Abstract
Background
Metformin (Met) has antitumor effects on various cancers, but it is still unclear whether it exerts a reversible effect on endocrine resistance in breast cancer (BC) patients. In the present survey, metformin’s effects on tamoxifen-resistant MCF cells (The cell line was established at the Michigan Cancer Foundation, hence the name MCF cell) were evaluated and the molecular mechanism was explored.
Methods
We constructed a tamoxifen-resistant BC cell line MCF-7R, then applied Cell Counting Kit-8 (CCK-8), flow cytometry, and EdU assessments to determine the growth and apoptosis of MCF-7R cells. Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot were used to evaluate the expression level of lncRNA GAS5 and the mTOR-associated proteins. Overexpression and downregulation of lncRNA GAS5 were carried out to explore the molecular mechanism of Met.
Results
The combined effect of Met and OHT (4-hydroxytamoxifen, main active metabolite of tamoxifen) was to remarkably reduce the growth and increase the apoptosis of MCF-7R cells compared with OHT alone. The levels of the mTOR, p-mTOR and p-P70S6K proteins in MCF-7R cells decreased after Met treatment, but the p-AMPK2 and PTEN proteins significantly increased. The overexpression of lncRNA GAS5 in MCF-7R cells inhibited cell growth with decreased mTOR, p-mTOR, PCNA, and Bcl-2 proteins. Metformin had no significant effect on MCF-7R cells with lncRNA GAS5 knockdown but contrarily activated p-mTOR.
Conclusions
Metformin can inhibit overactivation of the mTOR signaling pathway through upregulating lncRNA GAS5 expression, thereby inhibiting the growth and inducing the apoptosis of BC cells, providing a new clinical treatment for BC.
Keywords: Breast Cancer (BC), drug resistance, GAS5 long non-coding RNA, metformin, mTOR protein
Introduction
Breast cancer (BC), the leading tumor in females, is a public health challenge and a fundamental cause of many deaths annually (1,2). Most BC patients (70%) express the estrogen receptor (ER+), and for these patients, a selective estrogen receptor modulator (SERM), such as tamoxifen, is one of the main treatments (3,4); however, a significant proportion have a poor response or even resistance to tamoxifen (5,6). Consequently, it is crucial and necessary to discover an efficacious treatment to ameliorate endocrine resistance in BC patients.
Metformin (Met), a biguanide, has been a common first-line therapeutic for type 2 diabetes mellitus (T2DM) for decades (7,8). Various epidemiological studies have showed that metformin can lower the risk of different kind of cancers in T2DM patients by 30–50% (9,10). Further research has indicated that Met has antitumor effects. Both in vivo and in vitro results were consistent for its effect on pancreatic cancer (11). Its antitumor role is mainly mediated through the I/IGF and AMP-inducible protein kinase (AMPK) signaling pathways (10). Overactivation of the mTOR pathway is a key factor in endocrine resistance in BC (7,12,13), and the AMPK signaling pathway is mainly involved in inhibiting activation of the mTOR pathway by AMPK-mediated phosphorylation, thus inhibiting the growth of tumor cells. However, it is still unclear whether Met can reverse endocrine resistance through the mTOR signaling pathway, which is a new research direction.
Long-noncoding RNA (lncRNA) GAS5 is expressed at low levels in various cancer types (e.g., bladder, breast, cervical and ovarian cancers). It is a tumor suppressor gene through its promotion of cell apoptosis and inhibition of cell growth (14-25). Research has shown that overexpression of lncRNA GAS5 will hinder the growth of gastric cancer or esophageal cancer cells by inhibiting the AKT/mTOR signaling pathway (26,27). In addition, overexpression of lncRNA GAS5 can also increase the chemosensitivity of triple-negative BC cells and induce cell apoptosis (28). Therefore, a potential target for chemotherapy resistance in BC is lncRNA GAS5; however, its specific mechanism is still unclear.
The studies on metformin and BC that have been carried out mainly focus on the proliferation and inhibition of tumor cells and their stem cells, and the sensitization of chemotherapeutic drugs, but there is no report on the reversal of endocrine therapy resistance. In this study, we firstly constructed a tamoxifen-resistant MCF-7 cell line (MCF-7R), and studied the ability of Met to reverse tamoxifen resistance. Furthermore, we explored the molecular mechanism using overexpression and downregulation of lncRNA GAS5. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-795/rc).
Methods
Chemical reagents and cell lines
Metformin and 4-hydroxytamoxifen (OHT, the main active metabolite of tamoxifen) were acquired from Sigma-Aldrich (St. Louis, MO, USA) and the purity of each was at least 95.0% as determined by high-performance liquid chromatography (HPLC). Metformin was dissolved in 1× phosphate-buffered saline in 5 M stock solution, and OHT was dissolved in dimethyl sulfoxide (DMSO) in 25 mM stock solution. Both Met and tamoxifen were stored at –80 ℃, and diluted with respective fresh medium immediately before use. Rapamycin (RAPA) (purity ≥95%, HPLC) was obtained from Sigma-Aldrich and was dissolved in DMSO in a 20 nM solution for all experiments. The anti-GAS5 small interfering RNA (siRNA) and pLenti-GAS5 were obtained from RiboBio (Guangzhou, China). The primary antibodies against mTOR, p-mTOR, p-P70S6K, p-AMPK2, AMPK2, PCNA, Bcl-2 and tubulin were procured from Cell Signaling Technology (Danvers, MA, USA). The primary antibody against PTEN was purchased from Abcam (Cambridge, UK). Human BC cell line MCF-7 was procured from the American Type Culture Collection (ATCC, USA) and cultivated in DMEM supplemented with 1% glutamine, 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco, USA) at 37 ℃ in a 5% CO2 humidity incubator.
Tamoxifen-resistant MCF-7 cell line
MCF-7 cells were grown in estrogen-deprived medium [phenol red-free RPMI 1640 supplemented with 10% charcoal-stripped FBS (Gibco, USA)] and then OHT added to medium at given concentration (1 μM) and cells left to grow for 21 days. The tamoxifen-resistant MCF-7 cell line (MCF-7R) was continued until the growth of the MCF-7R cells was no longer inhibited, after which the MCF-7R cells were cultured with 0.1 μM OHT for at least 4 months before the downstream studies.
Quantitative real-time polymerase chain reaction (qRT-PCR), Lenti-EGFP-GAS5 and antiGAS5-siRNA
We isolated the total RNA from MCF-7R cells using TRIzol reagent (Invitrogen, USA). qRT-PCR of RNA reverse transcribed cDNA was performed with Revertaid First Strand cDNA Synthesis Kit (Invitrogen, USA). Next, PowerUpTM SYBRTM Green Master Mix (Invitrogen) was used to conduct qPCR reactions on a Bio-Rad CFX Manager system (Bio-Rad, USA). ACTB served as the endogenous control and 2−∆∆Ct was calculated for the relative expression of lncRNA GAS5. The primer sequences used were: lncRNA GAS5-F: 5'-TCC CCA AGG AAG GAT GAG AA-3', R: 5'-CCA GGA GCA GAA CCA TTA AGC-3'; ACTB-F: 5'-GGC ACT CTT CCA GCC TTC C-3', R: 5'-GAG CCG CCG ATC CAC AC-3'.
Retroviral particles overexpressing GAS5, also known as Lenti-EGFP-GAS5, were infected with MCF-7R cells, and a stable overexpressed cell line was obtained by 2μg/mL puromycin screening. AntiGAS5-siRNA was transfected into the MCF-7R cells to knockdown GAS5 using Lipofectamine™ 3000 Transfection Reagent (Invitrogen, USA), following the protocol of the manufacturer.
Cell Counting Kit-8 (CCK-8) assay
MCF-7 and MCF-7R cells in the logarithmic phase of growth were plated onto 96-well plates (1×104 cells/well) and incubated for 24 h. Following processing with different drugs [5 mM Met, OHT (0, 0.1, 1, 10, 20, 40, 60, and 100 μM), 20 nM RAPA] for 48 h, the cells were incubated for 2 h with 10 μL/well CCK8 solution. Absorbance was recorded at 450 nm using a Multiskan MK3 microplate reader (Thermo Fisher Scientific, USA) to appraise the inhibitory concentration (IC50) and resistance index (RI = IC50 of MCF-7R cells/IC50 of MCF-7 cells).
EdU assay
MCF-7R cells (5×104 cells/well) were seeded onto 24-well plates and processed with the different drugs (5 mM Met, 1 μM OHT, 20 nM RAPA) for 48 h. The cells were then exposed to 5-ethynyl-2’-deoxyuridine (EdU, RiboBio, China) for 2 h. Next, the cells were fixed with 4% formaldehyde for 30 min and permeabilized with 0.5% Triton-X-100, and then incubated with 100 μL/well of 1× Apollo® reaction cocktail at room temperature for 30 min. The cells were treated with 1× Hoechst 33342 (Beyotime, China) for 30 min to stain the DNA contents, and finally visualized using a fluorescence microscope.
Apoptosis detection
MCF-7R cells were processed with the different drugs (5 mM Met, 1 μM OHT, 20 nM RAPA) for 48 h, then were accumulated and resuspended in 1× diluted binding buffer. Annexin V-FITC (BD Biosciences, USA) and 7-Amino-Actinomycin D (7-AAD) (BD Biosciences, USA) were mixed to stain the cells in the dark. After incubation for 15 min, the cells were scrutinized by flow cytometry and the percentage of apoptotic cells was evaluated.
Western blot
The extraction of total protein in MCF-7R cells was performed using boiling loading buffer. The protein lysates were separated by 10% SDS-PAGE and subsequently transferred to PVDF membrane. After that, non-specific proteins were blocked by 5% non-fat milk, and the PVDF membranes were incubated with primary antibodies at 4 ℃ overnight. One day later, incubation of the membranes was completed with the aid of the secondary antibodies at ambient temperature for 90 min. Following rinsing with TBST buffer, the protein membranes were signaled through the use of SuperSignal™ West Dura Extended Duration Substrate and subjected to X-ray imaging.
Statistical analysis
The statistical outcomes are given as mean ± SD and calculated with SPSS 22.0 and GraphPad Prism 7.00 software. For comparison between groups, the unpaired Student’s t-test was applied. Assessments were repeated at least three times and P<0.05 was regarded as a meaningful difference.
Results
Effects of OHT and Met on the viability of MCF-7 and MCF-7R cells
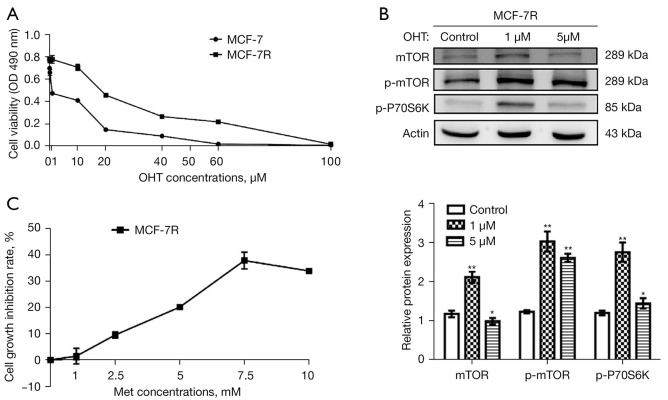
The data indicated that the growth of MCF-7R and MCF-7 cells was significantly hindered by OHT in a dose-dependent manner. However, the MCF-7R cells showed a lower rate of inhibition compared with the MCF-7 cells, indicating tamoxifen resistance (Figure 1A). The IC50 of tamoxifen-treated MCF-7R and MCF-7 cells for 48 h was 65.53 μM and 16.11 μM, respectively, and the drug RI of the MCF-7R cells was 4.06. These findings indicated that we had successfully constructed a tamoxifen-resistant BC cell line MCF-7R.
Figure 1.
Effects of metformin (Met) and 4-hydroxytamoxifen (OHT) on the growth of MCF-7 and MCF-7R cells. (A) Cell Counting Kit-8 (CCK-8) analysis of the growth of MCF-7 and MCF-7R cells treated using OHT (0, 0.1, 1, 10, 20, 40, 60, and 100 µM) for 48 h. (B) Western blot conducted to evaluate the expressions of mTOR signaling-related proteins in MCF-7R cells constructed with different concentrations of OHT. (C) CCK-8 assessment for examining the growth of MCF-7R cells following administration of Met (0, 1, 2.5, 5, 7.5, and 10 mM) for 48 h. *, P<0.05, **, P<0.01 compared with control group (without any treatment).
We then detected the expression of mTOR signaling pathway associated proteins in MCF-7R cells using a concentration gradient of OHT. MCF-7R cells of 1 μM OHT, 5 μM OHT and control group were constructed respectively. The levels of expression of the mTOR, p-mTOR and p-P70S6k proteins in MCF-7R cells constructed by 1 μM OHT were notably higher than in the cells in the control group and 5 μM group (Figure 1B). In addition, the cells constructed by 1 μM OHT were in better condition than those with 5 μM OHT. Therefore, the 1 μM OHT constructed cells best met the experimental requirements and were used for the follow-up assessments.
As shown in Figure 1C, the growth of MCF-7R cells was inhibited by Met in a dose-dependent manner: 7.5 mM was the most effective. The IC50 of MCF-7R cells treated with Met for 48 h was 14.02 mM. After comprehensive consideration, 5 mM Met was used in subsequent experiments for additional examination of its effects on BC cells.
Effect of Met on the growth of MCF-7R cells
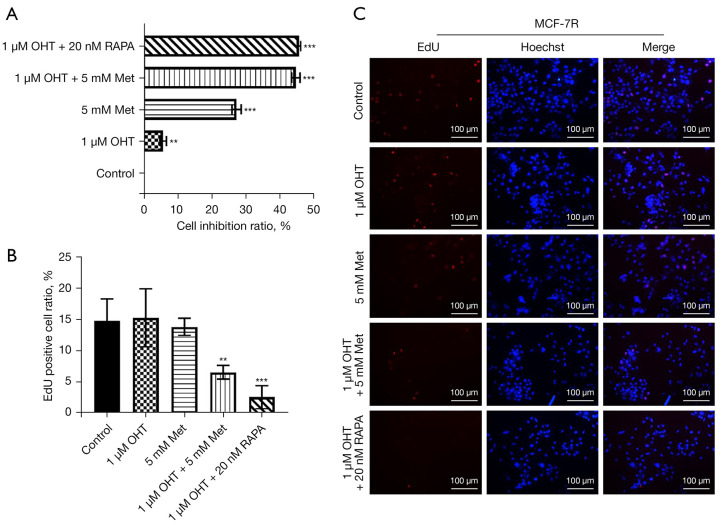
MCF-7R cells were further treated with Met and OHT for 48 h and their growth was assessed by CCK8 assay. As shown in Figure 2A, 1 μM OHT had almost no effect on depressing the growth of MCF-7R cells, but 5 mM Met showed a substantial effect. Combined treatment with Met and OHT significantly depressed the growth of cells, which was similar to the effect of the combination of the mTOR inhibitor RAPA and OHT.
Figure 2.
Effect of metformin on the growth of MCF-7R cells. MCF-7R cells were processed with different drugs [5 mM metformin (Met), 1 µM 4-hydroxytamoxifen (OHT) and 20 nM rapamycin (RAPA)] for 48 h. (A) CCK8 assessment evaluated cell growth. (B,C) EdU analysis of the growth of MCF-7R cells (red shows EdU-positive cells and blue shows the cells’ nuclei, immunofluorescent staining). Scale =100 µm. **, P<0.01, ***, P<0.001 compared with control group (without any treatment). Outcomes are given as mean ± SD of three independent assessments (n≥3).
We used the EdU assay to determine the DNA replication of cells as a reflection of cell growth. Compared with the control group without any treatment, there was no substantial alternation in the growth of MCF-7R cells after treatment with 1 μM OHT or 5 mM Met. However, the DNA replication of MCF-7R cells was significantly depressed after treatment with OHT + Met (Figure 2B,2C). In conclusion, these findings showed that Met hindered the growth of MCF-7R cells and further reduced the resistance of MCF-7R cells to tamoxifen.
Effect of metformin on apoptosis of MCF-7R cells
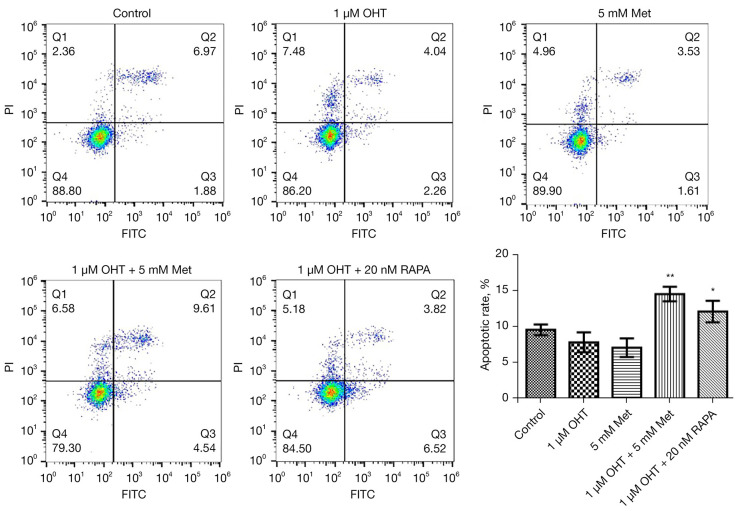
The effects of Met and OHT on apoptosis of MCF-7R cells were studied by flow cytometry. The combination of Met and OHT induced significant apoptosis of MCF-7R as compared with OHT alone. This combination had a similar effect as the combination of RAPA and OHT (Figure 3).
Figure 3.
Induction of apoptosis of MCF-7R cells by metformin (Met). Flow cytometry was used to visualize the apoptosis of MCF-7R cells following processing with different drugs [5 mM Met, 1 µM 4-hydroxy tamoxifen (OHT) and 20 nM rapamycin (RAPA)] for 48 h. **, P<0.01 vs. control (without any treatment). Outcomes are given as mean ± SD of three independent assessments (n≥3).
Effect of Met on lncRNA GAS5 and mTOR signaling pathway
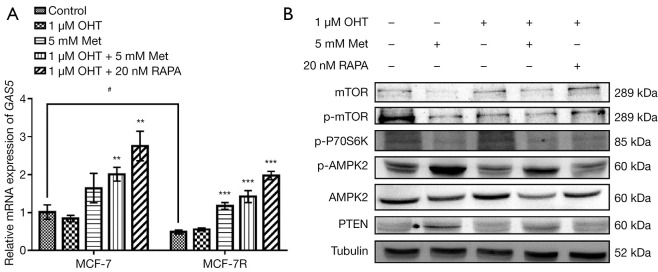
To investigate the interactions between lncRNA GAS5 and tamoxifen resistance, the expression levels of lncRNA GAS5 in both MCF-7 and MCF-7R cells were evaluated, and we found lncRNA GAS5 was significantly reduced in MCF-7R cells, whereas the reduction in MCF-7 cells was not notable. OHT had no remarkable effect on lncRNA GAS5 expression levels in either cell line, but the combination of Met and OHT significantly increased its expression in both cells (Figure 4A).
Figure 4.
Effect of metformin on lncRNA GAS5 and mTOR signaling pathway. (A) Quantitative real-time PCR was applied for appraising the mRNA expression of lncRNA GAS5 in MCF-7 and MCF-7R cells under different drug treatments [5 mM metformin (Met), 1µM 4-hydroxytamoxifen (OHT) and 20 nM rapamycin (RAPA)] for 48 h. (B) Western blot was used to evaluate the mTOR signaling-related proteins’ expression levels in MCF-7R cells after different drug treatments. **, P<0.01; ***, P<0.001 compared with control (without any processing); #, P<0.05 compared with MCF-7 cells. Outcomes are given as mean ± SD of three independent assessments (n≥3). “+” indicated that the substance has been added; “−” indicated that the substance was not added.
In addition, we detected the mTOR signaling-related proteins using western blot. As shown in Figure 4B, the p-AMPK2 and PTEN protein expression levels were remarkably increased in Met-treated MCF-7R cells, and the expression levels of p-P70S6K, mTOR, and p-mTOR proteins were significantly decreased. The combination of Met and OHT also activated the p-AMPK2 protein and inhibited the mTOR protein and its phosphorylation, but did not demonstrate a significant effect on the total expression level of AMPK2 protein in MCF-7R cells. Collectively, these results showed that Met promoted the expression of lncRNA GAS5 while inhibiting the mTOR signaling pathway in MCF-7R cells. Therefore, we speculate that lncRNA GAS5 is involved in the regulation of mTOR signaling by Met.
Effect of lncRNA GAS5 overexpression on MCF-7R cells
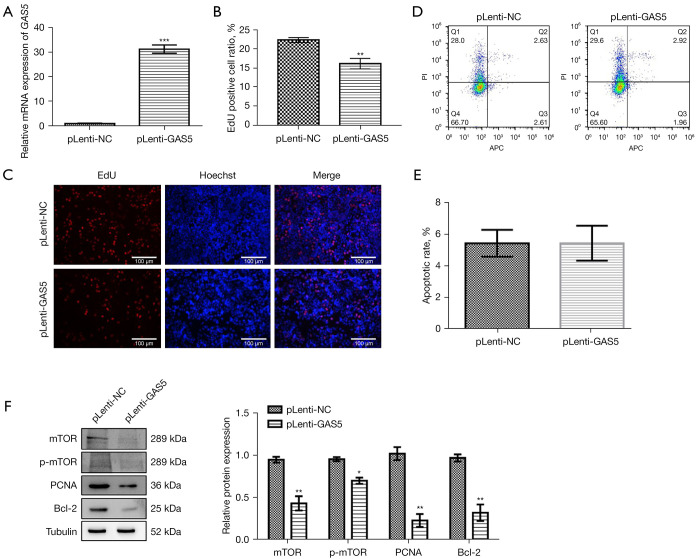
After overexpressing lncRNA GAS5 in MCF-7R cells (Figure 5A), we found that the growth of lncRNA GAS5-overexpressed MCF-7R cells was significantly reduced according to EdU assay (Figure 5B,5C), but there was no effect on cell apoptosis (Figure 5D,5E). The western blot results indicated that the mTOR and p-mTOR proteins’ expression levels were substantially decreased, and the expression of PCNA, a growth-associated protein, was also significantly decreased (Figure 5B,5C), as was the expression level of the apoptosis-suppressor protein Bcl-2 (Figure 5F).
Figure 5.
Effect of overexpression of lncRNA GAS5 on MCF-7R cells. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of the expression level of lncRNA GAS5 in MCF-7R cells following lncRNA GAS5 overexpression. (B,C) EdU analysis of the growth of MCF-7R cells after lncRNA GAS5 overexpression (red shows EdU-positive cells and blue shows the cells’ nuclei, immunofluorescent staining). Scale bar =100 µm. (D,E) Flow cytometry to evaluate apoptosis of MCF-7R cells after lncRNA GAS5 overexpression. (F) Western blot results for the expression levels of mTOR signaling-related proteins in MCF-7R cells after lncRNA GAS5 overexpression. *, P<0.05, **, P<0.01 and ***, P<0.001 vs. pLenti-NC. Outcomes are given as mean ± SD of three independent assessments (n≥3).
Effects of lncRNA GAS5 knockdown on MCF-7R cells
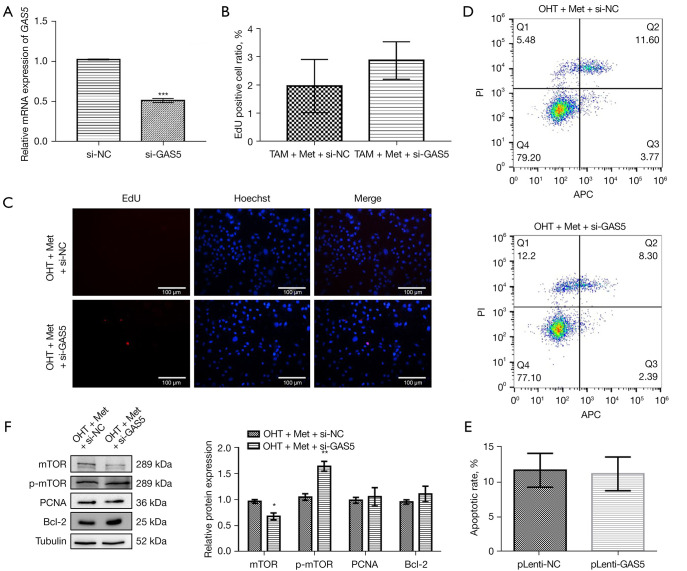
To further understand the effects of lncRNA GAS5 in the Met-regulated mTOR signaling pathway, we established lncRNA GAS5-knockdown MCF-7R cell lines (Figure 6A). The combination of OHT and Met was administered to the lncRNA GAS5-knockdown MCF-7R cells, which promoted a growth trend of MCF-7R cells, but had no effect on the apoptosis rate (Figure 6B-6E). Western blot also revealed that lncRNA GAS5-silenced MCF-7R cells substantially reduced the expression level of mTOR, but enhanced the expression level of p-mTOR protein. Meanwhile, the expression levels of the Bcl-2 and PCNA proteins tended to increase (Figure 6F).
Figure 6.
Effect of lncRNA GAS5 knockdown on MCF-7R cells. lncRNA GAS5-knockdown MCF-7R cells were processed with OHT and metformin (Met) in combination. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of the expression level of lncRNA GAS5 in MCF-7R cells following lncRNA GAS5 knockdown. (B,C) EdU analysis of the DNA replication activity in MCF-7R cells after lncRNA GAS5 knockdown (red shows EdU-positive cells and blue shows the cells’ nuclei, immunofluorescent staining). Scale bar =100 µm. (D,E) Flow cytometry for apoptosis of MCF-7R cells following lncRNA GAS5 knockdown. (F) Western blot to detect mTOR signaling-related proteins’ expression in MCF-7R cells after lncRNA GAS5 knockdown. **, P<0.01 vs. OHT + Met + si-NC; ***, P<0.001 vs. si-NC. Outcomes are given as mean ± SD of three independent assessments (n≥3). si-NC mean normal control group.
Discussion
Met is a lipophilic biguanide that is used as the first-line treatment for glucose control in patients with T2DM, due to its safety, efficacy and tolerability. According to the results of large-scale observational and cohort studies, administration of Met is related to a lowered risk of cancer and has a significant sensitization effect in chemotherapy (7,29,30). However, because its effect on reversing endocrine resistance remains unexplained, we investigated its molecular mechanism using endocrine-resistant BC cells. Our results showed that Met depressed the overactivation of the mTOR signaling pathway by upregulating the expression level of lncRNA GAS5, resulting in inhibition of the growth of BC cells, increased apoptosis, and ultimately, reversing the endocrine resistance of BC cells.
It is important to note that our results implied that the growth of tamoxifen-resistant MCF-7R cells was not significantly inhibited when treated with OHT alone, was significantly affected when Met was added. This finding further supported the view that Met may reverse the endocrine resistance of BC cells, by an as yet unspecified mechanism.
Overactivation of the mTOR signaling pathway is a key process in the advancement of endocrine resistance in BC (12,13). mTOR, a serine/threonine kinase, is fundamental to cell growth, involving the integration of diverse extracellular signals of energy, nutrition, and growth factors and participation in the biological processes of gene transcription, ribosome synthesis, protein translation, etc. (31,32). At present, the mTOR inhibitor everolimus has been used in clinical practice. Everolimus combined with endocrine drugs can reverse endocrine drug resistance, which has obvious advantages. However, the existing mTOR inhibitors have disadvantages such as large side effects and high price. Therefore, it is very important to find inexpensive and safe mTOR signaling pathway inhibitors that can produce good socioeconomic effects. Our results elucidated that the expression of both the mTOR and p-mTOR proteins in MCF-7R cells was significantly decreased after treatment with Met, as was the expression of the downstream effector p-P70S6K, but the expression of p-AMPK2 was substantially enhanced, which was in agreement with the outcomes reported by Ma et al. (33) AMPK, known as an energy sensor in cells, has been confirmed by many studies to be activated by Met and hinder the expression of mTOR (7,34). As our results showed, Met may stimulate the activation of AMPK2, and p-AMPK2 then inhibits the expression of mTOR in MCF-7R cells. Ribosomal S6 protein kinase (P70S6K), one of the mTOR effectors, is responsible for ribosomal protein synthesis (35). With inhibition of mTOR protein expression, the phosphorylation of its downstream protein P70S6K is also depressed. But how to ultimately reverse endocrine resistance needs further research.
LncRNA is involved in the regulation of various processes in BC cells, which is closely related to the occurrence, development and prognosis of BC, and is a new target for the treatment of BC. LncRNA GAS5, a 5’ terminal oligopyrimidine (5’-TOP) RNA, has low levels of expression in various cancers, but it can not only hinder the growth but also boost the apoptosis of tumor cells (14-25). In our study, qPCR analysis revealed that the expression level of lncRNA GAS5 in MCF-7R cells was decreased with statistical significance compared with MCF-7 cells. It did not increase significantly when treated with OHT until Met was added. These results are supported by those of Gu et al. who delineated that lncRNA GAS5 overexpression might enhance sensitivity to tamoxifen (36).
Based on the above researches, we constructed MCF-7R cell lines that overexpressed lncRNA GAS5, among which the expression levels of mTOR and p-mTOR proteins related to the mTOR signaling pathway were found to be significantly decreased. This result was consistent with the investigation of Xue et al. (37) Moreover, Li et al. reported that upregulation of lncRNA GAS5 could notably inhibit the progression of triple-negative BC, induce cell apoptosis and enhance chemotherapy sensitivity (28). Our results also indicated that overexpression of lncRNA GAS5 significantly reduced the rate of fluorescing cells labeled with EdU among MCF-7R cells, indicating inhibited cell growth. Meanwhile, after lncRNA GAS5 was overexpressed, the expression levels of the PCNA protein related to growth and of the Bcl-2 protein related to inhibiting apoptosis were significantly decreased. That finding indicated that overexpressed lncRNA GAS5 can hinder the growth of MCF-7R cells, probably through the mTOR signaling pathway. To further identify whether Met inhibits cell growth and reverses endocrine resistance through regulation of the mTOR signaling pathway via lncRNA GAS5, we silenced lncRNA GAS5 in MCF-7R cells. After combined treatment with OHT and Met, the expression level of p-mTOR protein was significantly increased instead of being decreased. On the contrary, the mTOR signaling pathway was activated and cell proliferation was promoted to a certain extent, indicating that Met did not have a marked effect on MCF-7R cells with lncRNA GAS5 knockdown. It further proved that lncRNA GAS5 fulfills a key task in Met inhibiting the growth of MCF-7R cells. Therefore, when Met treated the MCF-7R cells, it may have activated the expression of AMPK and hindered the expression of mTOR, which reduced the phosphorylation of downstream P70S6K, thus in turn inhibiting the growth of cells and finally reversing resistance to tamoxifen. All of these were regulated by lncRNA GAS5.
However, cell apoptosis showed no significant changes with lncRNA GAS5 overexpression or knockdown. Although upregulation of lncRNA GAS5 affected the expression of Blc-2 protein, there was no statistical significance for the results of flow cytometry. Because apoptosis of cells is a very complex biological process, affected by many factors, further studies of its mechanism are needed.
Moreover, early studies reported that mTOR inhibitors could significantly increase the expression of lncRNA GAS5 and hinder the growth of hormone-sensitive cell lines (e.g., prostate and BC cells) (15,37,38). Therefore, in this study, we used RAPA as a positive control and found that the effect of Met was similar to that of RAPA, which further confirmed that Met may be an effective mTOR inhibitor.
Conclusions
Our study indicated that Met could depress the expansion and induce the apoptosis of endocrine-resistant BC cells, based on the upregulation of the expression level of lncRNA GAS5 and the inhibition of overactivation of the mTOR signaling pathway, thus reversing the endocrine resistance of BC cells. Met plays a positive role in delaying the drug resistance of BC and improving the prognosis of BC patients, which provides a safe, effective and economical treatment plan for endocrine drug resistant BC. Furthermore, the inhibiting influence on BC cells by Met was similar to that of the mTOR inhibitor RAPA. Both are expected to novel drugs in clinical practice for BC treatment. However, this study was carried out at the cellular level in vitro and does not reflect the processes in whole organisms. Further trials still need to be carried out in animals and clinical patients.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank all the research participants in the laboratory, and the Wenzhou Science & Technology Bureau for the funding of the study.
Funding: This project was supported by a grant from the Wenzhou Science & Technology Bureau (Y20180089).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-795/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-795/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-795/coif). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. 10.1146/annurev-med-070909-182917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani A, Stebbing J, Giamas G, et al. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front Endocrinol (Lausanne) 2019;10:245. 10.3389/fendo.2019.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar BN, Rajput S, Dey KK, et al. Celecoxib alleviates tamoxifen-instigated angiogenic effects by ROS-dependent VEGF/VEGFR2 autocrine signaling. BMC Cancer 2013;13:273. 10.1186/1471-2407-13-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov 2003;2:205-13. 10.1038/nrd1031 [DOI] [PubMed] [Google Scholar]
- 7.Faria J, Negalha G, Azevedo A, et al. Metformin and Breast Cancer: Molecular Targets. J Mammary Gland Biol Neoplasia 2019;24:111-23. 10.1007/s10911-019-09429-z [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 9.Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469-80. 10.1016/j.tem.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, et al. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017;60:1639-47. 10.1007/s00125-017-4372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zi F, Zi H, Li Y, et al. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol Lett 2018;15:683-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 Pathways in Endocrine Resistant HR+/HER2- Metastatic Breast Cancer: Biological Mechanisms and New Treatments. Cancers (Basel) 2019;11:1242. 10.3390/cancers11091242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysocki PJ, Wierusz-Wysocka B. Obesity, hyperinsulinemia and breast cancer: novel targets and a novel role for metformin. Expert Rev Mol Diagn 2010;10:509-19. 10.1586/erm.10.22 [DOI] [PubMed] [Google Scholar]
- 14.Goustin AS, Thepsuwan P, Kosir MA, et al. The Growth-Arrest-Specific (GAS)-5 Long Non-Coding RNA: A Fascinating lncRNA Widely Expressed in Cancers. Noncoding RNA 2019;5:46. 10.3390/ncrna5030046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickard MR, Williams GT. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat 2014;145:359-70. 10.1007/s10549-014-2974-y [DOI] [PubMed] [Google Scholar]
- 16.Arshi A, Sharifi FS, Khorramian Ghahfarokhi M, et al. Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol Ther Nucleic Acids 2018;12:751-7. 10.1016/j.omtn.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourtada-Maarabouni M, Pickard MR, Hedge VL, et al. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009;28:195-208. 10.1038/onc.2008.373 [DOI] [PubMed] [Google Scholar]
- 18.Du Z, Fei T, Verhaak RG, et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol 2013;20:908-13. 10.1038/nsmb.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Huang H, Li Y, et al. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep 2016;36:3241-50. 10.3892/or.2016.5200 [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Wang W, Jiang J, et al. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One 2013;8:e73991. 10.1371/journal.pone.0073991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma N, Li S, Zhang Q, et al. Long non-coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA-21 and SPRY2 expression. Exp Ther Med 2018;16:73-82. 10.3892/etm.2018.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansoori Y, Tabei MB, Askari A, et al. Expression levels of breast cancer-related GAS5 and LSINCT5 lncRNAs in cancer-free breast tissue: Molecular associations with age at menarche and obesity. Breast J 2018;24:876-82. 10.1111/tbj.13067 [DOI] [PubMed] [Google Scholar]
- 23.Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016;29:452-63. 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Q, Liu Y, Lyu H, et al. Long Noncoding RNA GAS5, Which Acts as a Tumor Suppressor via microRNA 21, Regulates Cisplatin Resistance Expression in Cervical Cancer. Int J Gynecol Cancer 2017;27:1096-108. 10.1097/IGC.0000000000001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta 2013;1832:1613-23. 10.1016/j.bbadis.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Dong S, Zhang X, Liu D. Overexpression of long noncoding RNA GAS5 suppresses tumorigenesis and development of gastric cancer by sponging miR-106a-5p through the Akt/mTOR pathway. Biol Open 2019;8:bio041343. 10.1242/bio.041343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Sun J, Zhao H, et al. Long Non-Coding RNA (lncRNA) Growth Arrest Specific 5 (GAS5) Suppresses Esophageal Squamous Cell Carcinoma Cell Proliferation and Migration by Inactivating Phosphatidylinositol 3-kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Signaling Pathway. Med Sci Monit 2018;24:7689-96. 10.12659/MSM.910867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Li L, Yuan H, et al. Up-regulated lncRNA GAS5 promotes chemosensitivity and apoptosis of triple-negative breast cancer cells. Cell Cycle 2019;18:1965-75. 10.1080/15384101.2019.1635870 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res 2011;17:3993-4005. 10.1158/1078-0432.CCR-10-2243 [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Lee J, Jang SY, et al. Anticancer effect of metformin on estrogen receptor-positive and tamoxifen-resistant breast cancer cell lines. Oncol Rep 2016;35:2553-60. 10.3892/or.2016.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017;168:960-76. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall MN. mTOR-what does it do? Transplant Proc 2008;40:S5-8. 10.1016/j.transproceed.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Guo Y, Chen S, et al. Metformin enhances tamoxifen-mediated tumor growth inhibition in ER-positive breast carcinoma. BMC Cancer 2014;14:172. 10.1186/1471-2407-14-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269-73. 10.1158/0008-5472.CAN-06-1500 [DOI] [PubMed] [Google Scholar]
- 35.Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992;69:1227-36. 10.1016/0092-8674(92)90643-Q [DOI] [PubMed] [Google Scholar]
- 36.Gu J, Wang Y, Wang X, et al. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer Lett 2018. Retraction in: Cancer Lett 2021;517:106. 10.1016/j.canlet.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 37.Xue D, Zhou C, Lu H, et al. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol 2016;37:16187-97. 10.1007/s13277-016-5429-8 [DOI] [PubMed] [Google Scholar]
- 38.Yacqub-Usman K, Pickard MR, Williams GT. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 2015;75:693-705. 10.1002/pros.22952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as