Abstract
Background
Coronary microvascular dysfunction (CMD) and obstructive coronary artery disease (CAD) are likely to exist side-by-side, thereby probably inducing angina pectoris symptoms of some patients not effectively relieved after revascularization. We aimed to evaluate the prevalence and characteristics exhibited by CMD in patients with recurrent chest pain who received percutaneous coronary intervention (PCI) before.
Methods
We conducted a single-center cross-sectional retrospective study. A total of 373 patients having received PCI were hospitalized for recurrent chest pain. Subsequently, they underwent coronary angiography and a rest/stress dynamic and routine gated myocardial perfusion imaging (MPI). At the vascular level, if any coronary artery stenosis <50% and myocardial flow reserve (MFR) <2.0 in the corresponding territory was considered to result from CMD. At the participant level, the CMD group was defined as one of the non-obstructive coronary arteries, in accordance with CMD at the vascular level.
Results
A total of 102 patients were finally recruited. At the vascular level, 274 vessels were eligible for inclusion, and the proportion of CMD was 43.1% (118/274). At the participant level, 49.0% (50/102) post-PCI patients with recurrent chest pain were indicated as CAD coexisting with CMD. Body mass index (BMI), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) in CMD patients exceeded those in controls. The stress myocardial blood flow (MBF) of CMD patients was significantly decreased than controls (stress MBF of left ventricle in CMD vs. control: 1.36±0.43 vs. 2.50±0.70, P<0.01). After age adjustment, multivariate logistic regression analysis revealed that increased BMI (OR: 1.405, 95% CI: 1.048–1.882) and LDL-C (OR: 3.094, 95% CI: 1.044–9.173) showed independent correlations with CMD (P<0.05).
Conclusions
The prevalence of CMD could be relatively high in post-PCI patients suffering from recurrent angina with no need for revascularization, and increased BMI and LDL-C could be independent predictors of CMD.
Keywords: Coronary microvascular dysfunction (CMD), myocardial flow reserve (MFR), coronary artery disease (CAD), percutaneous coronary intervention (PCI), single-photon emission computed tomography (SPECT)
Introduction
Vascular stenosis and/or dysfunction caused by epicardial coronary atherosclerosis is considered the primary pathogenesis of coronary artery disease (CAD). Thus, the corresponding treatment [e.g., percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG)] are primarily targeted at epicardial coronary arteries. The clinical practice here reported that angina pectoris symptoms of some patients with obstructive CAD were not effectively relieved after revascularization treatment (1). Existing studies (1-5) have reported that nearly half of the mentioned patients may have coronary microvascular dysfunction (CMD), and also several research literatures (6-8) revealed that CMD is correlated with poor long-term outcomes. With the deepening of cardiovascular research, the clinical significance of CMD has aroused increasing attention. Positron emission computed tomography (PET/CT)-based myocardial perfusion imaging (MPI) is capable of quantitatively calculating the myocardial blood flow (MBF) under rest and stress state of the heart, and then obtaining the myocardial flow reserve (MFR), i.e., ration of stress MBF and rest MBF and synonymous for coronary flow reserve (CFR). PET is considered the “gold” standard for noninvasive measurement of MFR. However, as impacted by the limitations of equipment and radiopharmaceuticals, it remains difficult for PET/CT quantitative MPI to be extensively carried out in clinical practice until now, and PET/CT MPI is currently limited to a few large cardiac centers. Over the past few years, a novel type of cardiac-dedicated single-photon emission computed tomography (SPECT), exploiting a semi-conductor detector termed cadmium zinc tellurium (CZT), exhibits high sensitivity, good spatial resolution, as well as fast temporal resolution. It is capable of performing rapid and dynamic tomographic imaging, thereby making it possible to quantitatively measure MBF. It has become more extensively used in clinical practice, and numerous up-to-date researches have indicated that CZT-SPECT-based MFR is more significantly correlated with PET/CT (9). However, the incidence of CAD coexisting with CMD using CZT dynamic SPECT imaging has been rarely reported. Accordingly, CZT-SPECT was employed to measure MFR in this study, and it aimed to evaluate the prevalence and characteristics exhibited by CMD in post-PCI patients with recurrent chest pain and to be referenced for clinical practice. We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-705/rc).
Methods
Study population
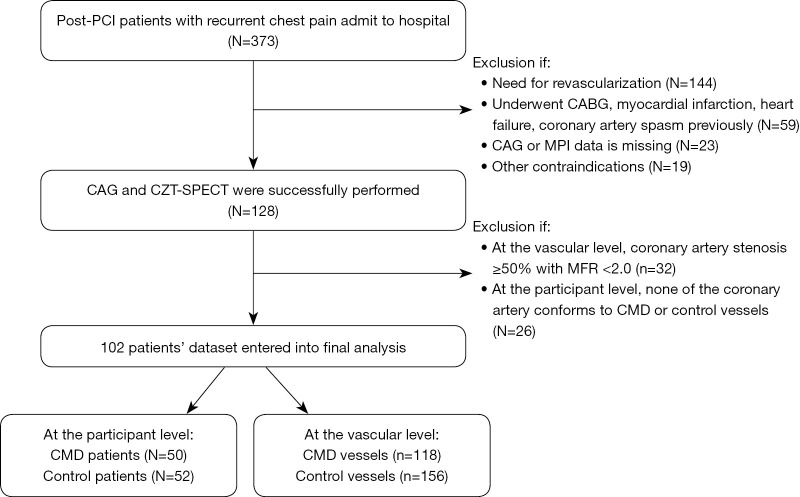
We conducted a single-center cross-sectional retrospective analysis of 373 consecutive CAD patients who had received PCI were hospitalized for recurrent angina at TEDA International Cardiovascular Hospital from January 2021 to March 2021. The inclusion criteria were as follows: patients (I) aged between 18 and 79 years; (II) providing written informed consent; (III) who had received CAG with no need for revascularization, and with SPECT used to evaluate the possibility of CMD; (IV) suitable for pharmacological stress MPI. Exclusion criteria: patients with contraindications to adenosine; prior CABG; myocardial infarction; severe lung diseases, heart failure, cardiomyopathy, valvular heart disease, malignant tumors, etc.; female patients during pregnancy or lactation. Figure 1 illustrates the flow of participants. Specific clinical data were collected, which included height, weight, past medical history, smoking history, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), serum creatinine (Cr), CAG, echocardiographic parameters, rest MBF (rMBF), stress MBF (sMBF) and MFR.
Figure 1.
Inclusion flow chart. PCI, percutaneous coronary intervention; CAG, coronary angiography; CZT, cadmium zinc tellurium; SPECT, single-photon emission computed tomography; MPI, myocardial perfusion imaging; CMD, coronary microvascular dysfunction; CABG, coronary artery bypass grafting; MFR, myocardial flow reserve.
The evaluation criteria of CAG
The left coronary artery CAG should be performed in at least four projection positions [Left Anterior Oblique (LAO) 45° + Cranial (CRA) 20°; Right Anterior Oblique (RAO) 30° + CRA 20°; LAO 45° + Caudal (CAU) 35°; RAO 30° + CAU 20°]; the right coronary artery CAG should be performed in at least two projection positions (LAO 45° and CRA 20°). Two experienced interventional surgeons evaluated the degree of coronary artery stenosis by quantitative coronary angiography (QCA) respectively. For the per-vessel analysis, obstructive coronary artery was defined as epicardial coronary stenosis ≥50%, and non-obstructive coronary artery (NOCA) was defined as epicardial coronary artery stenosis <50% or no stenosis.
Imaging equipment, methods, and image analysis
The imaging device was equipped with a CZT detector with 19 pinhole collimators (NM530c, GE Healthcare, Milwaukee, WI, USA). The imaging agent applied was 99mTc-methoxy isobutyl isonitrile (MIBI), which was provided by Beijing Senke Pharmaceutical Co., Ltd. or Atomic Hi-Tech Tianjin Pharmaceutical Co., Ltd. Imaging was performed using the one-day method: Adenosine load imaging was performed after resting. MBF is measured by MyoFlowQ 1.0.2 (Beijing Larkcloud Biomedical, Beijing, China) workstations. The dynamic list mode data were transferred to MyoFlowQ workstation and automatically re-binned into 18 frames consisting of 10×10 s, 5×20 s, 2×60 s, 1×280 s frames. The regions of interest for input function and myocardial radioactivity sampling were automatically or manually set to obtain the dynamic curve and fitting curve of the left ventricular blood pool and left ventricular myocardium, and to calculate the rMBF and sMBF of the left ventricle (LV). MFR was then obtained, calculated by the ratio of sMBF to rMBF. The stress blood flow of vasodilating drugs was not required to be corrected, while the rest blood flow measurement method was corrected according to RPP (the product of heart rate and blood pressure, i.e., the effect of the heart at rest, when RPP ≥1,000, the coefficient of 10,000/RPP was used for correction). For specific methods, please refer to reference (10). All patients discontinued vasoactive drugs, calcium antagonists, dipyridamole, adenosines, theophylline, as well as tea, coffee, or caffeinated beverages at least 24 h before the test.
CMD diagnostic criteria
The diagnostic criteria of CMD were defined as MFR <2.0 (11). At the vascular level, if any coronary artery stenosis (including the side branches) <50% and MFR <2.0 in this corresponding territory was considered to microvascular dysfunction; At the participant level, the CMD group were defined as one of the non-obstructive coronary arteries, in accordance with CMD at the vascular level; while MFR ≥2.0 of all three coronary arteries pertained to the normal control.
Statistical analysis
SPSS 23.0 statistical software package was used for statistical analysis. Continuous data were expressed as mean ± SD, and an independent sample t-test was used to compare the two groups. Categorical data were expressed as a percentage, and comparison between groups was performed by χ2 test or Fisher’s exact probability method. Univariate and multivariate logistic regression analyses were performed to determine the risk factors of CMD. A value of P<0.05 (two-sided) was considered statistically significant.
Ethical statement
This study was approved by the Institutional Ethics Board of TEDA International Cardiovascular Hospital, China (No. 2018-0626-3), and was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (as was revised in 2013). Informed consent was taken from all the patients.
Results
Comparison of baseline characteristics (Tables 1,2)
Table 1. Comparison between CMD and control vessels.
| Variable | CMD vessels (n=118) | Control vessels (n=156) | t or χ2 | P |
|---|---|---|---|---|
| PCI, n (%) | 60 (50.8) | 72 (46.2) | 0.296 | 0.586 |
| MFR | 1.46±0.34 | 3.01±0.73 | −15.120 | <0.001 |
| rMBF (mL/g/min) | 0.84±0.15 | 0.82±0.17 | 0.838 | 0.404 |
| sMBF (mL/g/min) | 1.22±0.37 | 2.47±0.80 | −11.069 | <0.001 |
Values was given as number of patients (%) or mean ± SD. CMD, coronary microvascular dysfunction; PCI, percutaneous coronary intervention; MFR, myocardial flow reserve; MBF, myocardial blood flow; rMBF, rest MBF; sMBF, stress MBF.
Table 2. Baseline characteristics of CMD and control patients.
| Variable | CMD patients (n=50) | Control patients (n=52) | t or χ2 | P |
|---|---|---|---|---|
| Female, n (%) | 22 (44.0) | 20 (38.5) | 0.091 | 0.763 |
| Age (years) | 61.22±7.91 | 62.93±8.03 | −0.770 | 0.445 |
| Hypertension, n (%) | 40 (80.0) | 40 (76.9) | 0.071 | 0.789 |
| Diabetes mellitus, n (%) | 10 (20.0) | 8 (15.4) | 1.116 | 0.572 |
| Smoking, n (%) | 24 (48.0) | 20 (38.5) | 1.018 | 0.313 |
| Double or triple vessel stenosis, n (%) | 28 (58.4) | 24 (46.1) | 2.318 | 0.314 |
| Residual coronary stenosis ≥50%, n (%) | 12 (24.0%) | 4 (7.7%) | 2.563 | 0.109 |
| BMI ≥24 kg/m2, n (%) | 40 (80.0) | 26 (50.0) | 5.023 | 0.025 |
| BMI (kg/m2) | 26.12±2.71 | 24.36±2.72 | 2.396 | 0.020 |
| TC (mmol/L) | 4.93±1.12 | 4.14±1.08 | 2.584 | 0.013 |
| LDL-C (mmol/L) | 3.04±0.88 | 2.42±1.00 | 3.478 | 0.001 |
| Cr (umol/L) | 62.25±16.8 | 65.5±14.7 | −0.748 | 0.458 |
| T wave inversion, n (%) | 22 (44.0) | 10 (19.2) | 3.632 | 0.047 |
| LAD (mm) | 36.53±3.52 | 36.32±3.13 | 0.270 | 0.788 |
| LVEDD (mm) | 45.74±3.36 | 45.18±4.52 | 0.546 | 0.588 |
| LVEF (%) | 64.51±5.63 | 62.55±3.81 | 1.478 | 0.146 |
Values was given as number of patients (%) or mean ± SD. CMD, coronary microvascular dysfunction; BMI, body mass index; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; Cr, creatinine; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
A total of 102 patients with an average age of 62.08±8.71 years were finally recruited. At the vascular level, excluding coronary arteries with stenosis ≥50%, 274 vessels were eligible for inclusion, and the proportion of CMD vessels was 43.1% (118/274). No significant difference was identified in the proportion of PCI between CMD vessels and the controls (P>0.05). At the participant level, 49.0% (50/102) post-PCI patients in all with recurrent chest pain were considered CAD coexists with CMD. No significant difference was identified in age, sex, hypertension, diabetes, smoking history, Cr, left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF) between the two groups (P>0.05). BMI, TC, LDL-C in CMD patients was higher than that in controls, and the proportion of patients with BMI ≥24 kg/m2, T wave inversion exceeded that in the control (80.0% vs. 50.0%) (all P<0.05).
Comparison of rMBF and sMBF between CMD patients and controls (Table 3)
Table 3. Comparison of myocardial blood flow between CMD and normal patients.
| Variable | CMD patients (n=50) | Control patients (n=52) | t | P |
|---|---|---|---|---|
| LAD rMBF (mL/g/min) | 0.88±0.14 | 0.81±0.17 | 1.569 | 0.123 |
| LAD sMBF (mL/g/min) | 1.32±0.33 | 2.47±0.74 | −7.192 | <0.001 |
| LCX rMBF (mL/g/min) | 0.80±0.15 | 0.83±0.15 | −0.677 | 0.502 |
| LCX sMBF (mL/g/min) | 1.12±0.43 | 2.35±0.66 | −7.882 | <0.001 |
| RCA rMBF (mL/g/min) | 0.84±0.16 | 0.80±0.19 | −0.736 | 0.465 |
| RCA sMBF (mL/g/min) | 1.41±0.47 | 2.66±0.96 | −5.876 | <0.001 |
Values are given as mean ± SD. CMD, coronary microvascular dysfunction; MBF, myocardial blood flow; rMBF, rest MBF; sMBF, stress MBF; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery.
No significant difference was identified in the rMBF of the respective coronary artery between the two groups, while the sMBF of the CMD group was significantly decreased [CMD vs. control: the sMBF (mL/g/min) of the left ventricle was 1.36±0.43 vs. 2.50±0.70, and the sMBF (mL/g/min) of RCA was 1.41±0.47 vs. 2.66±0.96, respectively (P<0.01)].
Correlation of MFR between LAD and LCX, LAD and RCA, RCA and LCX (Figure 2)
Figure 2.
Scatter diagram: correlations of MFR between LAD and LCX (A), LAD and RCA (B), RCA and LCX (C). MFR of LAD, LCX and RCA were positively correlated with each other in CAD patients (r=0.952, 0.862, 0.829, respectively, all P<0.01). MFR, myocardial flow reserve; LAD, left atrial diameter; LCX, left circumflex; RCA, right coronary artery; CAD, coronary artery disease.
MFR of LAD, LCX and RCA were positively correlated with each other in CAD patients (r=0.952, 0.862, 0.829, respectively, all P<0.01). In the CMD patients, MFR decreased in 38 cases (76.0%) with three coronary arteries, 8 cases (16.0%) with two coronary arteries, and 4 cases with a single coronary artery. Figure 3 presents a typical patient image.
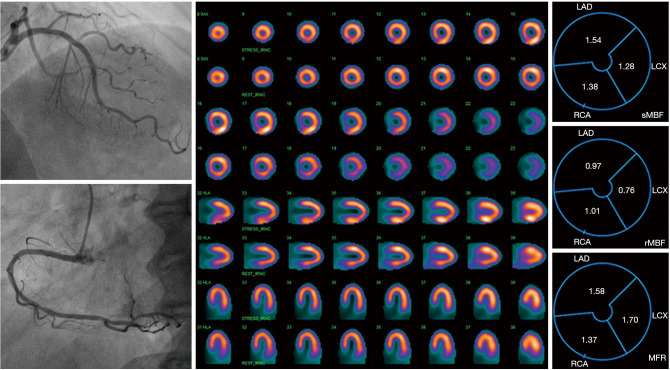
Figure 3.
A case report. A 58-year-old female patient with chief complaint of intermittent angina attack who received percutaneous coronary intervention three months ago was admitted to our hospital. Left figures: CAG showed no significant coronary stenosis and no obvious spasm. Central figure: routine adenosine stress plus rest serial tomographic images (row 1/3/5/7 was stress images, and row 2/4/6/8 was rest images) showed there was no significant change in the reversibility of the imaging agent. Right figures: it was showed that MFR was significantly decreased in the three territories of coronary arteries (LAD-MFR =1.58; LCX-MFR =1.70; RCA-MFR =1.37), meeting the diagnostic criteria of CMD. After nicorandil given to improve microvascular function, the patient’s symptoms were gradually relieved. CAG, coronary angiography; MFR, myocardial flow reserve; LAD, left atrial diameter; LCX, left circumflex; RCA, right coronary artery; CMD, coronary microvascular dysfunction.
Multivariate logistic regression analysis of CMD-related factors (Table 4)
Table 4. Multivariate logistic regression analysis for the risk factors of CMD.
| Variable | β | SE | Wald χ2 | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Age | −0.052 | 0.050 | 1.085 | 0.949 | 0.861–1.047 | 0.298 |
| Double or three vessel stenosis | 0.272 | 0.672 | 0.164 | 1.313 | 0.352–4.897 | 0.686 |
| Residual coronary stenosis ≥50% | −1.467 | 1.094 | 1.800 | 0.231 | 0.027–1.967 | 0.180 |
| TC | 0.135 | 0.413 | 0.106 | 1.144 | 0.509–2.570 | 0.745 |
| LDL-C | 1.129 | 0.555 | 4.149 | 3.094 | 1.044–9.173 | 0.042 |
| BMI | 0.340 | 0.149 | 5.185 | 1.405 | 1.048–1.882 | 0.023 |
| T wave inversion | −0.781 | 0.842 | 0.859 | 0.458 | 0.088–2.387 | 0.354 |
CMD, coronary microvascular dysfunction; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; BMI, body mass index.
Age, TC, LDL-C, coronary multi-vessel lesions, residual stenosis ≥50%, and T-wave inversion were substituted into the logistic regression equation. After the correction, increased BMI (OR: 1.405, 95% CI: 1.048–1.882) and LDL-C (OR: 3.094, 95% CI: 1.044–9.173) were used as independent predictors of CMD occurrence (P<0.05).
Discussion
CAD can seriously threaten people’s health, and its incidence rate remains high. Revascularization therapy (PCI and CABG) has been recognized as a mature treatment for obstructive CAD, whereas the inflection point of cardiovascular disease prevention and treatment has not yet arrived. According to the ISCHEMIA study (12), among patients with stable coronary disease and moderate or severe ischemia, invasive treatment did not significantly lower the risk of ischemic cardiovascular events or all-cause death compared with conservative treatment in the follow-up period (median follow-up time: 3.2 years). CMD refers to a clinical syndrome with objective evidence of exertional angina pectoris or myocardial ischemia caused by structural and/or functional abnormalities of anterior coronary arterioles and arterioles due to multiple pathogenic factors (13-15). The diagnosis and clinical significance of CMD have aroused rising attention from experts and scholars. Micrangium exhibiting a diameter <500 µm takes up over 90% of the coronary artery system, and microvascular dysfunction may be critical to developing cardiovascular diseases (3,13,14). The value of nuclear medicine MPI in the noninvasive function evaluation of CAD has been recognized internationally (15). With the continuous innovation of nuclear medicine imaging equipment as a novel technology, CZT-SPECT quantitative myocardial flow technique shows a great application prospect in clinical MFR evaluation (10,15).
Thus far, data from extensive sample epidemiological studies on CMD remain lacked (3). The clinical study conducted by Wu et al. (16) reported that at the vascular level, the proportion of non-obstructive coronary diagnosis of CMD was higher in the obstructive CAD group than in the non-obstructive CAD group, which indicated that CMD can be reported in patients with obstructive CAD and non-obstructive CAD, and the incidence might be higher in the former. As opposed to the mentioned, no significant difference was identified in the incidence of CMD at the participant level between the two groups. According to this study, the proportion of CAD coexisted with CMD in post-PCI patients with recurrent chest pain of 49.0%, and at the vascular level, the proportion of CMD accounted for 43.1%, relatively higher than patients with NOCA disease chest pain in our previous study (17), which reported 30% patients with CMD.
According to the results above, in patients with obstructive CAD, although the pericardial coronary arteries have been fully revascularization therapy, numerous patients still had microcirculation dysfunction, and targeted intensive drug therapy might mitigate the symptoms of angina and long-term prognosis. However, in routine outpatient follow-up, considerable post-PCI patients with recurrent chest pain had normal CAG, as recognized by doctors as “non-cardiogenic chest pain” and ignored, thereby causing repeated visits without adequate treatment (18). CZT-SPECT-based MFR could help doctors differentiate and identify high-risk patients, thereby being worthy of widespread clinical application (10,19). Figure 3 illustrates a typical case. The female patient with the chief complaint of intermittent angina attack after PCI, was admitted to the hospital of the authors. No significant coronary stenosis was found by CAG reexamination. CZT-SPECT did not show any significant reversible ischemic variations, and MFR significantly decreased in the three perfusion areas of the coronary artery (i.e., LAD-MFR =1.58; LCX-MFR =1.70; RCA-MFR =1.37), which was consistent with the diagnostic criteria of CMD. After nicorandil were given to improve microcirculation, the symptoms were progressively relieved.
We considered whether PCI could damage the microvascular function of culprit coronary arteries. At the vascular level, 50.8% (60/118) of vessels received PCI in the CMD group, which indicated no statistical difference from the control. From the results of our study, no difference was found in MFR between culprit or non-culprit coronary arteries. Importantly, it was not excluded that microvascular dysfunction might occur in the short term after PCI (e.g., within one week), which should be studied further.
Twenty-four percent (12/50) patients included in this study that had CMD also had obstructive CAD in another territory, whereas we did not consider that this result was related to the patient’s symptoms for reasons below. At the first step, patients with severe stenosis requiring revascularization were excluded from the screening. At the second step, we excluded vessels with stenosis ≥50% and MFR <2.0 at the vascular level in screened patients since we could not identify this coronary artery and its supplying area with the possibility of CMD by MFR. However, if coronary artery stenosis ≥50% but MFR >2.0, we considered that there was no ischemia in the area supplied by this coronary artery. Thus, for CMD patients, even if there was obstructive CAD with MFR >2.0 in another area, we would not considered that it was the cause of the patient’s symptoms, and CMD might be the potential cause.
T wave represents the “repolarization” of the ventricle, and T wave inversion is a common electrocardiography (EKG) manifestation in clinic, which indicates the possibility of myocardial ischemia. As indicated by the results of this study, the proportion of T wave inversion was 44% in the CMD group and 19.2% in control. According to the scatter diagram, the majority of CMD patients reduced MFR in three coronary arteries. Given the possibility of diffuse myocardial ischemia, T-wave inversion might be one of the EKG manifestations, which could be the underlying cause.
No significant difference was identified in the rMBF of the respective coronary artery between the CMD group and the normal control, but the sMBF was significantly decreased (P<0.05). MFR is the ratio of sMBF to rMBF, and a relative increase in rest blood flow or a decrease in stress blood flow can cause a decrease in MFR. Given the results of this study, the reduction of MFR in CMD patients is primarily caused by the significant reduction of sMBF, which indicated a significant decline in microvascular reserve function. De Vita et al. (20) evaluated the microvascular function in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) and non-obstructive CAD after intravenous infusion with ergonovine and adenosine, respectively. According to the results, patients with NSTE-ACS and non-obstructive CAD exhibited a significant coronary dysfunction, consisting of increased microvascular contractility and reduced microvascular dilation function, both persisting at 12-month follow-up. Safdar et al. (21) examined the incidence and clinical characteristics exhibited by CMD in 195 patients suffering from acute chest pain, excluding several factors (e.g., pulmonary embolism, aortic dissection, pericarditis and myocarditis). They reported that 42% patients had CMD, 36% patients had CAD and 22% patients had normal flows, which indicated that conventional risk factors of cardiovascular disease were not correlated with CMD. However, most of the patients recruited here were low-risk patients developing atypical chest pain symptoms, with an average age of 54.6 years. Moreover, 69.7% of the patients were female. Furthermore, CTA/CAG examination was lacked to clarify the condition of epicardial vessels. The grouping of CAD and CMD might be ambiguous, and the patients of CAD coexisting with CMD in the CAD group were not considered. In our study, patients with CAD and PCI history showed relatively severe epicardial atherosclerosis and more traditional cardiovascular disease risk factors. As indicated by the results, patients with CAD combined with CMD accounted for a relatively high proportion, and SPECT showed significant coronary dysfunction. Increased BMI and LCD-C were correlated with CMD, and the probable mechanism might be considered that atherosclerosis at the microvascular level in CAD patients may cause microvascular dysfunction and decreased coronary microvascular reserve function. It was indicated that poor control of traditional cardiovascular risk factors might be one of the causes of CAD patients coexisting with microvascular dysfunction. Existing studies (3,4) generally found that CMD and atherosclerosis share various common risk factors (e.g., diabetes, hypertension, hyperlipidemia, smoking, chronic inflammation), causing microvascular dysfunction via endothelial dysfunction cell-dependent and independent mechanisms, which is manifested as reduced MFR. The mentioned factors may improve the occurrence and development of microvascular dysfunction. However, several studies have revealed that the occurrence of CMD cannot be clarified by atherosclerosis’s risk factors in non-obstructive CAD patients, gender, psycho-psychological factors, stress state, inflammation and other non-traditional pathogenic factors might be correlated with CMD, which are likely to be more vital pathogenic factors and should be explored in depth. According to existing studies (22), every 10 kg/m2 increase in BMI is correlated with a 20% increase in cardiovascular events. Although the relationship between the increase in BMI and CMD is not clear, the increase in BMI is directly proportional to blood lipid, blood glucose and inflammatory factors, which may be a common factor that accelerates microvascular dysfunction.
This study still had some limitations. First, the sample size was relatively small, and it pertained to a retrospective study failing to comprehensively represent the accurate incidence of CMD in the obstructive CAD patients and the correlation of various risk factors. Second, there were certain deviations in the selected patients, and the positive rate of abnormal blood flow quantification was higher after the patients’ self-report and doctors’ screening. Third, we lacked certain methods of eliminating coronary spasm (acetylcholine tests). For this reason, the involvement of coronary microvascular spasm could not be overall excluded, and the effect on the diagnosis of CMD was difficult to avoid. Subsequent research should cover more samples on the incidence of CMD in patients suffering from obstructive CAD and the value of CTZ-SPECT in CMD evaluation.
In brief, we reported that the prevalence of CMD was relatively high in post-PCI patients suffering from recurrent angina with no need for revascularization. The decrease of MFR in CMD patients was primarily caused by the significant reduction of sMBF, which indicated a significant decline in microvascular reserve function. The increase in BMI and LDL-C were used as independent predictors of CMD. Poorly control of traditional cardiovascular risk factors might be one of the causes of CAD patients coexisting with microvascular dysfunction. CZT-SPECT quantitative determination of MFR shows a promising application in diagnosing CMD in post-PCI patients suffering from recurrent chest pain.
Acknowledgments
Funding: This work was supported by Tianjin Medical Key Discipline (Specialty) Construction Project.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Ethics Board of TEDA International Cardiovascular Hospital, China (No. 2018-0626-3), and was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (as was revised in 2013). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-705/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-705/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-705/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-705/coif). The authors have no conflicts of interest to declare.
References
- 1.Corcoran D, Young R, Adlam D, et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: The CE-MARC 2 coronary physiology sub-study. Int J Cardiol 2018;266:7-14. 10.1016/j.ijcard.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv 2015;8:1445-53. 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 3.Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2625-41. 10.1016/j.jacc.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sechtem U, Brown D, Godo S, et al. Coronary microvascular dysfunction in stable ischaemic heart disease (non-obstructive coronary artery disease and obstructive coronary artery disease). Cardiovasc Res 2020;116:771-86. 10.1093/cvr/cvaa005 [DOI] [PubMed] [Google Scholar]
- 5.Campisi R, Marengo FD. Coronary microvascular dysfunction in women with nonobstructive ischemic heart disease as assessed by positron emission tomography. Cardiovasc Diagn Ther 2017;7:196-205. 10.21037/cdt.2017.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brainin P, Frestad D, Prescott E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int J Cardiol 2018;254:1-9. 10.1016/j.ijcard.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation 2017;136:2325-36. 10.1161/CIRCULATIONAHA.117.029992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vita A, Milo M, Sestito A, et al. Association of coronary microvascular dysfunction with restenosis of left anterior descending coronary artery disease treated by percutaneous intervention. Int J Cardiol 2016;219:322-5. 10.1016/j.ijcard.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 9.Cantoni V, Green R, Acampa W, et al. Diagnostic performance of myocardial perfusion imaging with conventional and CZT single-photon emission computed tomography in detecting coronary artery disease: A meta-analysis. J Nucl Cardiol 2021;28:698-715. 10.1007/s12350-019-01747-3 [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Li S, Chen W, et al. Diagnostic efficiency of quantification of myocardial blood flow and coronary flow reserve with CZT dynamic SPECT imaging for patients with suspected coronary artery disease: a comparative study with traditional semi-quantitative evaluation. Cardiovasc Diagn Ther 2021;11:56-67. 10.21037/cdt-20-728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailly M, Thibault F, Courtehoux M, et al. Myocardial Flow Reserve Measurement During CZT-SPECT Perfusion Imaging for Coronary Artery Disease Screening: Correlation With Clinical Findings and Invasive Coronary Angiography-The CFR-OR Study. Front Med (Lausanne) 2021;8:691893. 10.3389/fmed.2021.691893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395-407. 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godo S, Suda A, Takahashi J, et al. Coronary Microvascular Dysfunction. Arterioscler Thromb Vasc Biol 2021;41:1625-37. 10.1161/ATVBAHA.121.316025 [DOI] [PubMed] [Google Scholar]
- 14.Padro T, Manfrini O, Bugiardini R, et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res 2020;116:741-55. 10.1093/cvr/cvaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler TH, Dilsizian V. Coronary Microvascular Dysfunction: Clinical Considerations and Noninvasive Diagnosis. JACC Cardiovasc Imaging 2020;13:140-55. 10.1016/j.jcmg.2018.11.036 [DOI] [PubMed] [Google Scholar]
- 16.Wu P, Guo XS, Zhang X, et al. Value of absolute quantification of myocardial perfusion by PET in detecting coronary microvascular disease in patients with non-obstructive coronaries. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:205-10. [DOI] [PubMed] [Google Scholar]
- 17.Wang YD, Chen WQ, Li Y, et al. Related risk factors of PET/CT detected coronary microvascular disease in patients with chest pain and no obstructive coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:942-7. [DOI] [PubMed] [Google Scholar]
- 18.Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504-20. 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang Z, Wang J, Li S, et al. Diagnostic analysis of new quantitative parameters of low-dose dynamic myocardial perfusion imaging with CZT SPECT in the detection of suspected or known coronary artery disease. Int J Cardiovasc Imaging 2021;37:367-78. 10.1007/s10554-020-01962-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vita A, Manfredonia L, Lamendola P, et al. Coronary microvascular dysfunction in patients with acute coronary syndrome and no obstructive coronary artery disease. Clin Res Cardiol 2019;108:1364-70. 10.1007/s00392-019-01472-4 [DOI] [PubMed] [Google Scholar]
- 21.Safdar B, D'Onofrio G, Dziura J, et al. Prevalence and characteristics of coronary microvascular dysfunction among chest pain patients in the emergency department. Eur Heart J Acute Cardiovasc Care 2020;9:5-13. 10.1177/2048872618764418 [DOI] [PubMed] [Google Scholar]
- 22.Bajaj NS, Osborne MT, Gupta A, et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol 2018;72:707-17. 10.1016/j.jacc.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]