Abstract
Introduction
Outcomes of immune checkpoint inhibitor (ICI) rechallenge in NSCLC remain uncertain. This study estimated the safety and efficacy of ICI rechallenge and compared rechallenge benefit among different reasons of initial ICI discontinuation in NSCLC.
Methods
PubMed, EMBASE, and Cochrane Library were searched for studies on NSCLC retreated with ICI. Immune-related adverse events (irAEs), overall response rate (ORR), disease control rate (DCR), and progression-free survival (PFS) at initial ICI and rechallenge were analyzed.
Results
A total of 15 studies including 442 patients between 2018 and 2022 were eligible for meta-analysis. The incidence of grade 3 or 4 irAE was lower in rechallenge than initial ICI (8.6% versus 17.8%, p < 0.001). Patients rechallenged with ICI had lower ORR and DCR than initial ICI (13.2% versus 42.4%, p < 0.001; 51.1% versus 74.0%, p < 0.001). The ORR and DCR to ICI rechallenge were both higher in patients who experienced disease progression after stopping ICI or irAE than patients with disease progression during ICI treatment (ORR: 46.2% versus 20% versus 11.4%, p = 0.003; DCR: 84.6% versus 90.0% versus 55.0%, p = 0.002). In addition, 34.7% of 69 patients with individual response to ICI and PFS experienced the same or better response to ICI rechallenge in comparison with initial ICI, although PFS in initial ICI was longer than that in ICI rechallenge (median: 8.90 versus 3.67 mo, hazard ratio = 0.44, 95% confidence interval: 0.33–0.59).
Conclusions
ICI rechallenge had less severe toxicity than initial ICI treatment. Patients undergoing disease progression after ICI cessation or ICI discontinuation owing to irAE are more likely to benefit from ICI rechallenge in NSCLC.
Keywords: Immune checkpoint inhibitors, Non–small cell lung cancer, Rechallenge, Immune-related adverse events, Prognosis
Introduction
Immune checkpoint therapy with antibodies targeting PD-1/PD-L1 and CTLA4 is a new cornerstone of cancer treatment and has been found to have a therapeutic efficacy across various types of cancer in the past 5 years.1 The number of patients with NSCLC who received immune checkpoint inhibitors (ICIs) is rapidly increasing because growing evidence suggests that ICI induces durable treatment response and prolongs survival in advanced NSCLC, especially to patients with high PD-L1 expression.2,3 Recently, immune checkpoint therapy or that plus chemotherapy was further recommended as first-line regimen in metastatic NSCLC without driver oncogene, regardless of PD-L1 levels.4
Nevertheless, immune checkpoint therapy was eventually discontinued in many patients with advanced NSCLC owing to disease progression.5 Even in patients with favorable therapeutic efficacy, ICI treatment may not last long owing to severe toxicities.6 In addition, the discontinuation of ICI in some patients is attributed to clinical decision after a defined time frame treatment such as 2 years or 35 cycles of anti–PD-1.7
Along with accumulating experience and evolving understanding of ICI, rechallenge of ICI is emerging in patients with NSCLC who discontinued ICI treatment owing to immune-related adverse events (irAEs) or disease progression during ICI treatment or after stopping ICI therapy after a defined number of cycles or a long period.8, 9, 10 Nevertheless, the reported small number of patients undergoing ICI rechallenge provided limited or heterogeneous evidence for ICI rechallenge. Thus, the risks and benefits of ICI rechallenge remained uncertain and inconclusive. The purpose of this meta-analysis was to synthesize available data on the safety and efficacy of ICI rechallenge and compare rechallenge benefit among different reasons of initial ICI discontinuation in patients with advanced NSCLC.
Materials and Methods
Data Sources and Study Selection
Study search and selection were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11 A comprehensive literature search was conducted to identify all relevant articles. The studies were searched in the databases of PubMed, EMBASE, and Cochrane Library until January 20, 2022. The search terms were the following Medical Subject Headings and their synonyms: “carcinoma, non-small-cell lung” and “immune checkpoint inhibitor” and “rechallenge.”
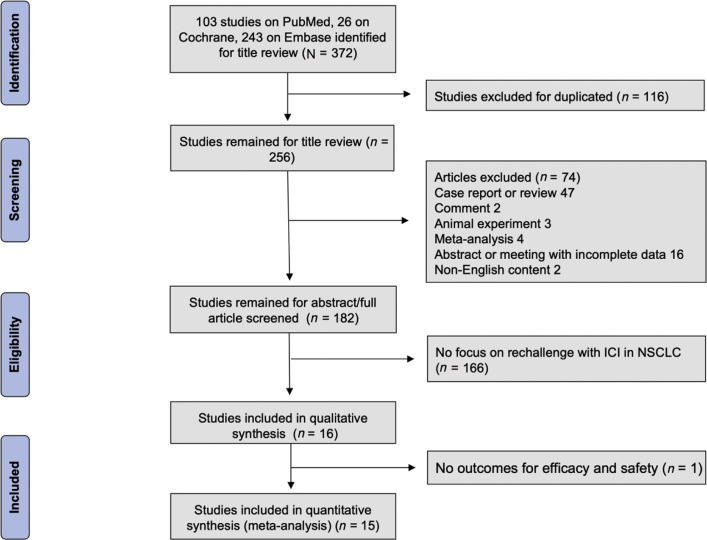
The studies were reviewed to evaluate the title, abstract, and full publication sequentially. The inclusion criteria were as follows: (1) clinical characteristics and prognosis parameters were described in patients with NSCLC; (2) ICI was used in both the initial treatment and retreatment (Fig. 1). Duplicate studies were excluded using the “remove duplicates” function in Endnote Online. Case reports, reviews, comments, animal experiments, meta-analyses, abstracts, and meetings with incomplete data or non-English content were also excluded.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. ICI, immune checkpoint inhibitor.
Data Extraction and Quality Assessment
A standardized data template was used to extract data from studies, and all discrepancies were resolved by consensus between two reviewers. The following information was extracted: first author, year of publication, country, study design, treatment period, number, sex and mean age of patients at initial treatment, regimens and therapy line of initial ICI and ICI rechallenge, tumor proportion status, best response to ICI treatment, number of patients who experienced grade 3 or 4 irAE after initial treatment and after rechallenge, median progression-free survival (PFS) (in mo), the cessation reasons of initial ICI, and interval time between initial ICI and ICI rechallenge (Table 1).13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Study quality assessments were performed according to the Newcastle-Ottawa Scale, which evaluated the study design based on eight questions regarding population selection, comparability, and exposure.12
Table 1.
Study Characteristics
| Study | Country | Study Design | Treatment Period | No.of Patient | Male, % | Mean Age (y) | Initial ICI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen(i) | Therapy Line | TPS ≥ 50% (%)a | PFSi (M) | Best Response |
G3/4 IrAE | Cessation Reason | |||||||||||
| CR | PR | Stable Disease | PD | NR | |||||||||||||
| Bernard et al., 201813 | France | Retrospective | 2012.5–2017.10 | 1 | NR | NR | Anti–PD-(L)1 | NR | NR | 19.9 | 0 | 1 | 0 | 0 | 0 | 0 | PD |
| Fujita et al., 201814 | Japan | Retrospective | 2015.12–2018.3 | 12 | 66.7 | 70.8 | Nivo | Second∼ | 41.7 | 6.2 | 0 | 7 | 2 | 3 | 0 | 2 | PD |
| Niki et al., 201815 | Japan | Retrospective | 2015.12–2017.12 | 11 | 81.8 | 66 | Nivo | NR | NR | 4.9 | 0 | 5 | 2 | 4 | 0 | 0 | PD |
| Santini et al., 201816 | America | Retrospective | 2011.4–2016.5 | 38 | 52.6 | 64 | Anti–PD(L)1/anti-CTLA4 | First∼ | NR | NR | 18 | 20 | 0 | 6 | irAE | ||
| Fujita et al., 201917 | Japan | Retrospective | 2018.1–2018.12 | 18 | 61.1 | 71 | Nivo/Pemb | Second | 50 | NR | 0 | 8 | 4 | 5 | 1 | 0 | NR |
| Watanabe et al., 201918 | Japan | Retrospective | 2015.12–2017.12 | 14 | 57.1 | 61.5 | Atezo/nivo/pemb | NR | 50 | 3.7 | 0 | 3 | 5 | 6 | 0 | 0 | PD |
| Mouri et al., 201919 | Japan | Retrospective | 2015.12–2018.8 | 21 | 90.5 | 69.6 | Nivo | Second∼ | NR | 13.3 | 1 | 12 | 8 | 0 | 0 | 7 | irAE |
| Fujita et al., 202020 | Japan | Retrospective | 2018.1–2019.8 | 15 | 93.3 | 71.4 | Atezo/durva | Second∼ | 0 | 3 | 0 | 0 | 5 | 9 | 1 | 0 | PD |
| Gobbini et al., 202021 | France | Retrospective | 2010–2018 | 144 | 67.4 | 63 | Anti–PD-(L)1 | First∼ | 14.6 | 13 | 10 | 61 | 38 | 26 | 9 | 27 | irAE, PD, clinical decision |
| Herbst et al., 202022 | Keynote 10 | Retrospective | 2013.8–2015.2 | 14 | NR | NR | Pemb | First∼ | NR | NR | 0 | 13 | 0 | 0 | 1 | NR | Clinical decision |
| Katayama et al., 202023 | Japan | Retrospective | 2017.4–2018.11 | 35 | 68.6 | 70 | Nivo/pemb/atezo | Third | 40 | 4 | 0 | 12 | 12 | 10 | 1 | NR | PD |
| Kitagawa et al., 202024 | Japan | Retrospective | 2018.4–2019.9 | 17 | 64.7 | 69 | Anti–PD-(L)1 | First∼ | 17.6 | 9.7 | 0 | 6 | 9 | 2 | 0 | 3 | PD |
| Furuya et al., 202125 | Japan | Retrospective | 2018.4–2019.2 | 38 | NR | NR | Nivo/pemb | Second∼ | NR | NR | 0 | 8 | 16 | 11 | 3 | NR | irAE, PD |
| Takahara et al., 202226 | Japan | Retrospective | 2016.8–2021.7 | 24 | 66.7 | NR | Durva/pemb/nivo | NR | 45.8 | NR | NR | 4 | irAE, PD | ||||
| Xu et al., 202227 | People's Republic of China | Retrospective | 2018.12–2021.6 | 40 | 77.5 | NR | Anti–PD-1 | NR | NR | 5.7 | 0 | 14 | 19 | 7 | 0 | NR | PD |
| Study | Interval Time (Median mo) | ICI Rechallenge |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen(r) | Therapy Line | TPS ≥ 50% (%)a | PFSr (M) | Best Response |
G3/4 irAE | ||||||
| CR | PR | Stable Disease | PD | NR | |||||||
| Bernard et al., 201813 | 10 | Anti–PD-(L)1 | NR | NR | 35.4 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fujita et al., 201814 | NR | Pemb | NR | 50 | 3.1 | 0 | 1 | 4 | 6 | 1 | 0 |
| Niki et al., 201815 | 4.2 | Nivo/pemb | NR | NR | 2.7 | 0 | 3 | 2 | 6 | 0 | 0 |
| Santini et al., 201816 | NR | Anti–PD-(L)1/anti–CTLA4 | NR | NR | NR | NR | 8 | ||||
| Fujita et al., 201917 | NR | Atezo | Third∼ | NR | NR | 0 | 0 | 7 | 11 | 0 | 2 |
| Watanabe et al., 201918 | 6.5 | Atezo/nivo/pemb | NR | NR | 1.6 | 0 | 1 | 2 | 11 | 0 | 0 |
| Mouri et al., 201919 | NR | Nivo | NR | NR | 7.4 | 0 | 4 | 14 | 2 | 1 | 2 |
| Fujita et al., 202020 | NR | Nivo/pemb | NR | NR | 2.4 | 0 | 0 | 4 | 9 | 2 | 2 |
| Gobbini et al., 202021 | NR | Anti–PD-(L)1 | Second∼ | NR | 4.4 | 5 | 18 | 45 | 54 | 22 | 4 |
| Herbst et al., 202022 | NR | Pemb | NR | NR | NR | 0 | 6 | 5 | 2 | 1 | NR |
| Katayama et al., 202023 | 5.2 | Nivo/pemb/atezo | Fourth | NR | 2.7 | 0 | 1 | 14 | 18 | 2 | NR |
| Kitagawa et al., 202024 | NR | Anti–PD-(L)1 | Second∼ | NR | 4 | 0 | 1 | 9 | 7 | 0 | 2 |
| Furuya et al., 202125 | NR | Atezo | NR | NR | NR | 0 | 1 | 12 | 20 | 5 | NR |
| Takahara et al., 202226 | NR | Nivo/atezo/pemb | NR | NR | NR | 0 | 2 | 9 | 13 | 0 | 3 |
| Xu et al., 202227 | NR | Anti–PD-1 | NR | NR | 6.8 | 0 | 9 | 25 | 6 | 0 | NR |
Anti–PD-(L)1, immune checkpoint blockade targeting programmed cell death-(ligand)1; Atezo, atezolizumab; CR, complete response; Durva, durvalumab; G3/4 irAE, grade 3/4 immune-related adverse event; ICI, immune checkpoint inhibitor; Nivo, nivolumab; NR, not reported; PD, progressive disease; Pemb, pembrolizumab; PFSi, progression-free survival of initial ICI; PFSr, progression-free survival of ICI rechallenge; PR, partial response; Regimen(i), initial ICI regimen; Regimen(r), ICI rechallenge regimen; TPS, tumor proportion score.
Percentage of patients whose TPS is equals to or more than 50%.
Outcome Assessment and Statistical Analysis
The best response to treatment was accessed as complete response, partial response (PR), stable disease, progressive disease (PD), or not estimated according to the Response Evaluation Criteria in Solid Tumors version 1.1. The overall response rate (ORR) was defined as the percentage of complete response and PR obtained as best response, whereas the disease control rate (DCR) included the ORR and percentage of achieved stable disease. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. PFS of initial ICI was defined as the time from the start of initial ICI treatment to objective disease progression. PFS of ICI rechallenge was defined as the time from the date of rechallenge of ICI to the date of disease progression or death from any cause. Hazard ratio for PFS was estimated using Inverse Variance in RevMan version 5.4.
Fixed effect model was used in our meta-analysis when p value is greater than 0.1, I2 is less than 50% in tests for heterogeneity, otherwise random effect model was adopted. Each study in the fixed/random effect analysis was weighted based on its sample size. The pooled OR with 95% confidence interval (CI) was calculated to evaluate the safety and efficacy of ICI rechallenge in patients with NSCLC who received ICI treatment. Publication bias was evaluated using funnel plots. Differences of categorical data between groups were analyzed using Pearson’s chi-square test or Fisher’s exact test. All the statistical analyses were performed using RevMan version 5.4 (www.training.cochrane.org/), MedCalc software (https://www.medcalc.org), or SPSS version 22.0 (IBM Corp., Armonk, NY) software. A p value less than 0.05 was considered statistically significant.
Results
Eligible Studies and Quality Assessment
Our search retrieved a total of 372 publications from the databases of PubMed, EMBASE, and Cochrane Library. After excluding duplicates and screening titles of the studies, 182 articles were selected based on the relevance to the study topic. The study selection scheme is illustrated in Figure 1. In total, 15 retrospective studies were included in the meta-analysis after review of abstract and full article for final qualitative and quantitative analyses.
The Newcastle-Ottawa Quality Assessment Scale was used to evaluate quality of the included studies. All studies scored either 7 or 8. The quality assessment results for the individual studies are found in Supplementary Table 1. There was no evidence of publication bias in the funnel plots of irAE, ORR, DCR, or PFS among the included studies (Supplementary Fig. 1).
Characteristics of Studies Included in Meta-Analysis
A total of 442 patients who received ICI rechallenge after initial ICI treatment from the 15 eligible studies were included in the meta-analysis. The mean age of patients was 65.8 years, and 69% of the patients were male. The main clinical characteristics and outcomes, including grade 3/4 irAE, the best response to ICI, and PFS, are found in Table 1.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Among them, the discontinuation of initial ICI occurred in 131 patients owing to different grades of irAE, 251 patients owing to disease progression during ICI treatment, and 42 patients owing to clinical decision such as after a defined period or cycles of ICI treatment, and no informative reason of cessation was provided in 18 patients. Eight of 15 studies reported systemic therapy (docetaxel + ramucirumab, carboplatin + nanoparticle albumin-bound paclitaxel/pemetrexed, gemcitabine, etc.) or local therapy (radiation therapy or surgery) between initial ICI and ICI rechallenge.
Pooled Analysis of irAE in Initial ICI Treatment Versus ICI Rechallenge
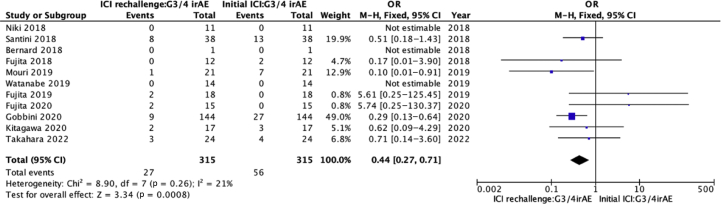
Of the 442 patients, information for grade 3/4 (or not) was reported for 315 patients with both initial ICI treatment and ICI rechallenge. Among them, initial ICI was discontinued in 131 patients owing to irAE. The incidence of grade 3/4 irAE was lower when ICI was rechallenged, compared with initial ICI (8.6% versus 17.8%, p = 0.001). The odds of grade 3/4 irAE occurrence was significantly lower in ICI rechallenge than initial ICI treatment (0.44, 95% CI: 0.27–0.71, p < 0.001) (Fig. 2).
Figure 2.
Pooled OR of G3/4 irAE in ICI rechallenge versus initial ICI. Lower OR represents lower incidence of irAE. CI, confidence interval; G3/4, grade 3/4; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; M-H, Mantel–Haenszel.
Among 79 patients with detailed irAE records, 32 patients who discontinued the initial ICI owing to irAE experienced grade 3/4 toxicity and only 4 of the 32 patients developed grade 3/4 irAE again during ICI rechallenge.19,21
Pooled Analysis of Therapeutic Responses and PFS in Initial ICI Treatment Versus ICI Rechallenge
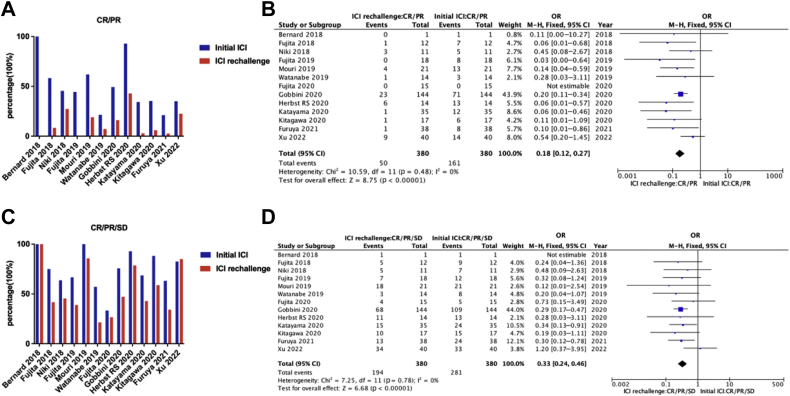
Among the 442 patients, information on therapeutic response in both initial ICI treatment and ICI rechallenge was available in 380 patients of 13 studies. Patients rechallenged with ICI were found to have a decreased ORR (13.2% versus 42.4%, p < 0.001) (Fig. 3A and B). DCR after ICI rechallenge reached 51.1%, although it is lower than the rate of 74.0% in the initial ICI treatment (p < 0.001) (Fig. 3C and D).
Figure 3.
Efficacy analysis of initial ICI versus ICI rechallenge. (A) Overall response rates of initial ICI versus ICI rechallenge in studies with related information. (B) Pooled OR of overall response (CR/PR) in ICI rechallenge versus initial ICI. Lower OR represents lower incidence of overall response. (C) Disease control rates of initial ICI versus ICI rechallenge in studies with related information. (D) Pooled OR of disease control (CR/PR/stable disease) in ICI rechallenge versus initial ICI. Lower OR represents lower disease control rate. CI, confidence interval; CR, complete response; ICI, immune checkpoint inhibitor; M-H, Mantel–Haenszel; PR, partial response.
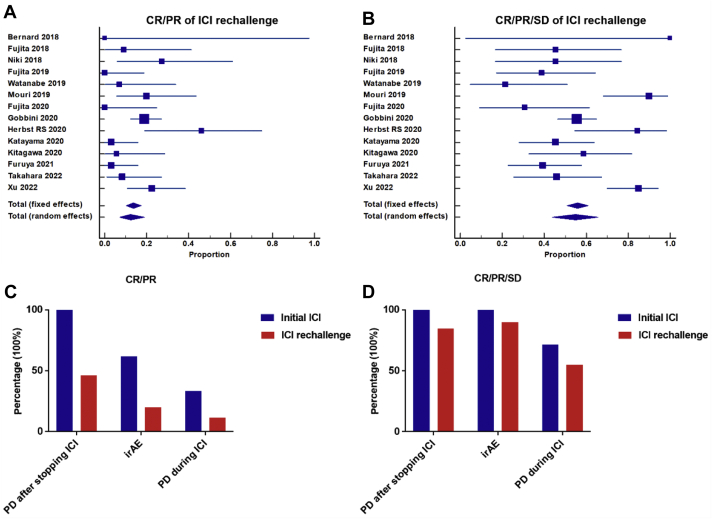
ORR and DCR of ICI rechallenge were analyzed using meta-analysis of proportion among 404 patients in 14 studies which provided exact therapeutic response of ICI rechallenge. The pooled ORR at ICI rechallenge was 12.4%. Nevertheless, the pooled DCR of ICI rechallenge reached 54.9% (Fig. 4A and B). Especially, the therapeutic response of ICI rechallenge was also compared among 10 studies with 180 patients who both had the specific discontinuation reasons of initial ICI (disease progression after stopping ICI therapy, irAE, and PD during initial ICI treatment) and their corresponding therapeutic response. The ORR and DCR of ICI rechallenge were both higher in patients who experienced disease progression after stopping ICI treatment or irAE than in patients with disease progression during ICI treatment (ORR: 46.2% versus 20% versus 11.4%, p = 0.003; DCR: 84.6% versus 90.0% versus 55.0%, p = 0.002) (Fig. 4C and D).
Figure 4.
Therapeutic responses of ICI rechallenge and efficacy comparison based on different initial ICI discontinuation reasons (PD after stopping ICI therapy versus irAE versus PD during ICI). (A) Pooled ORR (CR/PR) of ICI rechallenge in meta-analysis of proportion (12.4%, p = 0.0016, random effects). (B) Pooled DCR (CR/PR/stable disease) of ICI rechallenge in meta-analysis of proportion (54.9%, p < 0.0001, random effects). (C) ORR of initial ICI versus ICI rechallenge based on different initial ICI discontinuation reasons in 10 studies. The ORR of ICI rechallenge was higher in patients who experienced PD after stopping ICI treatment or irAE than in patients with PD during ICI treatment (ORR: 46.2% versus 20% versus 11.4%, p = 0.003). (D) DCR of initial ICI versus ICI rechallenge based on different initial ICI discontinuation reasons. The DCR of ICI rechallenge was higher in patients who experienced PD after stopping ICI treatment or irAE than in patients with PD during ICI treatment DCR: 84.6% versus 90.0% versus 55.0%, p = 0.002. CR, complete response; DCR, disease control rate; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; ORR, overall response rate; PD, progressive disease; PR, partial response.
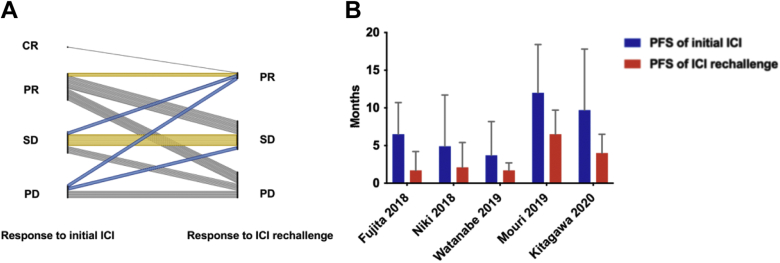
Furthermore, among 69 patients for whom survival information was available from 5 of the total 15 studies, 13% of the patients were found to have better therapeutic response with longer PFS in ICI rechallenge than in their initial ICI (median: 4.8 versus 2.5 mo, p < 0.001). In addition, 21.7% of the patients maintained the same response (PR, stable disease) in ICI rechallenge as in their initial ICI. Overall, the pooled PFS in initial ICI was longer than that in ICI rechallenge (median: 8.9 versus 3.7 mo; hazard ratio = 0.44, 95% CI: 0.33–0.59) (Fig. 5A and B and Supplementary Fig. 2).
Figure 5.
Therapeutic responses and PFS in initial ICI versus ICI rechallenge among 69 patients. (A) Therapeutic response change from initial ICI to ICI rechallenge. (B) The pooled PFS of initial ICI and ICI rechallenge. CR, complete response; ICI, immune checkpoint inhibitor; PD, progression disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Discussion
This is the first meta-analysis in which available data on the safety and efficacy of ICI rechallenge were estimated and rechallenge benefit among different reasons of initial ICI discontinuation was compared in NSCLC. This study revealed that ICI rechallenge was less effective but had a lower incidence of irAE than initial ICI. Patients with disease progression in treatment-free period after initial ICI and patients who experienced irAE in ICI had better therapeutic response to ICI rechallenge, compared with those undergoing PD during initial ICI treatment. Importantly, this study suggests that patients who experience irAE or disease progression after stopping ICI treatment may be potential candidates for successful rechallenge and achieving disease control.
Lung cancer accounts for the highest number of cancer-related deaths worldwide.28 ICI rechallenge could represent an attractive option in NSCLC, yet no systemic and conclusive analysis supporting this strategy is available. Overall, the current findings indicate that ICI rechallenge in patients with NSCLC is generally safe. Recurrent or new irAE after ICI retreatment seems mild and manageable in comparison with initial immune checkpoint therapy.29,30 On the basis of the durable response in previous ICI treatment, patients with disease progression in treatment-free period are supposed to have a more favorable efficacy at ICI rechallenge. Consistently, the present pooled study revealed that patients with disease progression in treatment-free period after ICI treatment had the best therapeutic efficacy of ICI rechallenge.
Clinical irAE, which was associated with the immunotherapeutic efficacy, may represent a clinical biomarker for ICI response.31 The mechanism of irAE may reflect the bystander effect from activated T cells, and patients responding to ICIs may have greater likelihood of autoimmune toxicities owing to a more competent/treatment-responsive immune system or cross-reactivity between the tumor and host tissue.32 Interestingly, our pooled analysis found that patients with irAE at initial ICI treatment had high therapeutic efficacy at ICI rechallenge among different reasons of initial ICI discontinuation (Fig. 4). Nevertheless, the ORR of ICI rechallenge in our NSCLC study is still lower than that in the pan-cancer studies.30,33
Although recent studies have begun to evaluate the clinical outcomes of ICI rechallenge in patients with cancer who had previously discontinued ICI treatment, the uncertain risks and benefits of ICI retreatment may impede the decision to resume ICI as an alternative therapy option in the clinical settings.34 In the studies included in the present meta-analysis, the switch from PD-1/PD-L1 to PD-L1/PD-1 at disease progression that occurred during ICI treatment revealed limited clinical efficacy.14,17,20,23,24 Theoretically, a switch from anti–PD-(L)1 to anti–CTLA-4 therapy or vice versa may be reasonable. CTLA-4 inhibition works by increasing the diversity of the antitumor immune response in the lymph node and perhaps by depleting highly CTLA-4–expressing regulatory T cells in the tumor microenvironment, whereas PD-1/PD-L1 blockade mostly works at the tumor site by locally reactivating the exhausted tumor-infiltrating lymphocytes.35 Outcomes of ICI rechallenge have also been reported in patients with melanoma.36,37 Nivolumab and pembrolizumab in ipilimumab-refractory patients were found to have an ORR of 20% to 30%, although response rates were lower with anti–PD-1 rechallenge after prior anti–PD-1. Owing to these nonoverlapping mechanisms, treatment sequencing from one class to the other or their combination may be feasible and beneficial for patients.
Although this study is the first meta-analysis published to date analyzing the safety and efficacy of ICI rechallenge after initial ICI treatment in NSCLC, there were some limitations to this study. First, the meta-analysis was based on retrospective studies, which have their inherent biases. For example, recurrent or new irAE after ICI retreatment seems mild and manageable in comparison with initial ICI, but physicians seemed to select the patients for rechallenging of ICI more safely, that is, selection bias. Larger scale prospective studies are warranted to validate the findings of this study. Second, there were insufficient raw data to conduct meta-analysis for long-term survival after ICI rechallenge. Many studies lacked mature PFS data necessary for meta-analysis. Third, ideally, the safety and efficacy of ICI rechallenge should be compared with second- or further-line docetaxel or pemetrexed, but this was not possible, as the data on the safety and efficacy of ICI rechallenge were compared with those of initial ICI in the previous studies included in this meta-analysis.
To conclude, ICI rechallenge should be considered on an individual scenario. Rechallenge with ICI is a reasonable therapeutic option for those who underwent disease progression after stopping ICI treatment or who discontinued treatment owing to toxicity. Additional studies are needed to better understand the molecular characteristics of responding patients.
CRediT Authorship Contribution Statement
Takehito Shukuya: Conceptualization.
Shiting Xu, Takehito Shukuya: Methodology.
Shiting Xu, Jun Tamura: Software.
Jun Tamura, Kouji Yamamoto: Data curation.
Shiting Xu: Writing - original draft preparation.
Takehito Shukuya, Shiting Xu: Formal analysis, Visualization.
Kazuhisa Takahashi, Takehito Shukuya: Supervision.
Kouji Yamamoto: Validation.
Shoko Shimamura, Kana Kurokawa, Keita Miura, Taichi Miyawaki, Daisuke Hayakawa, Tetsuhiko Asao, Kazuhisa Takahashi: Writing - review & editing.
Acknowledgments
Dr. Xu received Japan-China Sasakawa Medical Fellowship from the Sasakawa Memorial Health Foundation.
Footnotes
Disclosure: Dr. Shukuya received grants from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, MSD and honoraria from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, MSD, Taiho Pharma, Daiichi-Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Kayaku, Pfizer outside of the submitted work. Dr. Miura received honoraria from Chugai Pharmaceutical and Taiho Pharmaceutical outside of the submitted work. Dr. Asao received honoraria from AstraZeneca, Eli Lilly Japan, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD, Ono Pharmaceutical and Takeda Pharmaceutical outside of the submitted work. Dr. Yamamoto received grants from Chugai Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Takeda Pharmaceutical, Bayer Yakuhin, Daiichi-Sankyo, Astellas, Kyowa Kirin and honoraria from Chugai Pharmaceutical, Otsuka Pharmaceutical, CMIC holdings, J-Pharma, Craif, Johokiko, Triceps and Kanagawa Prefectural Hospital Organization outside of the submitted work. Dr. Takahashi received grants from Chugai Pharmaceutical, Nippon Boehringer Ingelheim, MSD, Glaxo SmithKline Consumer Healthcare Japan, NIPPON SHINYAKU, TSUMURA & CO, Pfizer Inc, Taiho Pharmaceutical, Daiichi-Sankyo, Astellas Pharma, KYORIN Pharmaceutical, KYOWA Hakko Kirin, TEIJIN PHARMA LIMITED, Sanofi, Ono Pharumaceutical, Shionogi & Co., Ltd, Novartis Pharma, Eli Lilly Japan, Actelion Pharmaceuticals Japan, NIPRO PHARMA CORPORATION, Takeda Pharmaceutical Company Limited, Bayer Yakuhin, Torii Pharmaceutical and honoraria from Chugai Pharmaceutical, Nippon Boehringer Ingelheim, MSD, Pfizer, AstraZeneca, Taiho Pharumaceutical, KYORIN Pharmaceutical, Ono Pharumaceutical, Bristol-Myers Squibb Company, Novartis Pharma, Eli Lilly Japan, Meiji Seika Pharma and Abbott Japan LLC outside of the submitted work. Drs. Xu, Tamura, Shimamura, Kurokawa, Miyawaki and Hayakawa declared no conflict of interest.
Cite this article as: Xu S, Shukuya T, Tamura J, et al. Heterogeneous outcomes of immune checkpoint inhibitor rechallenge in patients with NSCLC: a systematic review and meta-analysis. JTO Clin Res Rep. 2022;3:100309.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100309.
Supplementary Data
References
- 1.Sharma P., Allison J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20:75–76. doi: 10.1038/s41577-020-0275-8. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger D.S., Wood D.E., Aisner D.L., et al. NCCN Guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19:254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 5.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 8.Dolladille C., Ederhy S., Sassier M., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giaccone G., Felip E., Cobo M., et al. 120MO Activity of OSE-2101 in HLA-A2+ non-small cell lung cancer (NSCLC) patients after failure to immune checkpoint inhibitors (ICI): Step 1 results of phase III ATALANTE-1 randomised trial. Ann Oncol. 2020;31(suppl):S814–S815. [Google Scholar]
- 10.Leal T.A., Berz D., Rybkin I., et al. 1191O MRTX-500: phase II trial of sitravatinib (sitra) + nivolumab (nivo) in patients (pts) with non-squamous (NSQ) non-small cell lung cancer (NSCLC) progressing on or after prior checkpoint inhibitor (CPI) therapy. Ann Oncol. 2021;32(suppl):S949. [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G.A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed January 20, 2022.
- 13.Bernard-Tessier A., Baldini C., Martin P., et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer. 2018;101:160–164. doi: 10.1016/j.ejca.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Fujita K., Uchida N., Kanai O., Okamura M., Nakatani K., Mio T. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother Pharmacol. 2018;81:1105–1109. doi: 10.1007/s00280-018-3585-9. [DOI] [PubMed] [Google Scholar]
- 15.Niki M., Nakaya A., Kurata T., et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. 2018;9:32298–32304. doi: 10.18632/oncotarget.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini F.C., Rizvi H., Plodkowski A.J., et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6:1093–1099. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K., Uchida N., Yamamoto Y., et al. Retreatment with anti-PD-L1 antibody in advanced non-small cell lung cancer previously treated with anti-PD-1 antibodies. Anticancer Res. 2019;39:3917–3921. doi: 10.21873/anticanres.13543. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H., Kubo T., Ninomiya K., et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol. 2019;49:762–765. doi: 10.1093/jjco/hyz066. [DOI] [PubMed] [Google Scholar]
- 19.Mouri A., Kaira K., Yamaguchi O., et al. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol. 2019;84:873–880. doi: 10.1007/s00280-019-03926-y. [DOI] [PubMed] [Google Scholar]
- 20.Fujita K., Yamamoto Y., Kanai O., et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer. 2020;11:15–18. doi: 10.1111/1759-7714.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbini E., Toffart A.C., Pérol M., et al. Immune checkpoint inhibitors rechallenge efficacy in non-small-cell lung cancer patients. Clin Lung Cancer. 2020;21:e497–e510. doi: 10.1016/j.cllc.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Herbst R.S., Garon E.B., Kim D.W., et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced nonsmall-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38:1580–1590. doi: 10.1200/JCO.19.02446. [DOI] [PubMed] [Google Scholar]
- 23.Katayama Y., Shimamoto T., Yamada T., et al. Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med. 2019;9:102. doi: 10.3390/jcm9010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa S., Hakozaki T., Kitadai R., Hosomi Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: case series and literature review. Thorac Cancer. 2020;11:1927–1933. doi: 10.1111/1759-7714.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuya N., Nishino M., Wakuda K., et al. Real-world efficacy of atezolizumab in non-small cell lung cancer: a multicenter cohort study focused on performance status and retreatment after failure of anti-PD-1 antibody. Thorac Cancer. 2021;12:613–618. doi: 10.1111/1759-7714.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahara Y., Tanaka T., Ishige Y., et al. Efficacy and predictors of rechallenge with immune checkpoint inhibitors in non-small cell lung cancer. Thorac Cancer. 2022;13:624–630. doi: 10.1111/1759-7714.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Hao X, Yang K, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study [e-pub ahead of print]. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-021-03901-2. Accessed January 20, 2022. [DOI] [PMC free article] [PubMed]
- 28.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 29.Simonaggio A., Michot J.M., Voisin A.L., et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310–1317. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q., Zhang J., Xu L., et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol. 2021;12:730320. doi: 10.3389/fimmu.2021.730320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricciuti B., Genova C., De Giglio A., et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
- 32.Das S., Johnson D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inno A., Roviello G., Ghidini A., et al. Rechallenge of immune checkpoint inhibitors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;165:103434. doi: 10.1016/j.critrevonc.2021.103434. [DOI] [PubMed] [Google Scholar]
- 34.Haanen J., Ernstoff M., Wang Y., et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 36.Ribas A., Puzanov I., Dummer R., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber J.S., D’Angelo S.P., Minor D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.