Key Points
Question
Is cabozantinib as monotherapy effective and tolerated in patients with untreated metastatic collecting duct carcinoma?
Findings
This phase 2, single-arm clinical trial of 25 patients met its primary end point showing objective response rate of 35% per Response Evaluation Criteria in Solid Tumors (version 1.1) in metastatic collecting duct carcinoma.
Meaning
Cabozantinib may be considered a therapeutic option in first-line treatment for patients with metastatic collecting duct carcinoma.
This preliminary analysis of data from the BONSAI clinical trial examines the safety and efficacy of cabozantinib as monotherapy in patients with untreated metastatic collecting duct carcinoma.
Abstract
Importance
Metastatic collecting duct carcinoma (mCDC) is a rare type of non–clear cell renal cell carcinoma (ncRCC) with poor prognosis and no standard treatments. Despite retrospective series that have documented the benefit of cabozantinib in ncRCC, no prospective trials have evaluated this treatment in mCDC.
Objective
To determine whether cabozantinib is an active treatment in patients with mCDC.
Design, Setting, and Participants
The caBozantinib in cOllectiNg ductS Renal Cell cArcInoma (BONSAI) trial was an open-label, single-arm, phase 2 clinical trial carried out between January 2018 and November 2020 at a single academic center with data cut off in September 2021 on behalf of the the Italian Network for Research in Urologic-Oncology (Meet-URO 2). Eligible patients had histologic diagnosis of centrally confirmed mCDC with measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). In total, 25 patients were screened.
Interventions
Patients received cabozantinib, 60 mg orally once daily, until disease progression, unacceptable toxic effects, or withdrawal of consent.
Main Outcomes and Measures
The primary end point was objective response rate (ORR) per RECIST, version 1.1.
Results
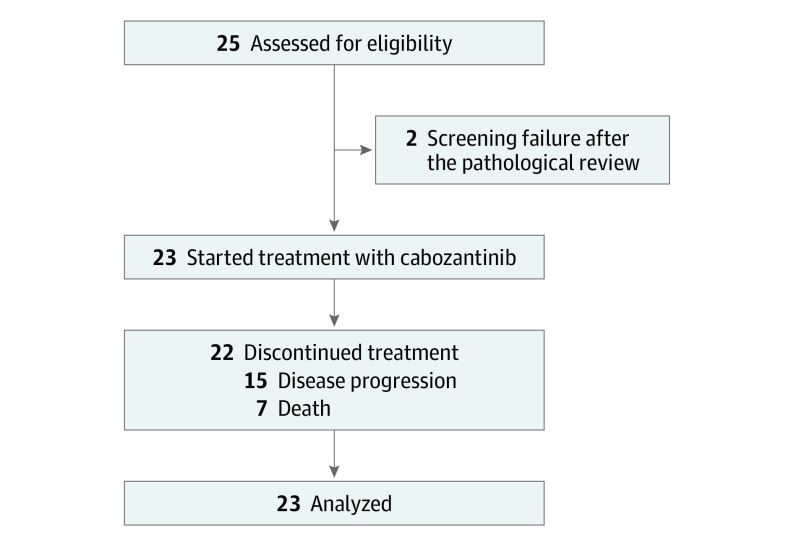
At data cut off, of 25 patients enrolled, 23 started treatment because 2 were excluded after failing the screening process at pathologic review. The median follow-up cannot be estimated using the reverse Kaplan-Meier estimator. The median time to censoring was 11 months (95% CI, 0-22 months). Median (range) age was 66 (53-74) years. As best overall response, 3 patients presented stable disease, 1 patient achieved a complete response, and 7 a partial response. The ORR was 35% (95% CI, 16%-57%). The median progression-free survival was 4 months (95% CI, 3-13 months). The median OS was 7 months (95% CI, 3-31 months). All patients reported at least 1 grade (G) 1 to 2 adverse event (AE). The most common G1 to G2 AEs were fatigue (14 [60%]), anorexia (9 [39%]), hand-foot syndrome (7 [30%]), hypothyroidism (7 [30%]), mucositis (7 [30%]), diarrhea (5 [22%]), and hypertension (3 [13%]). Six G3 AEs were reported: 2 arterial hyperthension, 1 pulmonary thromboembolism, 1 bleeding, and 2 fatigue. There were no permanent discontinuations from the study owing to AEs. Four patients (17%) required dose reduction to 40 mg, and 4 (17%) required a transitory interruption to manage toxic effects.
Conclusions and Relevance
The study met the ORR primary end point, showing encouraging efficacy of cabozantinib in untreated patients with mCDC. Further investigations to advance the molecular understanding of this tumor are ongoing.
Trial Registration
ClinicalTrials.gov Identifier: NCT03354884
Introduction
Collecting duct carcinoma (CDC) is a rare type of non–clear cell renal cell carcinoma (RCC) with poor prognosis and no standard treatment.1 To date, the treatment of metastatic disease remains unsatisfactory because evidence from randomized histology-specific trials is lacking. Cabozantinib is an inhibitor of multiple tyrosine kinase receptors approved in RCC based on the results of 2 prospective trials limited to a population with clear cell RCC.2,3 Despite case reports and retrospective series documenting encouraging activity of cabozantinib in non–clear cell RCC, no prospective trials have evaluated this treatment in patients with metastatic CDC (mCDC).4,5 Herein, we report the primary efficacy and safety results of the prospective phase 2 caBozantinib in cOllectiNg ductS Renal Cell cArcInoma (BONSAI) trial of cabozantinib in patients with untreated mCDC.
Methods
The BONSAI trial was an open-label, single-arm phase 2 clinical trial conducted at Istituto Nazionale dei Tumori (Milan, Italy). The trial protocol is available in Supplement 1.
Eligible patients had histologic confirmation of mCDC and must not have received previous treatment. Further inclusion criteria were Eastern Cooperative Oncology Group performance status of 2 or lower and measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Pathologic review by a genitourinary pathologist was required to confirm histologic results before starting therapy.
Cabozantinib was administered orally at the dose of 60 mg once daily until disease progression, unacceptable toxic effects, or withdrawal of consent.
The primary end point was objective response rate (ORR) defined as the proportion of patients who had as their best overall response a complete (CR) or partial response (PR) according to RECIST, version 1.1, by investigator assessment (G.P., P.S., and an outside radiologist). Secondary end points were progression-free survival (PFS), defined as time from first study dose to first documentation of disease progression or death; overall survival (OS), defined as time from first study dose to death from any cause; safety; and tolerability.
The trial was conducted on behalf of the Italian Network For Research In Urologic-Oncology (Meet-URO) and in accordance with the International Council for Harmonisation Good Clinical Practice Guidelines and Declaration of Helsinki. Institutional review boards or independent ethics committees approved the study. All patients provided written informed consent before study procedures.
The study design was based on a Simon’s 2-stage optimal design: to reject an ORR equal to 15% with a 1-sided alpha error of 10%, and to detect an ORR equal to 35% with a power of 80%. At least 2 responses in 9 patients were needed to proceed to the second stage, where at least 6 responses out of a total of 23 patients were needed to prove activity of cabozantinib.
Results
From January 1, 2018, to November 27, 2020, the study enrolled 25 patients with previously untreated mCDC. Two patients never started the therapy owing to screening failure after the pathologic review. Twenty-three patients started treatment and were included in the analysis (Figure 1). Baseline characteristics are summarized in the Table.
Figure 1. Patient Disposition.
Table. Baseline Characteristics of Patients.
| Characteristic | No. (%) |
|---|---|
| Total | 23 |
| Age, median (range), y | 66 (53-74) |
| Sex | |
| Male | 19 (83) |
| Female | 4 (17) |
| Nephrectomy | 19 (83) |
| No. of metastatic sites | |
| 1 | 9 (39) |
| 2 | 8 (35) |
| >2 | 6 (26) |
| Disease locations | |
| Nodes | 15 (65) |
| Bone | 13 (56) |
| Lung | 10 (43) |
| Liver | 4 (17) |
At data cutoff (September 21, 2021), the median follow-up cannot be estimated using the reverse Kaplan-Meier estimator. Four patients remained in study follow-up (1 patient continued receiving cabozantinib), 18 patients (78%) died, and 1 patient was lost to follow-up. The median time to censoring was 11 months (95% CI, 0-22 months).
Overall, 22 patients were evaluable for radiological response. One patient did not receive a restaging owing to rapid clinical progression. As best overall response, 3 patients presented stable disease, 1 patient achieved a confirmed CR and 7 a PR with an ORR of 35%; (95% CI, 16%-57%). The median (IQR) time to ORR was 2.5 (2-6) months. Moreover, 11 patients exhibited progressive disease as best response. For the secondary end point of PFS, there were 22 of 23 patients (97%) with progression events or death. The median PFS was 4 months (95% CI, 3-13 months). The median OS was 7 months (95% CI, 3-31 months) (Figure 2). At the 12-month time point, 10 patients (43%) were alive and 5 patients (23%) were in ongoing treatment.
Figure 2. Kaplan-Meier Curves .
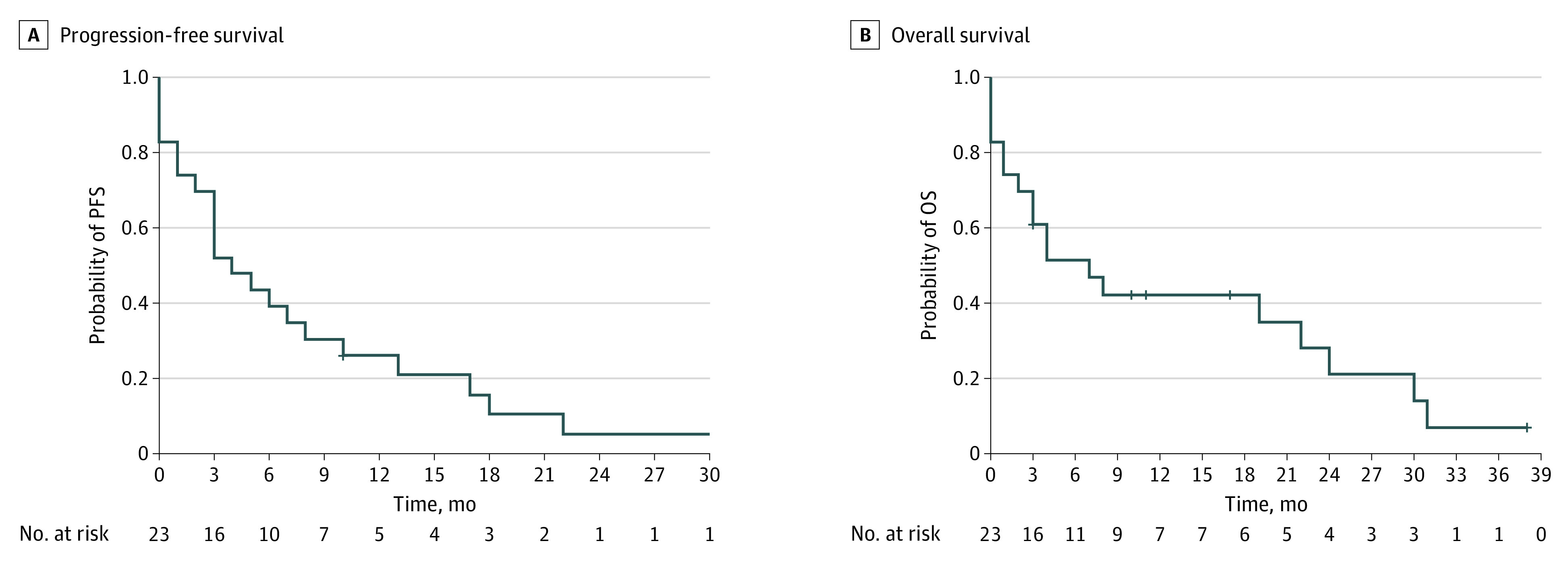
A, Progression-free survival (PFS). B, Overall survival (OS).
The most common therapies received by patients who discontinued the study drug for progressive disease were nivolumab (5 [23%]), cisplatin plus gemcitabine chemotherapy (1 [4%]), and supportive care (16 [73%]).
All patients reported at least 1 grade (G) 1 to 2 adverse event (AE). The most common G1 to G2 AEs were fatigue (14 [60%]), anorexia (9 [39%]), hand-foot syndrome (7 [30%]), hypothyroidism (7 [30%]), mucositis (7 [30%]), diarrhea (5 [22%]) and hypertension (3 [13%]). Six G3 AEs were reported: 2 arterial hypertension, 1 pulmonary thromboembolism, 1 bleeding, 2 fatigue. There were no permanent discontinuations owing to AEs. Four patients (17%) required dose reduction to 40 mg, and 4 (17%) a transitory interruption to manage toxic effects.
Discussion
Although the treatment landscape of clear cell RCC has evolved dramatically over the past 10 years,6,7,8,9,10 CDC was excluded from large randomized, prospective trials owing to its rarity and poor prognosis, and it still represents an orphan disease. Although expected outcomes are poor, platinum-based chemotherapy is suggested by international guidelines as a first-line option given the biological similarity between CDC and urothelial tumors,11 whereas the incorporation of novel agents in the therapeutic algorithm of mCDC is mostly based on retrospective series and case reports.4,5,12,13 The BONSAI trial is the first prospective trial evaluating the efficacy of the multitargeted oral tyrosine kinase inhibitor cabozantinib in untreated patients with mCDC. The study met the primary end point, reaching a noticeable ORR of 35%, while showing a manageable safety profile. Placed in the context of the little prospective evidence available, the results of the BONSAI trial are promising. In a multicenter, phase 2 trial14 that enrolled 23 patients with treatment-naive mCDC, first-line chemotherapy with carboplatin/cisplatin plus gemcitabine showed an ORR of 26%, a median PFS of 7.1 months, and a median OS of 10.5 months. Grade 3 to 4 neutropenia and thrombocytopenia were reported in 52% and 43% of patients, respectively. A subsequent multicenter, phase 2 trial published in 201815 investigated the addition of the multikinase inhibitor sorafenib to first-line cisplatin plus gemcitabine in an Asian population of 26 patients with mCDC. The biochemotherapy regimen showed an ORR of 30.8%, a median PFS of 8.8 months, and a median OS of 12.5 months. Grade 3 to 4 leukopenia, thrombocytopenia, and anemia were reported in 26.9%, 23.1%, and 11.5% of patients, whereas palmar-plantar erythrodysesthesia was reported in 7.7% of patients. Considering all the limitations and the caution in the interpretation of an indirect comparison, the cabozantinib monotherapy investigated in the BONSAI trial appeared to be more active than platinum-gemcitabine chemotherapy for the first-line treatment of mCDC, and resulted in similar antitumor activity compared with the combination of platinum-based chemotherapy plus sorafenib. Of note, the incidence of grade 3 or higher AEs in the BONSAI trial was lower than previously observed for platinum-gemcitabine with or withouit sorafenib, and no grade 4 or higher AEs were reported. Concerning survival outcomes, median PFS and OS of cabozantinib monotherapy appeared similar to those previously reported for platinum-gemcitabine chemotherapy, and slightly inferior to those observed with platinum-gemcitabine plus sorafenib, even if the 12-month OS rate was comparable (43% in the BONSAI trial vs 54% with platinum-gemcitabine plus sorafenib). However, the 3 phase 2 trials were not designed to properly evaluate PFS and/or OS, thus preventing any conclusion in this regard.
Limitations
The limited number of patients in this study make definitive conclusions on safety and efficacy difficult to draw.
Conclusions
The BONSAI trial met its ORR primary end point, suggesting that cabozantinib may be considered as a therapeutic option for the first-line treatment of patients with mCDC.
Trial Protocol
Data Sharing Statement
References
- 1.Pagani F, Colecchia M, Sepe P, et al. Collecting ducts carcinoma: an orphan disease. Literature overview and future perspectives. Cancer Treat Rev. 2019;79:101891. doi: 10.1016/j.ctrv.2019.101891 [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Escudier B, Powles T, et al. ; METEOR investigators . Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917-927. doi: 10.1016/S1470-2045(16)30107-3 [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591-597. doi: 10.1200/JCO.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mennitto A, Verzoni E, Peverelli G, Alessi A, Procopio G. Management of metastatic collecting duct carcinoma: an encouraging result in a patient treated with cabozantinib. Clin Genitourin Cancer. 2018;16(3):e521-e523. doi: 10.1016/j.clgc.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Procopio G, Verzoni E, Iacovelli R, Colecchia M, Torelli T, Mariani L. Is there a role for targeted therapies in the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective analysis of 7 cases. Clin Exp Nephrol. 2012;16(3):464-467. doi: 10.1007/s10157-012-0589-3 [DOI] [PubMed] [Google Scholar]
- 6.Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi: 10.1136/esmoopen-2020-001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch L, Martinez Chanza N, Farah S, et al. Clinical activity and safety of cabozantinib for brain metastases in patients with renal cell carcinoma. JAMA Oncol. 2021;7(12):1815-1823. doi: 10.1001/jamaoncol.2021.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators . Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Powles T, Burotto M, et al. ; CheckMate 9ER Investigators . Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi: 10.1056/NEJMoa2026982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer R, Alekseev B, Rha SY, et al. ; CLEAR Trial Investigators . Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi: 10.1056/NEJMoa2035716 [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Porta C, Schmidinger M, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706-720. doi: 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 12.Pécuchet N, Bigot F, Gachet J, et al. Triple combination of bevacizumab, gemcitabine and platinum salt in metastatic collecting duct carcinoma. Ann Oncol. 2013;24(12):2963-2967. doi: 10.1093/annonc/mdt423 [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol. 2017;71(3):426-436. doi: 10.1016/j.eururo.2016.11.020 [DOI] [PubMed] [Google Scholar]
- 14.Oudard S, Banu E, Vieillefond A, et al. ; GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) . Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) study. J Urol. 2007;177(5):1698-1702. doi: 10.1016/j.juro.2007.01.063 [DOI] [PubMed] [Google Scholar]
- 15.Sheng X, Cao D, Yuan J, et al. Sorafenib in combination with gemcitabine plus cisplatin chemotherapy in metastatic renal collecting duct carcinoma: a prospective, multicentre, single-arm, phase 2 study. Eur J Cancer. 2018;100:1-7. doi: 10.1016/j.ejca.2018.04.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement