Highlights
-
•
BML of the retroperitoneal lymph nodes is a rare, benign disease.
-
•
BML can mimic metastatic lymphadenopathy in the setting of concurrent malignancy.
-
•
BML in the retroperitoneal lymph nodes is a diagnosis of exclusion.
-
•
Consider biopsy if suspect metastatic disease in patients with BML risk factors.
Keywords: Cervical cancer, Benign metastasizing leiomyoma, Leiomyoma, Lymphadenopathy
Abstract
Extrauterine leiomyomas can present as benign metastasizing leiomyoma involving lymph nodes, which can be mistaken for metastatic malignancy. We report a case of a 52-year-old female who presented with postmenopausal bleeding and was found to have an endocervical mass. Imaging demonstrated retroperitoneal lymphadenopathy and biopsy of the cervical mass showed adenocarcinoma of either uterine or cervical origin. Patient underwent hysterectomy, bilateral salpingo-oophorectomy and lymphadenectomy for bulky pelvic and para-aortic lymph nodes. Final pathology was consistent with FIGO 2019 stage IB2 adenocarcinoma of the cervix with concurrent and benign metastasizing leiomyomas involving retroperitoneal lymph nodes.
1. Introduction
Benign metastasizing leiomyoma (BML) is a rare, benign disease that can be easily mistaken for a malignant disease process. BML is the growth of well-differentiated, hormonally dependent smooth muscle tissue in extrauterine sites (Barnaś et al., 2017, Jautzke et al., 1996). It is characterized as staining positive for smooth muscle actin, estrogen receptors and progesterone receptors (Jautzke et al., 1996, Williams et al., 2016, Kim et al., 2018). BML is most commonly seen in the lung, however, it has also been documented in the heart, bones, pelvis and lymph nodes (Barnaś et al., 2017, Williams et al., 2016, Kolaczyk et al., 2015). Most cases of BML occur in premenopausal or perimenopausal women after a primary surgery for leiomyomas (hysterectomy or myomectomy) (Williams et al., 2016). However, there are a few documented cases of BML occurring in patients without prior leiomyoma surgeries (Barnaś et al., 2017, Kim et al., 2018, Jo and Baek, 2018) It is hypothesized that there is seeding of the peritoneum, which allows for intravascular or lymphatic invasions of the leiomyomas.
Due to its largely benign nature, BML needs to be distinguished from malignant processes. A directed needle biopsy can be performed by interventional radiologist. The main stay of treatment is either surgical removal of the mass(s) followed by a thorough histopathologic evaluation or hormonal therapy (Williams et al., 2016, Aad et al., 2020). Due to the high likelihood of recurrence, patients are counseled extensively on the potential need for additional future surgical procedures.
There are no published cases of BML in the setting of concurrent cervical cancer. The incidence of cervical cancer in the United States is 7.6%, with an estimated 13,800 new cases in 2020 (Siegel et al., 2020). Cervical cancer is the fourth leading cause of cancer in female patients worldwide with a mortality rate of 2.3% (Siegel et al., 2020, Abu-Rustum et al., 2020). Early stage cervical cancer may be asymptomatic, found by cervical cytology or biopsies. Patients can present with watery discharge, post coital bleeding or vaginal spotting (Abu-Rustum et al., 2020). Cervical cancer staging is done clinically with the use of physical examination, biopsies, and imaging. While the treatment of cervical cancer varies with the stage at the time of diagnosis, for stage 1B cervical cancer, radiation therapy, or radical hysterectomy with pelvic lymphadenectomy is considered the gold standard in therapy due to its curative nature with an overall response rate of approximately 80–90% (Abu-Rustum et al., 2020, Landoni et al., 1997, Han et al., 2003).
Cervical cancer can spread by direct extension or by hematogenous and/or lymphatic dissemination. The presence of lymph node involvement is associated with worse prognosis and impacts decisions regarding radiotherapy fields (Singh and Arif, 2004). Incidence of lymph node metastasis depends on tumor size; histology, and stromal invasion, with up to 12% risk of lymph node metastasis in early stage cervical cancer (Hughes, 1988). The 2019 FIGO staging system was recently changed to include lymph node status to reflect the increased access to PET and MRI imaging (Bhatla et al., 2019).
The goal of this case report is to present a case of BML concurrent with carcinoma of the cervix. This case is valuable as it demonstrates an unique presentation of these conditions, which lead to an alteration in surgical management and treatment.
2. Case description
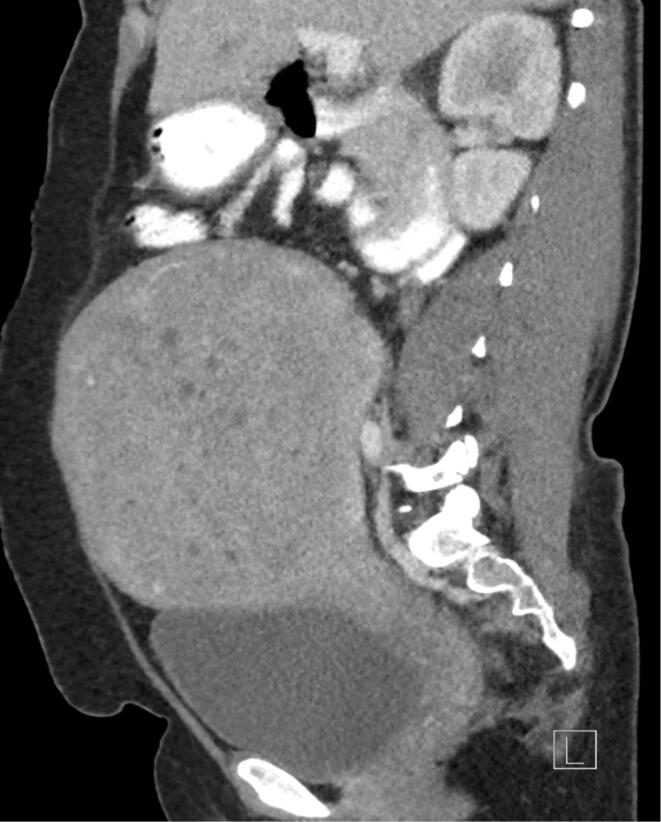
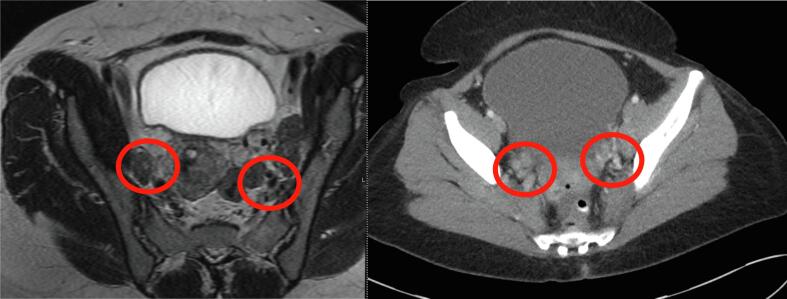
A 52-year-old woman (gravida 3, para 3) with history of abnormal pap smear with high-grade squamous intraepithelial lesion (HGSIL) and cervical intraepithelial neoplasia (CIN) II/III on colposcopic biopsy at a referring institution, admitted to the hospital with abnormal uterine bleeding requiring multiple transfusions of packed red blood cells. Abdominal examination demonstrated a 22 cm bulky uterus. On pelvic exam, patient had a normal ectocervix with no parametrial, vaginal, or rectal involvement. Computed tomography (CT) demonstrated a large heterogeneous mass in the anterior uterus measuring 17 × 12 × 16 cm with bilateral pelvic sidewall lymphadenopathy ranging from 1.4 to 2.9 cm (Fig. 1). There was no evidence of para-aortic lymphadenopathy, ascites, upper abdominal or distant disease. Magnetic resonance imaging (MRI) demonstrated a large intrauterine mass without parametrial invasion and pelvic sidewall and para-aortic adenopathy (Fig. 2). Repeated cervical and endometrial biopsies were done and demonstrated poorly differentiated adenosquamous carcinoma. Given the presence of a large uterine mass with extensive vaginal bleeding, no visible cervical mass, and large retroperitoneal lymphadenopathy, the patient was consented for a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy with presumed diagnosis of uterine or cervical malignancy. The case was discussed with the radiation oncologist preoperatively and MRI images were reviewed. Given the large size of the uterus, external beam radiation therapy would result in significantly increased toxicity. In addition, the tandem applicator for intracavitary high-dose-rate brachytherapy will not cover the uterus adequately. Therefore, it was recommended to proceed with surgical resection.
Fig. 1.
CT sagittal view of pelvic mass.
Fig. 2.
MRI of mass showing bilateral pelvic lymphadenopathy adjacent to the external and internal iliac arteries.
On laparotomy, washings were performed, the upper and lower abdomen were explored and no abdominal or pelvic peritoneal disease was noted. There was a 22 cm uterus with a dominant fibroid. Significant para-aortic and pelvic lymphadenopathy was noted with largest lesion measuring 5 cm located in the left obturator space. Given high concern for lymphadenopathy representing a malignant process, thus necessitating adjuvant radiation therapy, the decision was made to proceed with simple rather than radical hysterectomy to minimize postoperative morbidity. Patient underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy and removal of all bulky retroperitoneal masses. Intra-operative pathologic evaluation of the cervical mass was consistent with adenosquamous carcinoma, the uterine mass was leiomyoma, and the left obturator mass was found to be leiomyoma. Her post-operative course was uncomplicated, and she was discharged to home on post-operative day four after meeting appropriate milestones.
Gross pathology evaluation demonstrated a uterus measuring 22.5 × 19 × 19 cm with scant parametrium and a large spherical mass arising from myometrium measuring 19 cm predominantly within the anterior portion of the uterus. The ectocervix was grossly uninvolved, the endocervix contained an exophytic cervical mass measuring 3.9 cm in greatest dimension. The 19 cm myometrial mass had pale white whorled cut surface with focal areas of hemorrhage. The endometrial mucosa was unremarkable. The gross evaluation of the retroperitoneal masses demonstrated a white whorled and bulging cut surface.
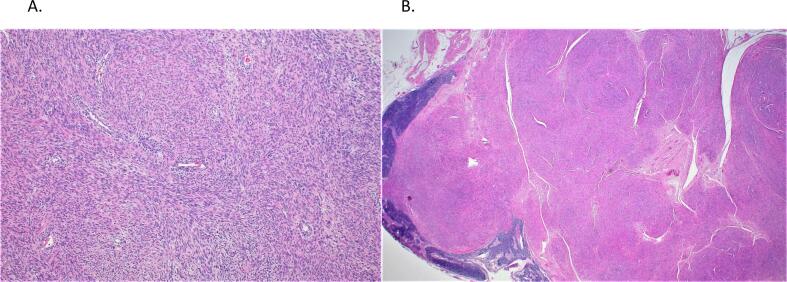
Histological evaluation of the cervical mass demonstrated an invasive cervical carcinoma with adenosquamous and stratisfied mucin-producing features (moderately to poorly differentiated) with 60% stromal invasion (0.9 of 1.5 cm), negative parametria, vaginal, and deep margins, and a focus suspicious of lymphovascular invasion. The tumor cells were diffusely positive for CK7 and p16. Immunostains for p40 and CK5/6 were positive in the squamous component. Mucicarmine highlighted intracytoplasmic mucin focally in the glandular component. The uterine mass demonstrated a large variably cellular cytologically uniform smooth muscle neoplasm with degenerative changes (infarctive/hyaline necrosis). The spindle cells of the smooth muscle neoplasm were positive for desmin and SMA, while negative for CD10. The stains for CK8/18 and CAM5.2 showed focal non-specific staining (Fig. 3a). The histopathological evaluation of all the retroperitoneal masses demonstrated bland smooth muscle neoplasms with some areas showing adenofibromatous differentiation. Again, the spindle cells were positive for desmin and SMA, while CK8/18, CAM5.2, and CD10 showed focal nonspecific staining (Fig. 3b).
Fig. 3.
A) Uterine Leiomyoma B) Retroperitoneal lymph node tumor with smooth muscle cells.
Given the features of the smooth muscle neoplasm in both the uterus and lymph nodes, the diagnosis was consistent with uterine leiomyoma with benign metastasizing leiomyoma of the retroperitoneal lymph nodes. After getting a second option John’s Hopkins, it was determined that some of the benign metastasizing leiomyomas were a form of a peculiar adenofibromatous differentiation in some of nodules in the lymph nodes. Thus, the patient was diagnosed with stage IB2 adenosquamous carcinoma of the cervix and concurrent benign metastasizing leiomyomas of the retroperitoneal lymph nodes. Patient was counseled extensively regarding recurrence rates of BML. Due to presence of lymphovascular space invasion, presence of middle stromal involvement, and tumor size greater than 2 cm, patient was recommended adjuvant treatment with radiation therapy in accordance with Sedlis’s criteria (Abu-Rustum et al., 2020). The patient was planned to undergo intensity-modulated radiation therapy to the pelvis to 50.4 Gy.
3. Discussion
This is the first case report of concurrent presentation of benign metastasizing leiomyomas of the retroperitoneal lymph nodes and early stage cervical cancer. Patient’s clinical and radiological findings of large retroperitoneal lymphadenopathy suggested presence of metastatic disease. While the preoperative biopsies were not conclusive, patient was still counseled initially regarding role of curative radiation therapy with concurrent chemotherapy, given the high suspicion for node-positive locally advanced cervical malignancy. The radiation oncologist was consulted and given the presence of a large 22 cm uterus, external beam radiation to the pelvis and para-aortic chain would result in significant toxicity. In addition, intracavitary high-dose rate brachytherapy would be suboptimal in achieving adequate coverage. CT-guided lymph node biopsy could have been performed for histologic assessment preoperatively, however, would not have changed the management of this patient. Diagnosis and treatment was achieved with surgical debulking and pelvic and para-aortic lymph node dissection. Furthermore, patient was having extensive vaginal bleeding necessitating blood product replacement, thus leading towards urgent surgical intervention.
At the time of surgery, simple hysterectomy was done instead of radical hysterectomy, as would be recommended for stage IB2 cervical cancer (Abu-Rustum et al., 2020). This decision was based on clinically high suspicion of the retroperitoneal nodes representing metastatic disease. The surgeons felt that performing a radical hysterectomy in a patient who already meets Peter’s criteria for adjuvant chemoradiation, would increase the morbidity of triple modality approach (Peters et al., 2000). Therefore, a simple hysterectomy was performed. In retrospect, given that patient’s met Sedlis’ criteria, she still was recommended to undergo adjuvant radiation, therefore the fact that she had a less aggressive surgery would not affect her prognosis while decreasing her postoperative morbidity.
4. Conclusions
Benign metastasizing leiomyomas of the retroperitoneal lymph nodes is a rare, benign disease that can mimic metastatic lymphadenopathy in the setting of concurrent malignancy. In this case report, the surgical approach to the patient’s cervical cancer was impacted by the presence of BML of the retroperitoneal lymph nodes. While, in this patient, this misdiagnosis may not have changed the treatment regimen or prognostic outcome, it may have impacted treatment decisions for other patients. Further exploration of the nodules through biopsy guided imaging or surgical planning for debulking with intraoperative pathology could help to avoid this rare diagnosis. In the future, BML in the retroperitoneal lymph nodes, while exceptionally rare, should be considered as a diagnosis of exclusion on the differential in surgical planning for patients, particularly female patients with concurrent metastatic disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aad G., Abbott B., Abbott D.C., et al. Search for heavy resonances decaying into a photon and a hadronically decaying Higgs boson in pp collisions at sqrt[s]=13 TeV with the ATLAS detector. Phys. Rev. Lett. 2020;125(25) doi: 10.1103/PhysRevLett.125.251802. [DOI] [PubMed] [Google Scholar]
- Abu-Rustum N.R., Yashar C.M., Bean S., et al. NCCN guidelines insights: cervical cancer, version 1.2020. J. Natl. Compr. Canc. Netw. 2020;18(6):660–666. doi: 10.6004/jnccn.2020.0027. [DOI] [PubMed] [Google Scholar]
- Barnas E., Ksiazek M., Ras R., Skret A., Skret-Magierlo J., Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: A review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017;12(4):e0175875. doi: 10.1371/journal.pone.0175875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla N., Berek J.S., Cuello Fredes M., Denny L.A., Grenman S., Karunaratne K., Kehoe S.T., Konishi I., Olawaiye A.B., Prat J., Sankaranarayanan R. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019;145(1):129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- Han C.C., Low J.J., Yeo R., et al. FIGO stage 1B2 cervical carcinoma–the KK Women's and Children's Hospital experience. Ann. Acad. Med. Singap. 2003;32(5):665–669. [PubMed] [Google Scholar]
- Hughes J.R. Accuracy of cervical cytology screening. Br. Med. J. (Clin. Res. Ed.) 1988;296(6635):1534. doi: 10.1136/bmj.296.6635.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jautzke G., Muller-Ruchholtz E., Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol. Res. Pract. 1996;192(3):215–223. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- Jo H.C., Baek J.C. Case of pulmonary benign metastasizing leiomyoma from synchronous uterine leiomyoma in a postmenopausal woman. Gynecol. Oncol. Rep. 2018;26:33–36. doi: 10.1016/j.gore.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-N., Eoh K.J., Lee J.-Y., Nam E.J., Kim S., Kim S.W., Kim Y.T. Aberrant uterine leiomyomas with extrauterine manifestation: intravenous leiomyomatosis and benign metastasizing leiomyomas. Obstet. Gynecol. Sci. 2018;61(4):509. doi: 10.5468/ogs.2018.61.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczyk K., Chamier-Cieminska K., Walecka A., et al. Pulmonary benign metastasizing leiomyoma from the uterine leiomyoma: a case report. Pol J Radiol. 2015;80:107–110. doi: 10.12659/PJR.892733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoni F., Maneo A., Colombo A., Placa F., Milani R., Perego P., Favini G., Ferri L., Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- Peters W.A., Liu P.Y., Barrett R.J., Stock R.J., Monk B.J., Berek J.S., Souhami L., Grigsby P., Gordon W., Alberts D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Singh N., Arif S. Histopathologic parameters of prognosis in cervical cancer–a review. Int. J. Gynecol. Cancer. 2004;14(5):741–750. doi: 10.1111/j.1048-891X.2004.014504.x. [DOI] [PubMed] [Google Scholar]
- Williams M., Salerno T., Panos A.L. Right ventricular and epicardial tumors from benign metastasizing uterine leiomyoma. J. Thorac. Cardiovasc. Surg. 2016;151(2):e21–e24. doi: 10.1016/j.jtcvs.2015.09.059. [DOI] [PubMed] [Google Scholar]