Abstract
Background
Magnetic resonance imaging (MRI) is a multi-sequence imaging technique. Although MRI is the most sensitive method for detecting breast cancer, it is limited in evaluating the malignant possibility of non-mass enhanced (NME) breast lesions. It is also rarely reported whether MRI can further indicate the invasion of the lesions. In this article, we explore the differentiation of MRI characteristics between benign and malignant NME lesions and determine which features are associated with invasion.
Methods
The MRI findings of 118 NME lesions were evaluated retrospectively to explore the characteristics of the benign and malignant NME lesions in different MRI sequences including dynamic contrast-enhanced (DCE) MRI and diffusion-weighted imaging (DWI). The difference of MRI findings between benign and malignant NME lesions were determined by Pearson χ2 test or Fisher’s exact test, and the diagnostic value of features for malignancy was evaluated by receiver operating characteristic (ROC) curve.
Results
This study included 118 NME lesions (62 benign and 56 malignant) in 118 patients. We found a segmental distribution, clustered-ring enhancement, wash-out dynamic curve, and lower apparent diffusion coefficient (ADC) value (P=0.01, <0.001, 0.02, 0.001) were associated with malignancy. Wash-out dynamic curves, diffusion restriction on DWI, lower ADC values were more advantageous in distinguishing invasive NME cancer from benign lesions than ductal carcinoma in situ (DCIS) (P<0.001, <0.001, 0.027). Further analysis showed that there were statistical differences between invasive carcinoma and carcinoma in situ in terms of wash-out dynamic curves, diffusion restriction on DWI and lower ADC values (P=0.001, 0.014, 0.024).
Conclusions
MRI is a valuable way to identify malignant NME lesions and could predict the invasion of the lesions. Compared with carcinoma in situ, some sequences have more advantages in distinguishing invasive carcinoma from benign lesions.
Keywords: Breast, magnetic resonance imaging (MRI), non-mass enhancement, invasion
Introduction
According to the Breast Imaging-Reporting and Data System (BI-RADS), non-mass enhanced (NME) breast lesions on magnetic resonance imaging (MRI) is defined as an enhancement mode without space occupation effect, which can be distinguished from normal surrounding enhanced breast parenchyma. NME lesions include a series of entities, such as intraductal papilloma, atypical ductal hyperplasia, apocrine metaplasia, radial scar, and complex sclerosing lesions (1) While MRI is the most sensitive method to detect breast cancers, its diagnostic value is limited to NME breast lesions because of overlapping imaging findings between benign and malignant lesions, which may lead to unnecessary biopsy (2,3).
MRI is a multi-sequence imaging technique. Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) is the most important method for the detection of breast cancer, which can provide morphological and dynamic characteristics of the lesions. With the development of technology, diffusion-weighted imaging (DWI) has become a reliable auxiliary method for DCE-MR. However, compared with enhanced masses, DCE-MRI and DWI may have lower sensitivity and specificity in the diagnosis of NME lesions, which means that the diagnostic value is limited (4,5). The value of DCE-MRI and DWI in the diagnosis of invasion of NME lesions has also been rarely studied. Since post-operative carcinoma in situ may require reoperation if invasive elements are found, it is important to determine the invasion of the lesion. Our aim was to explore the characteristics of benign and malignant NME lesions and to determine which features are associated with invasions. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-503/rc).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived in this retrospective study. The ethical approval was waived by the Ethics committee of the First Medical Center of Chinese PLA General Hospital. All breast lesions from March 1, 2018 to March 1, 2021 were retrospectively searched in The First Medical Center of Chinese PLA General Hospital. The inclusion criteria were as follows: (I) presence as NME (BI-RADS 3–5) on MRI; (II) breast lesions with pathological results (Table 1); and (III) available clinical data. The exclusion criteria were as follows: (I) lesions being treated prior to examination; and (II) images that could not be accurately evaluated. Eventually, 118 patients with 118 lesions were enrolled and the NME lesions were diagnosed using excision biopsy or US-guided core needle biopsy (US-CNB). The 118 lesions included 56 malignant NME lesions (32 DCIS, 24 invasive carcinoma) and 62 benign NME lesions (14 adenosis, 8 fibroadenoma, 24 intraductal papilloma, and 16 inflammation).
Table 1. Pathologic category of NME lesions.
| Category | No. |
|---|---|
| Malignant | 56 |
| Ductal carcinoma in situ | 32 |
| Invasive ductal carcinoma | 24 |
| Benign | 62 |
| Adenosis | 14 |
| Fibroadenoma | 8 |
| Intraductal papilloma | 24 |
| Inflammation | 16 |
NME, non-mass enhancement.
MR imaging evaluation
The results were obtained with a 1.5 T Achieva (Philips, Netherland). All patients placed both breasts into a four-channel phased-array coil in the prone position using an MR protocol as follows: Axial turbo short time inversion recovery (STIR) T2-weighted Images (TR/TE, 8,000/60 ms; FOV read 350; 5 mm thickness; number of slices 20). The coronal spin-echo T1-weighted images (TR/TE, 500/10 ms; FOV read 350; thickness 2 mm; number of slices 30). The three-dimensional coronal T1-fast field echo (FFE) was used to acquire DCE-MR images after the intravenous injection of contrast agent Gd-DOTA (Gd-DTPA) at a rate of 2 mL/s followed by 20 mL of saline solution. The diffusion-weighted images (DWI) and apparent diffusion co-efficient (ADC) maps were acquired with the following parameters: TR ms/TE ms, 6,730/50; matrix 120×120; section thickness 5 mm; intersection gap 1.5 mm; Two b values of 50 and 1,000 s/mm2.
MRI findings were analyzed independently by two radiologists with at least 5 years of experience, and who had no access to the clinical data. In cases where disagreement occurred, another radiologist re-evaluated the images until consensus was reached. Imaging parameters were collected by the American College of Radiology Breast Imaging Reporting and Data System MR imaging criteria.
On MRI, the following imaging parameters were observed. The type of distribution (focal, linear, segmental, regional, multiple regional, diffuse) and internal enhancement (homogeneous, heterogeneous, clumped and clustered-ring enhancement) were assessed by post-contrast axial image, maximum intensity projection (MIP) images, and radial multi-planar reconstruction (MPR). Dynamic curve modes were divided into three categories: the persistent pattern (type I), in which signal intensity increased continuously over time; the plateau pattern (type II), in which signal intensity did not vary with time after an initial increase in the delay phase; and the wash-out pattern (type III), in which signal intensity decreased after reaching its initial increase peak in the delay phase. The ADC threshold was 1.3×10−3mm 2/s used as the cutoff between benign (>1.3×10−3 mm2/s) and malignant (≤1.3×10−3 mm2/s) lesions (6).
Statistical analysis
All statistical analyses were performed using SPSS, version 18.0 (SPSS, Chicago, IL, USA), and P<0.05 was considered to show a significant difference. Pearson χ2 test or Fisher’s exact test were used to determine differences in MRI features between benign and malignant breast lesions, P<0.05 (bilateral) is a prerequisite for Fisher's exact test. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic values of these features.
Results
We enrolled 118 patients into the study and their age ranged from 18–70 years old. Of the 118 NME breast lesions, there were 56 malignant lesions (32 DCIS, 24 invasive carcinoma) and 62 benign lesions (14 adenosis, 8 fibroadenoma, 24 intraductal papilloma, 16 inflammation).
MRI findings of the 118 breast lesions are shown in Table 2. Among the distribution characteristics of the NME breast lesions, segmental distribution and linear distribution were found to be statistically different between the benign and malignant lesions (P<0.05). The segmental distribution was more common in malignant NME lesions (24/56, 42.9%), higher than that in the benign lesions (13/62, 21%). Of the benign NME lesions, 12 (19.4%) showed linear distribution and only 1 (1.8%) of the malignant lesions showed linear distribution. Among the internal enhancement patterns, the frequency of cluster-ring enhancement (19/56, 33.9%) in malignancy was higher than in benign lesions, which was statistically significant (P<0.05). The sensitivity and specificity of internal enhancement model for predicting malignant NME lesions were 73.2% and 51.6%. Of the dynamic curves, the persistent type (type I) and wash-out type curve (type III) were statistically significant in benign and malignant NME lesions (P<0.05). The wash-out type curve (type III) was more common in malignancy (28/56, 50%), higher than that in the benign group (18/62, 29%). The incidence of type I was higher in benign lesions (18/62, 29%) than in malignant lesions (2/56, 3.6%) (P=0.02). The sensitivity and specificity of the kinetic curve model for predicting malignant NME lesions were 96.4% and 29.0%. In terms of DWI, diffusion restriction was observed in 48 (85.7%) of the malignant lesions, while 48 (77.4%) of benign lesions had diffusion restriction, which was not statistically significant (P=0.248). The ADC values of benign and malignant lesions were statistically different, and the ADC values of malignant NME lesions were mostly lower than those of benign lesions (Figures 1,2). The sensitivity and specificity of the ADC value (≤1.3×10−3mm2/s) for predicting malignant NME lesions were 83.9% and 45.2%.
Table 2. MR imaging descriptors in NME lesions.
| Descriptor | Malignant (n=56) (%) | Benign (n=62) (%) | P value |
|---|---|---|---|
| Distribution | |||
| Focal | 10 (17.9) | 15 (24.2) | 0.400 |
| Linear | 1 (1.8) | 12 (19.4) | 0.002* |
| Segmental | 24 (42.9) | 13 (21.0) | 0.010* |
| Reginal | 6 (10.7) | 10 (16.1) | 0.391 |
| Multiple regional | 5 (8.9) | 4 (6.5) | 0.874 |
| Diffuse | 10 (17.9) | 8 (12.9) | 0.455 |
| Internal enhancement patterns | |||
| Heterogeneous | 15 (21.8) | 32 (51.6) | 0.006* |
| Clustered-ring | 19 (33.9) | 3 (4.8) | <0.001* |
| Clumped | 22 (39.3) | 26 (41.9) | 0.770 |
| Homogeneous | 0 (0.0) | 1 (1.6) | 1.000 |
| Kinetic curve | |||
| Persistent | 2 (3.6) | 18 (29.0) | <0.001* |
| Plateau | 26 (46.4) | 26 (41.9) | 0.623 |
| Washout | 28 (50.0) | 18 (29.0) | 0.020* |
| ADC value | 0.001* | ||
| >1.3×10−3 mm2/s | 9 (16.1) | 28 (45.2) | |
| ≤1.3×10−3 mm2/s | 47 (83.9) | 34 (54.8) | |
| Diffusion restriction | 0.248 | ||
| Present | 48 (85.7) | 48 (77.4) | |
| Absent | 8 (14.3) | 14 (22.6) |
*, P<0.05. NME, non-mass enhancement; MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.
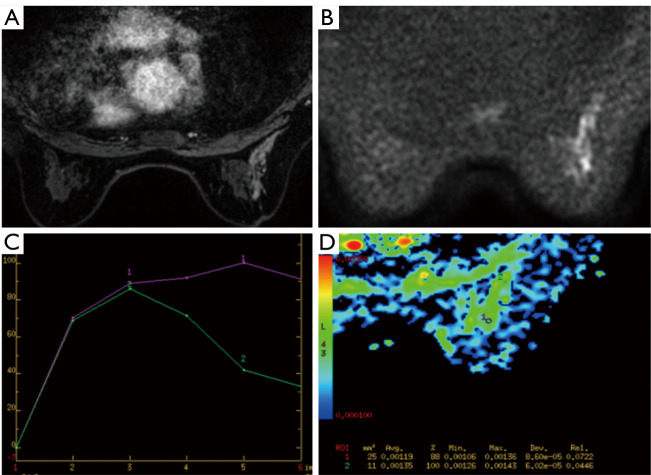
Figure 1.
MRI imaging in a 45-year-old woman. (A) Contrast enhanced breast MRI showed segmental distribution and clustered-ring enhancement; (B,C) the lesion showed diffusion restriction on DWI and wash-out dynamic curve; (D) the ADC value of this malignant lesion equals to 1.19×10−3 mm2/s. Final biopsy showed invasive ductal carcinoma. MRI, magnetic resonance imaging; DWI, diffusion-weighted images; ADC, apparent diffusion coefficient.
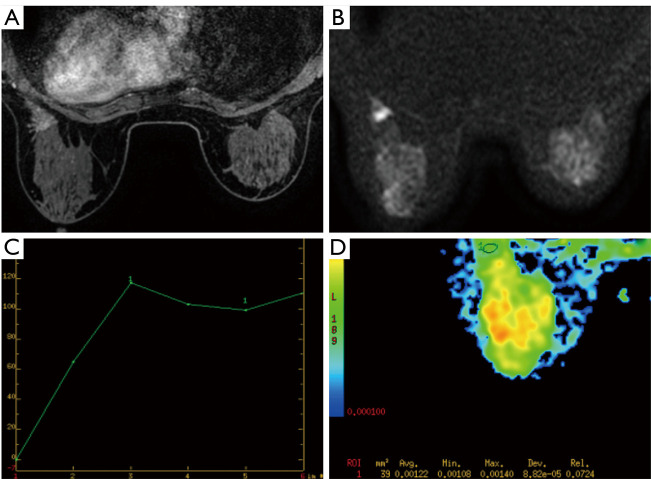
Figure 2.
MRI imaging in a 35-year-old woman. (A) Contrast enhanced breast MRI showed focal distribution and heterogeneous enhancement; (B,C) the lesion showed persistent curve without diffusion restriction on DWI; (D) the ADC value of this benign lesion equals to 1.22×10−3 mm2/s. Final biopsy showed intraductal papilloma. MRI, magnetic resonance imaging; DWI, diffusion-weighted images; ADC, apparent diffusion coefficient.
MRI features of DCIS and invasive cancer were further analyzed, and the results are shown in Table 3 and Table 4. Among the distribution characteristics and internal enhancement patterns, the segmental distribution and cluster-ring enhancement can help to distinguish between benign and malignant NME lesions, but there is no statistical difference between invasive carcinoma and carcinoma in situ. With respect to the dynamic curves, benign lesions were more likely to show type I than malignant lesions in two groups (P=0.003, 0.001). The wash-out curve (type III) was more frequent in invasive cancer (18/24, 75%), higher than that in the benign lesions (18/62, 29%) (P<0.05), while there was no statistical difference in type III curve between DCIS and benign lesions. All 24 (100%) of invasive cancer showed diffusion restriction and low ADC values, while 28 (45.2%) of benign lesions showed high ADC value. The difference was statistically significant (P<0.05). However, there was no significant difference of diffusion restriction on DWI and ADC value between DCIS and benign lesions. Further analysis showed dynamic curves, DWI, and ADC value were statistically different between invasive cancer and DCIS (P<0.05). The mean ADC value of invasive carcinoma (0.933×10−3mm2/s) is lower than that of carcinoma in situ (1.13×10−3mm2/s).
Table 3. MRI descriptors of between different pathological malignant NME lesions and benign lesions.
| Descriptor | Invasive carcinoma (n=24) (%) | Benign (n=62) (%) | P value | DCIS (n=32) (%) | Benign (n=62) (%) | P value |
|---|---|---|---|---|---|---|
| Distribution | ||||||
| Focal | 4 (16.7) | 15 (24.2) | 0.450 | 6 (18.8) | 15 (24.2) | 0.548 |
| Linear | 0 (0.0) | 12 (19.4) | 0.048* | 1 (3.1) | 12 (19.4) | 0.065 |
| Segmental | 11 (45.8) | 13 (21.0) | 0.021* | 13 (40.6) | 13 (21.0) | 0.043* |
| Reginal | 0 (0.0) | 10 (16.1) | 0.086 | 6 (18.8) | 10 (16.1) | 0.749 |
| Multiple regional | 1 (4.2) | 4 (6.5) | 1.000 | 4 (12.5) | 4 (6.5) | 0.545 |
| Diffuse | 8 (33.3) | 8 (12.9) | 0.061 | 2 (6.3) | 8 (12.9) | 0.523 |
| Internal enhancement patterns | ||||||
| Heterogeneous | 7 (29.2) | 32 (56.1) | 0.061 | 8 (25.0) | 32 (56.1) | 0.013* |
| Clustered-ring | 7 (29.2) | 3 (4.8) | 0.005* | 12 (37.5) | 3 (4.8) | <0.001* |
| Clumped | 10 (41.7) | 26 (41.9) | 0.982 | 12 (37.5) | 26 (41.9) | 0.678 |
| Homogeneous | 0 (0.0) | 1 (1.6) | 1.000 | 0 (0.0) | 1 (1.6) | 1.000 |
| Kinetic curve | ||||||
| Persistent | 0 (0.0) | 18 (29.0) | 0.003* | 2 (6.3) | 18 (29.0) | 0.001* |
| Plateau | 6 (25.0) | 26 (41.9) | 0.145 | 20 (62.5) | 26 (41.9) | 0.059 |
| Washout | 18 (75.0) | 18 (29.0) | <0.001* | 10 (31.3) | 18 (29.0) | 0.824 |
| ADC value | <0.001* | 0.109 | ||||
| >1.3×10−3 mm2/s | 0 (0.0) | 28 (45.2) | 9 (28.1) | 28 (45.2) | ||
| ≤1.3×10−3 mm2/s | 24 (100.0) | 34 (54.8) | 23 (71.9) | 34 (54.8) | ||
| Diffusion restriction | 0.027* | 0.793 | ||||
| Absent | 0 (0.0) | 14 (22.6) | 8 (25.0) | 14 (22.6) | ||
| Present | 24 (100.0) | 48 (77.4) | 24 (75.0) | 48 (77.4) |
*, P<0.05. NME, non-mass enhancement; MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.
Table 4. MRI features associated with invasion in NME lesions.
| Descriptor | Invasive carcinoma (n=24) (%) | Ductal carcinoma in situ (n=32) (%) | P value |
|---|---|---|---|
| Distribution | |||
| Focal | 4 (16.7) | 6 (18.8) | 1.000 |
| Linear | 0 (24.2) | 1 (3.1) | 1.000 |
| Segmental | 11 (45.8) | 13 (40.6) | 0.697 |
| Reginal | 0 (0.0) | 6 (18.8) | 0.071 |
| Multiple regional | 1 (4.2) | 4 (12.5) | 0.543 |
| Diffuse | 8 (33.3) | 2 (6.3) | 0.023* |
| Internal enhancement patterns | |||
| Heterogeneous | 7 (29.2) | 8 (25.0) | 0.728 |
| Clustered-ring | 7 (29.2) | 12 (37.5) | 0.515 |
| Clumped | 10 (37.5) | 12 (37.5) | 0.752 |
| Homogeneous | 0 (0.0) | 0 (0.0) | – |
| Kinetic curve | |||
| Persistent | 0 (0.0) | 2 (6.3) | 0.501 |
| Plateau | 6 (25.0) | 20 (62.5) | 0.005* |
| Washout | 18 (75.0) | 10 (31.3) | 0.001* |
| ADC value | 0.014* | ||
| >1.3×10−3 mm2/s | 0 (0.0) | 9 (28.1) | |
| ≤1.3×10−3 mm2/s | 24 (100.0) | 23 (71.9) | |
| Diffusion restriction | 0.024* | ||
| Absent | 0 (0.0) | 8 (25.0) | |
| Present | 24 (100.0) | 24 (75.0) |
*, P<0.05. NME, non-mass enhancement; MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient.
Discussion
DCE-MRI is an indispensable method to detect NME lesions, and morphologic features are the most important parameters (7). Asada et al. (8) found that segmental distribution was significantly associated with malignancy (P<0.05), which was similar to that seen in this study, in which the majority of malignant NME cases (24/56, 42.9%) presented as segmental distribution (P=0.01). We also found that linear distribution was more frequent in benign lesions (12/62, 19.4%), which was higher than that in the malignant NME group (1/56, 1.8%), and the difference was statistically significant (P=0.002). As for internal enhancement model, previous study had reported that cluster-ring enhancement could effectively identify malignant NME lesions (9). In this study, the cluster-ring enhancement was demonstrated in 33.9% (19/56) of the malignant NME lesions and 4.8 % (3/62) of the benign NME lesions, which was statistically significant (P<0.001). The sensitivity and specificity of internal enhancement model for predicting malignant NME lesions were 73.2% and 51.6%. Segmental distribution and cluster-ring enhancement were important predictors for NME malignancy, which was consistent with the findings of Lunkiewicz (10). The morphologic features could help to distinguish both invasive carcinoma and carcinoma in situ from benign lesions. Whether morphological features could indicate invasion of malignant lesions was further explored. Machida et al. observed 76 DCIS and 55 invasive breast cancers that presented as NME and found a clustered-ring was significantly associated with invasion (11). Hahn et al. also reported that a clustered-ring was more commonly observed in microinvasive ductal carcinoma than in pure DCIS (12). In the present study, there was no difference in internal enhancement between invasive carcinoma and DCIS, which may be due to the small sample size. In addition, we found that diffuse distribution was associated with invasion significantly (P=0.023), which may be because the average diameter of invasive lesions was much larger than that of DCIS.
Goto et al. in 2007 had reported that a kinetic curve was less effective for NME lesions than mass lesions (1). In this study, malignant NME lesions often showed a wash-out type curve, and benign NME lesions often showed a persistence dynamic curve, which was statistically significant (P<0.05). The sensitivity and specificity of the kinetic curve model for predicting malignant NME lesions were 96.4% and 29.0%. Malignant NME lesions were further divided into two groups (invasive carcinoma and DCIS), and invasive breast cancer of NME mostly showed type III compared with benign lesions, which was statistically significant (P<0.001). Most DCIS (62.5%) showed a plateau dynamic type, and there was no significant difference between DCIS and benign lesions. Thus, dynamic curves might help to differentiate invasive NME breast cancer from benign lesions, but do not effectively distinguish DCIS from benign lesions. Greenwood et al. showed that MRI evaluation of DCIS mainly depended on morphological features rather than kinetics, which was consistent with our results (13). In this study, the kind of dynamic curves between invasive cancer and DCIS were further evaluated, and DCIS showed persistent or plateau curves, which were more common than invasive breast cancer (P>0.05). The wash-out dynamic type was more common in invasive carcinoma (18/24, 75%), which was higher than in the DCIS group (10/32, 31.3%) (P=0.001). Thus, a wash-out curve may help identify invasive NME lesions from DCIS.
In this study, we also found that the parameters of DWI and ADC value were important in identifying malignant NME lesions. Malignant NME lesions mostly presented with lower ADC values (≤1.3×10−3 mm2/s) compared with benign lesions (P=0.001). The sensitivity and specificity of the ADC value (≤1.3×10−3 mm2/s) for predicting malignant NME lesions were 83.9% and 45.2%. The proportion of restricted diffusion on DWI in malignant NME lesions was higher than that in benign NME lesions, but there was no statistical difference (P=0.248). Sharma et al. showed malignant NME lesions had restricted diffusion on DWI at 1.5T, which was associated with increased cell density (14), while Avendano et al. found that the ADC value was limited in differentiating benign and malignant NME lesions, with 31% of NME lesions unable to be evaluated on DWI due to poor imaging quality (15). The differences of results might be due to the ROI measurement methods and ADC indicators in different studies. The potential of DWI and ADC value (b=1,000 s/mm2) to distinguish invasive cancer or DCIS from benign lesions to be explored. Invasive cancer showed restricted diffusion on DWI and a lower ADC value compared to benign NME lesions and the difference was statistically significant (P=0.027, <0.001). DCIS also showed restricted diffusion on DWI and lower ADC values compared to benign NME lesions, but the difference was not statistically significant (P=0.793, 0.109), indicating it is limited in identifying DCIS from benign NME lesions by DWI and ADC values. Thus, diffusion restriction on DWI and lower ADC value could help differentiate invasive malignant lesions from benign lesions compared with DCIS (P<0.001, 0.027). Greenwood et al. found that DWI and ADC values could distinguish DCIS from invasive disease, with invasive cancer having a lower average ADC value than DCIS (P<0.001) (b=1,000 s/mm2) (13). Our study also showed ADC values were lower in invasive cancer than in DCIS and invasive carcinoma was more likely to show restricted diffusion on DWI (P=0.014, 0.024).
This study had some limitations, including its retrospective nature and small sample size, and further studies with larger sample sizes are needed to confirm the conclusions. Moreover, there may be false negative results in the pathological results of percutaneous biopsy, which might affect the accuracy of our conclusions.
In conclusion, this study demonstrated MRI was useful in distinguishing malignant NME lesions. Segmental distribution, clustered-ring enhancement, washout dynamic type, and lower ADC value were associated with malignancy. Further analysis of MRI findings of malignant NME lesions found that wash-out dynamic type, diffusion restriction on DWI and more lower ADC value could help identify invasive lesions from DCIS. MRI is a valuable way to predict the malignancy and invasion of NME lesions.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by National Key Research and Development Program of China (Grant No. 2019YFC0118800).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived in this retrospective study. The ethical approval was waived by the Ethics committee of the First Medical Center of Chinese PLA General Hospital.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-503/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-503/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-503/coif). All authors report that this work was supported by National Key Research and Development Program of China (Grant No. 2019YFC0118800). The authors have no other conflicts of interest to declare.
(English Language Editor: B. Draper)
References
- 1.Goto M, Ito H, Akazawa K, et al. Diagnosis of breast tumors by contrast-enhanced MR imaging: comparison between the diagnostic performance of dynamic enhancement patterns and morphologic features. J Magn Reson Imaging 2007;25:104-12. 10.1002/jmri.20812 [DOI] [PubMed] [Google Scholar]
- 2.Edwards SD, Lipson JA, Ikeda DM, et al. Updates and revisions to the BI-RADS magnetic resonance imaging lexicon. Magn Reson Imaging Clin N Am 2013;21:483-93. 10.1016/j.mric.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23:8469-76. 10.1200/JCO.2004.00.4960 [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez RL, DeMartini WB, Eby PR, et al. BI-RADS lesion characteristics predict likelihood of malignancy in breast MRI for masses but not for nonmasslike enhancement. AJR Am J Roentgenol 2009;193:994-1000. 10.2214/AJR.08.1983 [DOI] [PubMed] [Google Scholar]
- 5.Pinker K, Moy L, Sutton EJ, et al. Diffusion-Weighted Imaging With Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone Parameter: Comparison With Dynamic Contrast-Enhanced and Multiparametric Magnetic Resonance Imaging. Invest Radiol 2018;53:587-95. 10.1097/RLI.0000000000000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinker K, Marino MA, Dr Meyer-Baese A, et al. Multiparametric and molecular imaging of breast tumors with MRI and PET/MRI. Radiologe 2016;56:612-21. 10.1007/s00117-016-0129-3 [DOI] [PubMed] [Google Scholar]
- 7.Newell D, Nie K, Chen JH, et al. Selection of diagnostic features on breast MRI to differentiate between malignant and benign lesions using computer-aided diagnosis: differences in lesions presenting as mass and non-mass-like enhancement. Eur Radiol 2010;20:771-81. 10.1007/s00330-009-1616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asada T, Yamada T, Kanemaki Y, et al. Grading system to categorize breast MRI using BI-RADS 5th edition: a statistical study of non-mass enhancement descriptors in terms of probability of malignancy. Jpn J Radiol 2018;36:200-8. [DOI] [PubMed] [Google Scholar]
- 9.Aydin H. The MRI characteristics of non-mass enhancement lesions of the breast: associations with malignancy. Br J Radiol 2019;92:20180464. 10.1259/bjr.20180464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunkiewicz M, Forte S, Freiwald B, et al. Interobserver variability and likelihood of malignancy for fifth edition BI-RADS MRI descriptors in non-mass breast lesions. Eur Radiol 2020;30:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machida Y, Shimauchi A, Tozaki M, et al. Descriptors of Malignant Non-mass Enhancement of Breast MRI: Their Correlation to the Presence of Invasion. Acad Radiol 2016;23:687-95. 10.1016/j.acra.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Hahn SY, Han BK, Ko EY, et al. MR features to suggest microinvasive ductal carcinoma of the breast: can it be differentiated from pure DCIS? Acta Radiol 2013;54:742-8. 10.1177/0284185113484640 [DOI] [PubMed] [Google Scholar]
- 13.Greenwood HI, Wilmes LJ, Kelil T, et al. Role of Breast MRI in the Evaluation and Detection of DCIS: Opportunities and Challenges. J Magn Reson Imaging 2020;52:697-709. 10.1002/jmri.26985 [DOI] [PubMed] [Google Scholar]
- 14.Sharma U, Danishad KK, Seenu V, et al. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 2009;22:104-13. 10.1002/nbm.1245 [DOI] [PubMed] [Google Scholar]
- 15.Avendano D, Marino MA, Leithner D, et al. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res 2019;21:136. 10.1186/s13058-019-1208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as