Abstract
Indinavir concentrations were determined in plasma and saliva over a random period of 4 h. On average, levels in saliva were 70% ± 38% of the corresponding levels in plasma. These findings suggest that saliva might serve as an appropriate specimen for monitoring of plasma indinavir levels in patients treated with indinavir.
The protease inhibitor indinavir (IDV) has been shown to be an important component of triple-drug regimens for the treatment of human immunodeficiency virus (HIV) infection and to have excellent efficacy (6, 7). However, its use may be associated with severe side effects such as the development of lipodystrophy (4) or renal calculi (8).
Pharmacokinetic studies of IDV have revealed considerable interindividual differences in such parameters as peak concentration or area under the concentration-time curve (AUC) (9, 12). To date, there is preliminary evidence that trough levels of indinavir in plasma correlate with a reduction in the viral load (1, 9, 12). However, an efficient and safe therapeutic range or desirable target levels are still under debate.
To establish such target levels, large studies with frequent blood sampling are required. These are cumbersome to perform and costly and necessitate the attendance of medical staff. In contrast, monitoring of IDV concentrations in saliva would have obvious advantages: sample collection is easy and inexpensive and can be carried out by the patient, even at home. The use of saliva samples would facilitate large-scale studies on the relation of drug levels to efficacy. Moreover, the risk of HIV transmission to the medical staff and discomfort for the patients would be minimized. The goal of our study was thus to evaluate the feasibility of using saliva for determination of IDV concentrations.
Patients and methods.
Ten asymptomatic HIV-infected male outpatients currently treated with antiretroviral combination therapies that included IDV were asked to participate in the study. None of the patients suffered from acute parotitis. The mean CD4 cell count was 337 ± 257/μl, and the patients' disease classifications according to the Centers for Disease Control and Prevention were 1xB3, 1xC1, and 8xC3. The mean age of the patients was 42 ± 9 years, and the mean weight was 71 ± 3.7 kg. Antiretroviral treatment was as follows: 7 patients received 800 mg of IDV three times a day (t.i.d.) in combination either with stavudine and lamivudine or with zidovudine and lamivudine; one patient received 1,200 mg of IDV t.i.d. with nevirapine and lamivudine; one patient received 300 mg of IDV two times a day (b.i.d.) with ritonavir, stavudine, didanosine, efavirenz, and hydroxyurea; and one patient received 400 mg of IDV b.i.d. with ritonavir, zidovudine, lamivudine, and efavirenz.
Blood and simultaneous saliva samples were collected every 60 min over a period of 4 h during a routine visit to the outpatient department. To avoid interruption of the therapeutic schedule, patients were advised to adhere to their routine drug regimen and to record the time of the last drug intake. Thus, the time lag between the last dose and the first sampling ranged from 0 to 6 h, and drug intake was not under observation for most patients. For all patients except those also taking ritonavir, IDV was administered while the patients were in the fasted state (1 h before or 2 h after a meal).
Blood samples (2 ml placed in tubes containing EDTA) were drawn with a peripheral indwelling catheter. Saliva was collected by having the patient spit into a collection tube. Blood and saliva samples were centrifuged within 2 h to remove cellular elements or mucous, and supernatants were stored at −80°C until IDV concentrations were measured.
The concentrations of IDV were determined by liquid chromatography-tandem mass spectrometry. Plasma and saliva samples were diluted 1:3 with ammonia phosphate buffer (0.1 M). N-Ethyl-diazepam was added as an internal standard, and online extraction was performed on an activated diol silica column (ADS-6; Merck, Darmstadt, Germany). An Eurospher RP-18 (30 mm) column was used as a filter between the LC-system (Merck-Hitachi, Darmstadt, Germany) and the mass spectrometry interface (API 365; PE-SCIEX, Langen, Germany). The lower limit of detection for both plasma and saliva was 0.5 ng/ml, the intra-assay variability was 2.8%, and the interassay variability was 6.5% (at 100 and 5,000 ng/ml). The method was linear between 20 and 20,000 ng/ml (M. Kurowski, M. Mueller, K. Arasteh, and C. Moecklinghoff, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 320, p. 13, 1999).
The maximum concentration of drug (Cmax) was determined by visual inspection of the time-concentration curve. The AUC was determined by the log-trapezoidal method. Values were interpreted by linear regression analysis and with the plots of Bland and Altman (3). IDV and reference material were kind gifts of MSD, Munich, Germany. The internal standard was purchased from Bio-Rad (Hercules, Calif.).
Results.
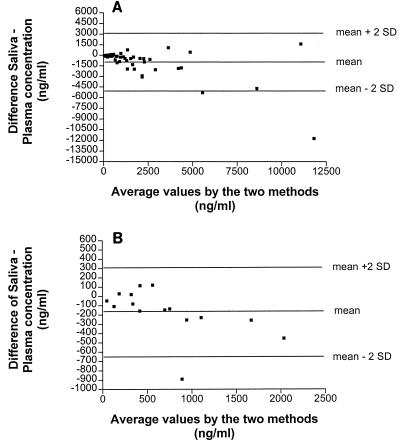
Forty-eight plasma IDV concentrations for 10 patients were related to the corresponding concentrations in saliva. The mean ± standard deviation (SD) Cmaxs in plasma and saliva were 5,074 ± 5,168 and 3,340 ± 3,558 ng/ml, respectively. The mean AUCs for plasma and saliva were 9,196 ± 10,452 and 5,432 ± 5,872 ng · h/ml (62% ± 26% of that for plasma), respectively. On average, the IDV concentrations in saliva were 70% ± 38% of the corresponding levels in plasma. Linear regression analysis (Fig. 1) yielded correlation coefficients of 0.80 (P < 0.0001) for single values and 0.82 (P = 0.0035) for the respective AUCs. However, analysis of data for different times after the last intake of the drug revealed that the best correlation between concentrations in plasma and saliva was for samples collected more than 5 h after dosing (Table 1; Fig. 1). To better evaluate the suitability of saliva as a substitute for determination of the IDV concentration in plasma, data were plotted as described by Bland and Altman (3) (Fig. 2A and B). Descriptive analysis of the plots shows that the differences in the concentrations in saliva and plasma tend to increase with increases in the concentrations in plasma, indicating a closer relation between the concentrations in saliva and plasma at lower concentrations, which occurs at the end of the dosing interval.
FIG. 1.
Scattergrams of IDV concentrations in plasma versus those in saliva. (A) Whole sampling time (n = 48). (B) Sampling time from >5 to 10 h after intake (n = 15).
TABLE 1.
Evaluation of the ratio of the concentration in saliva/concentration in plasma and linear regression analysis for the different time periods after intake of IDV
| Sampling time (h) | No. of samples | Ratio of concn in saliva/concn in plasma (%) | r value | P value |
|---|---|---|---|---|
| Overall | 48 | 70 ± 38 | 0.80 | <0.0001 |
| 0–2 | 14 | 54 ± 28 | 0.78 | 0.0005 |
| >2–5 | 19 | 71 ± 42 | 0.69 | 0.0030 |
| >5–10 | 15 | 81 ± 29 | 0.93 | <0.0001 |
FIG. 2.
Bland-Altman (3) plots of the difference in concentration in saliva and concentration in plasma versus the mean concentrations in saliva and plasma. (A) Whole sampling time (n = 48). (B) Sampling time from >5 to 10 h after intake (n = 15).
Discussion.
The feasibility of using saliva to monitor drug concentrations has been clearly demonstrated for a variety of drugs, e.g., phenytoin, zidovudine, and theophylline (2, 5, 10, 11).
The present study demonstrates that IDV enters the salivary compartment. On average, IDV concentrations in saliva were lower than those in plasma but higher than the assumed unbound fraction of the drug in plasma (approximately 40%) (13). Whether protein binding in saliva or blood-saliva barrier-related mechanisms contribute to this finding cannot be concluded from our data.
In general, there was a good agreement between the IDV levels in plasma and saliva. This was particularly true at the end of the sampling period suggesting a slow equilibration between the blood and the salivary gland compartment. Prediction of the levels in plasma shortly after drug intake seems to be less reliable. However, there is evidence in the literature that the AUC or trough levels of IDV are associated with antiretroviral efficacy (1, 12). Determination of the AUC, however, necessitates repeated blood sampling over prolonged periods of time, which is difficult to perform in an outpatient setting. Therefore, determination of the trough concentration is more convenient. As it is considered important to keep trough levels above approximately 200 ng/ml (1, 12), the small difference between the concentration in saliva and the concentrations in plasma (Bland-Altman plot [3]) at concentrations below 500 ng/ml (a difference of about 100 ng/ml) for samples gathered 5 to 10 h after IDV administration indicate that saliva might be a substitute with which monitoring of trough concentrations in plasma is easy to perform. Furthermore, determination of IDV concentrations in saliva could provide an easy method for determination of patient adherence to the treatment regimen.
Acknowledgments
We are indebted to the patients who participated in this study. We thank C. Moser-Juenemann (practice of E. Jaegel-Guedes and H. Jaeger) for excellent cooperation.
REFERENCES
- 1.Acosta E P, Henry K, Baken L, Page L M, Fletcher C V. Indinavir concentrations and antiviral effect. Pharmacotherapy. 1999;19:708–712. doi: 10.1592/phco.19.9.708.31544. [DOI] [PubMed] [Google Scholar]
- 2.Aviram M, Tal A, Ben-Zvi Z, Gorodischer R. Monitoring theophylline therapy using citric acid-stimulated saliva in infants and children with asthma. Pediatrics. 1987;80:894–897. [PubMed] [Google Scholar]
- 3.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Thorisdottir A, Kaufmann G R, Chisholm D J, Cooper D A. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 5.Danhof M, Breimer D D. Therapeutic drug monitoring in saliva. Clin Pharmacokinet. 1978;3:39–57. doi: 10.2165/00003088-197803010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif W A, Condra J H, Emini E A, Isaacs R, Chodakewitz J A, Richman D D. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection. JAMA. 1998;280:35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 8.Medical Economics Company, Inc. PDR generics. 4th ed. Montvale, N.J: Medical Economics Company, Inc.; 1998. [Google Scholar]
- 9.Murphy R L, Sommadossi J P, Lamson M, Hall D B, Myers M, Dusek A. Antiviral effect and pharmacokinetic interaction between nevirapine and indinavir in persons infected with human immunodeficiency virus type 1. J Infect Dis. 1999;179:1116–1123. doi: 10.1086/314703. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds F, Ziroyanis P N, Jones N F, Smith S E. Salivary phenytoin concentrations in epilepsy and in chronic renal failure. Lancet. 1976;ii:384–386. doi: 10.1016/s0140-6736(76)92404-1. [DOI] [PubMed] [Google Scholar]
- 11.Rolinski B, Wintergerst U, Matuschke A, Fuessl H, Goebel F D, Roscher A A, Belohradsky B H. Evaluation of saliva as a specimen for monitoring zidovudine therapy in HIV-infected patients. AIDS. 1991;5:885–888. doi: 10.1097/00002030-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Stein D S, Fish D G, Billelo J A, Preston S L, Martineau G L, Drusano G L. A 24 week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Yeh K C, Deutsch P J, Haddix H, Hesney M, Hoagland V, Ju W D, Justice S J, Osborne B, Sterret A T, Stone J A, Woolf E, Waldman S. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–338. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]