., 2010)). Given non-overlapping toxicities, combining AKT inhibitors with other agents is a concept being tested in other cancers that could be extended to USC in the future.
In summary, treatment with AKT inhibitor MK-2206 generated modest partial responses and disease stability (e.g., > 6 months PFS) in individual patients with USC, despite a lack of consistent activity in this population. Further study is warranted to understand the characteristics of USC patients who may attain benefit from AKT inhibition, either alone or in combination with other treatments.
Funding information
Stand Up to Cancer Dream Team Translational Research Grant (SU2C-AACR-DT0209) to S.N.W., R.R.B., V.M., J.L., R.L.C., F.M.B., C.A., R.C., G.B.M., L.C.C., U.M., A.P., NO1 (NO1-CM- 2011-00039) to MD Anderson Cancer Center, NCI Cancer Center Support Grant (P30 CA016672) to MD Anderson Cancer Center, NCI Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center (V.M.), NCI SPORE in Uterine Cancer (P50 CA098258) to S.N.W., R.R.B., R.L.C., G.B.M., K08 CA237871 to E.H.S., Andrew Sabin Family Fellowship to S.N.W., NIH K12 Calabresi Scholar Award (K12 CA088084) to S.N.W., GOG Foundation Scholar Investigator to S.N.W., R35 CA197588 to L.C.C. MK-2206 (Merck Pharmaceuticals) was provided by NCI/CTEP (Cancer Therapy Evaluation Program).
CRediT authorship contribution statement
Elizabeth H. Stover: Investigation, Writing – original draft, Writing – review & editing. Niya Xiong: Writing – original draft, Writing – review & editing. Andrea P. Myers: Conceptualization, Investigation, Supervision, Writing – review & editing. Nabihah Tayob: Writing – original draft, Writing – review & editing. Victoria Engvold: Project administration, Writing – review & editing. Madeline Polak: Project administration, Writing – review & editing. Russell R. Broaddus: Conceptualization, Investigation, Writing – review & editing. Vicky Makker: Conceptualization, Investigation, Writing – review & editing. Ronny Drapkin: Conceptualization, Investigation, Writing – review & editing. Joyce F. Liu: Conceptualization, Investigation, Writing – review & editing. Neil S. Horowitz: Conceptualization, Investigation, Writing – review & editing. Funda Meric-Bernstam: Conceptualization, Investigation, Writing – review & editing. Carol Aghajanian: Conceptualization, Investigation, Writing – review & editing. Robert L. Coleman: Conceptualization, Investigation, Writing – review & editing. Gordon B. Mills: Conceptualization, Investigation, Writing – review & editing. Lewis C. Cantley: Conceptualization, Investigation, Writing – review & editing. Ursula A. Matulonis: Conceptualization, Investigation, Supervision, Writing – review & editing. Shannon N. Westin: Conceptualization, Investigation, Supervision, Writing – review & editing. Panagiotis A. Konstantinopoulos: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare that they have no competing interests relevant to this study. For completeness, the following conflicts of interest disclosure is provided: A.P.M. is a full-time employee of Novartis. V.M. has participated in advisory boards for Novartis, Iteos, Eisai, Merck, Karyopharm, Clovis, and GSK. R.D. has been a consultant for Repare Therapeutics and Mersana Therapeutics, and is a founding member and has a financial interest in VOC Health. J.F.L. has received institutional funding for clinical trials from 2X Oncology, Aravive, Arch Oncology, AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, CytomX Therapeutics, GlaxoSmithKline, Regeneron, Surface Oncology, Tesaro, and Vigeo Therapeutics; and has participated in advisory boards for AstraZeneca, Clovis, Eisai, EpsilaBio, Genentech, GSK/Tesaro, and Regeneron Pharmaceuticals. F. M.-B. has received research support from Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co.,; has been a consultant for AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, and Zymeworks; has received honoraria from Chugai Biopharmaceuticals; and has participated in advisory boards for Black Diamond, Biovica, Eisai, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis. C.A. has received institutional funding for clinical trials from Abbvie, Clovis, Genentech, and AstraZeneca; has been a consultant for Eisai/Merck, Mersana Therapeutics, Roche/Genentech, Abbvie, AstraZeneca, Merck, and Repare Therapeutics; and has served on an advisory board for Blueprint Medicines. R.C. has received research support from AstraZeneca, Merck, Clovis, Genmab, Roche/Genentech, Janssen, Immunogen, and Genelux; and has been a consultant for AstraZeneca, GSK, Clovis, Genmab, Roche/Genentech, Janssen, Agenus, Regeneron, OncoQuest, Immunogen, Genelux, Onxerna, Onxeo, Deciphera, and Alkermes. G.B.M. has received funding for clinical trials from AstraZeneca, Genentech, GSK,and Eli Lilly; has been a consultant and/or advisory board member for Amphista, AstraZeneca, Chrysallis Biotechnology, GSK, ImmunoMET, Ionis, Lilly, PDX Pharmaceuticals, Signalchem Lifesciences, Symphogen, Tarveda, Turbine, and Zentalis Pharmaceuticals; and has a financial interest in Catena Pharmaceuticals, ImmunoMet, SignalChem, and Tarveda. L.C.C. has been a consultant for Novartis. U.A.M. has been a consultant for Merck, Novartis, and Astrazeneca; and has served on an advisory board for Symphogen. S.N.W. has received research funding from AstraZeneca, Bayer, Bio-Path, Clovis Oncology, GSK, Mereo, Novartis, OncXerna, Roche/Genentech, Zentalis; and has been a consultant for Agenus, AstraZeneca, Clovis Oncology, Eisai, ERQX, GSK, ImmunoGen, Merck, Mereo, Novartis, Pfizer, Roche/Genentech, Vincerx, and Zentalis. P.A.K. has been a consultant for Alkermes, AstraZeneca, Bayer, GSK, Merck, Pfizer, Tesaro, Mersana, Repare Therapeutics, and Kadmon.
References
- Bogani G., Ray-Coquard I., Concin N., Ngoi N.Y.L., Morice P., Enomoto T., Takehara K., Denys H., Nout R.A., Lorusso D., Vaughan M.M., Bini M., Takano M., Provencher D., Indini A., Sagae S., Wimberger P., Póka R., Segev Y., Kim S.I., Candido dos Reis F.J., Lopez S., Mariani A., Leitao M.M., Raspagliesi F., Panici P.B., Di Donato V., Muzii L., Colombo N., Scambia G., Pignata S., Monk B.J. Uterine serous carcinoma. Gynecol. Oncol. 2021;162(1):226–234. doi: 10.1016/j.ygyno.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, Kandoth C., Schultz N., et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.A., Devesa S.S., Harvey S.V., Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J. Clin. Oncol. 2019;37(22):1895–1908. doi: 10.1200/JCO.19.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader A.N., Roque D.M., Siegel E., Buza N., Hui P., Abdelghany O., Chambers S., Secord A.A., Havrilesky L., O'Malley D.M., Backes F.J., Nevadunsky N., Edraki B., Pikaart D., Lowery W., ElSahwi K., Celano P., Bellone S., Azodi M., Litkouhi B., Ratner E., Silasi D.-A., Schwartz P.E., Santin A.D. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020;26(15):3928–3935. doi: 10.1158/1078-0432.CCR-20-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Sootome H., Nakatsuru Y., Miyama K., Taguchi S., Tsujioka K., Ueno Y., Hatch H., Majumder P.K., Pan B.-S., Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Hua H., Zhang H., Chen J., Wang J., Liu J., Jiang Y. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021;14:128. doi: 10.1186/s13045-021-01137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E., Wu R.-C., Guan B., Wu G., Zhang J., Wang Y., Song L., Yuan X., Wei L., Roden R.B.S., Kuo K.-T., Nakayama K., Clarke B., Shaw P., Olvera N., Kurman R.J., Levine D.A., Wang T.-L., Shih I.-M. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J. Natl. Cancer Inst. 2012;104(19):1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.K., Fader A.N., Santin A.D., Liu J.F. Uterine serous carcinoma: Molecular features, clinical management, and new and future therapies. Gynecol. Oncol. 2021;160(1):322–332. doi: 10.1016/j.ygyno.2020.10.017. [DOI] [PubMed] [Google Scholar]
- Lu K.H., Broaddus R.R. Endometrial Cancer. N. Engl. J. Med. 2020;383(21):2053–2064. doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- Myers A.P. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway–the devil is in the details. Clin. Cancer Res. 2013;19(19):5264–5274. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- Myers A.P., Konstantinopoulos P.A., Barry W.T., Luo W., Broaddus R.R., Makker V., Drapkin R., Liu J., Doyle A., Horowitz N.S., Meric‐Bernstam F., Birrer M., Aghajanian C., Coleman R.L., Mills G.B., Cantley L.C., Matulonis U.A., Westin S.N. Phase II, 2-stage, 2-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer. Int. J. Cancer. 2020;147(2):413–422. doi: 10.1002/ijc.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Bode A.M., Dong Z., Lee M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019;79(6):1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Perry M.W.D., Brown J.R., André F., Okkenhaug K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021;20(10):741–769. doi: 10.1038/s41573-021-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin S.N., Labrie M., Litton J.K., Blucher A., Fang Y., Vellano C.P., Marszalek J.R., Feng N., Ma XiaoYan, Creason A., Fellman B., Yuan Y., Lee S., Kim T.-B., Liu J., Chelariu-Raicu A., Chen T.H., Kabil N., Soliman P.T., Frumovitz M., Schmeler K.M., Jazaeri A., Lu K.H., Murthy R., Meyer L.A., Sun C.C., Sood A.K., Coleman R.L., Mills G.B. Phase Ib Dose Expansion and Translational Analyses of Olaparib in Combination with Capivasertib in Recurrent Endometrial, Triple-Negative Breast, and Ovarian Cancer. Clin. Cancer Res. 2021;27(23):6354–6365. doi: 10.1158/1078-0432.CCR-21-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Choi M., Overton J.D., Bellone S., Roque D.M., Cocco E., Guzzo F., English D.P., Varughese J., Gasparrini S., Bortolomai I., Buza N., Hui P., Abu-Khalaf M., Ravaggi A., Bignotti E., Bandiera E., Romani C., Todeschini P., Tassi R., Zanotti L., Carrara L., Pecorelli S., Silasi D.-A., Ratner E., Azodi M., Schwartz P.E., Rutherford T.J., Stiegler A.L., Mane S., Boggon T.J., Schlessinger J., Lifton R.P., Santin A.D. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA. 2013;110(8):2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]