Abstract
Background
With long-term pharmacotherapy, Parkinson’s disease (PD) is expectedly to incur a significant healthcare burden. However, drug utilization and costing study is limited, so is the cost composition and its impact on resource allocation. This study took a healthcare provider’s perspective to quantify medical and drug expenses and the utilization of drugs for managing PD and its complications.
Methods
Medical resources use and associated cost of outpatient visits and inpatient admission episodes for PD patients were extracted from electronic medical records at a tertiary hospital in China from 1 January 2016 to 15 August 2018. Total and average direct medical (costs of outpatient visits and inpatient admission episodes) and drug costs were calculated during the study period and each calendar year. Drug cost was quantified by defined daily dose cost (DDDc) and levodopa equivalent dose cost (LEDc) per outpatient visit or inpatient admission episode for PD in Chinese yuan (¥), stratified by medication categories, and presented in descriptive statistics.
Results
Overall, 18,158 outpatient visits and 366 inpatient admissions were incurred by 2,640 outpatients and 330 inpatients, with a median age of 71.0 and 73.5 years, respectively. Drug cost accounted for 97.82% and 23.33% of outpatient and inpatient medical expenditure. The average cost of drugs for managing PD accounted for 60.48% (¥952.50) and 2.70% (¥564.90) of cost per outpatient visit and inpatient episode, while drugs for managing PD complications was 11.38% and 0.70%, respectively. The highest DDDc and LEDc of drugs for managing PD per outpatient visit or inpatient episode were incurred by pramipexole (¥56.90–72.70 and ¥227.48–290.67) and entacapone (¥37.70–45.70 and ¥228.64–276.77). The DDDc and LEDc of pramipexole is more than 10 times that of levodopa/benserazide (DDDc: ¥4.90–5.70; LEDc: ¥10.14–11.98) and carbidopa/levodopa (DDDc: ¥4.00–5.00; LEDc: ¥11.02–13.95).
Conclusions
The outpatient direct medical cost for patients with PD was predominantly attributed to drug cost for managing PD, but drug cost weighed less of the inpatient cost. After adjusting the dose and number of patients, drugs with indirect dopamine effects had an excessively higher cost than dopamine precursors. Their long-term cost-effectiveness in real-world settings warrants further studies.
Keywords: Parkinson’s disease (PD), direct medical cost, drug cost, medications for PD, pharmacoeconomic
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disease commonly prevalent in older people (1). In China, 1.7% of people over 65 years have PD (2), and it has been estimated that around 5 million people will have PD by 2030, accounting for almost 50% of the global PD population (3). With a rapidly ageing population, the management of PD and its coexisting non-motor symptoms and complications are associated with a rising disease burden to the healthcare system (4). Understanding the cost of care is vital to inform a sustainable healthcare policy to maximize allocation efficiency and service delivery (5). Nevertheless, estimating the economic burden of PD encompasses complex cost elements, e.g., direct medical costs (costs inquired from therapies), direct non-medical costs (patient’s out-of-pocket expenses) and indirect costs (society’s productivity lost), and the intangible costs (suffering associated with therapies), the latter three are difficult to measure due to methodological challenges (6).
Pharmacotherapy is the mainstay for managing PD (2). There are medications available for PD, such as dopamine precursors (e.g., levodopa and carbidopa), dopamine agonists (e.g., pramipexole, piribedil), anti-dyskinetic (e.g., amantadine), anticholinergics (e.g., trihexyphenidyl), monoamine-oxidase-B inhibitors (e.g., selegiline), and catechol-o-methyl-transferase inhibitors (e.g., entacapone). However, these medicines aim to alleviate symptoms rather than cure disease (7), and many incur intolerable side effects. Besides, with PD’s chronicity and degenerating nature, drug costs comprise the majority of medical expenses. As the disease progresses, it becomes an economic burden to patients, carers, and the healthcare system (8).
Nonetheless, studies are currently lacking on medication utilization for PD and the related cost despite published studies on PD’s direct and indirect, medical and non-medical costs in China and internationally (6,8-10). Moreover, evidence regarding cost comparisons of different medications for PD is scarce. Two previously published studies have investigated the direct medical costs to outpatients and inpatients with PD by questionnaire survey (8,9). Liu et al. [2016] found that drug costs accounted for 63% of the total direct medical cost (¥21,035) to patients with PD in 2014 (9); Li et al. [2021] reported that medications for PD accounted for 66–76% of direct medical costs at different disease phases (8). However, the surveys involved recall bias in data collection. Some previous studies conducted in other countries have explored the costs of PD through databases (6,10), such as administrative claims databases, from a healthcare payer’s perspective (10). Nevertheless, these studies only reported the total drug cost without presenting the quantities and frequencies of medication use (6,10,11), which is the key parameter to estimate the cost for overall economic evaluation.
The cost of medications for managing PD varies greatly (8), which may be the key determinant for their cost-effectiveness. Li et al. [2021] reported that dopamine precursors (the gold standard of symptomatic management of PD) only account for 3% of the cost of medications for PD, while dopamine agonists and monoamine-oxidase-B inhibitors comprised 65% and 28%, respectively (8). Selecting cost-effective medications for long-term PD management significantly affects the medical expense from the perspectives of healthcare providers and payers. Although the current Chinese clinical guidelines suggest that patients’ economic burden should be considered in choosing therapeutic regimens (2), there is no robust evidence indicating the cost-effectiveness of these types of drugs to inform clinical practice in China.
Therefore, this study aimed to identify the magnitude of drug cost over the total medical expense and measure medication usage and cost for managing PD disease and its complications, in order to inform future economic evaluations. As almost all the participants with PD initiated their treatments and prescriptions in the hospital under the current Chinese medical security systems, this study used a single hospital's data as a pilot study to inform further multi-center research. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1014/rc).
Methods
Study design and population
This cross-sectional study was conducted in 2018 using the electronic medical records from Peking University Third Hospital (PUTH), a 2,264-bed tertiary care medical center and teaching institution in Beijing, the capital of China. The study period was from 1 January 2016 to 15 August 2018, which yielded the most recent and available data when this study was initiated. From 2016 to 2018, there were 3.94–4.22 million outpatients and 100,549–137,655 inpatients in PUTH. Since December 2017, the hospital launched a joint clinic by physicians and pharmacists for patients with PD. Pharmacists who provided pharmaceutical services to patients with PD identified the financial burden during long-term drug treatment for PD (12).
The study population included patients with a newly diagnosed PD or a PD history who were admitted to PUTH or visited the outpatient clinics of PUTH during the study period. The diagnosis record of hospital admission episode or outpatient visit was screened by the International Classification of Diseases, Tenth Revision, Clinical Modification code (ICD-10 code: G20) and relevant terms (e.g., PD) to identify the study population.
Data source
This study took a healthcare provider’s perspective and a bottom-up approach to quantify medical resource use and attach a unit cost to calculate the medical and drug-related cost. Data related to patients’ hospital admission episodes or outpatient visits were identified from the hospital’s electronic medical records and calculated separately.
Each outpatient visit’s administrative information (i.e., the date and specialty, type of health insurance); patient demographics (i.e., age, gender) and disease diagnosis; and medical resource use (i.e., types of medical resources, items, and unit cost) and prescription (i.e., each drug’s branded/generic name, quantity, and unit cost) data were extracted. Likewise, the administrative information of each inpatients' hospitalization episode (i.e., start and end time, department of admission and discharge, type of discharge); patient demographics and diagnosis; and medical resource and drug use data were retrieved. There was no missing data of the above items.
Before further analysis, all data was pseudonymized by the principal investigator (ZMY) and stored in a password-protected computer. Only the research team at PUTH could access the data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Peking University Third Hospital (No. IRB00006761-M2018228). Individual consent for this retrospective analysis was waived.
Outcome measures
The primary outcomes were direct medical and drug costs for each outpatient visit or hospital admission episode in Chinese yuan (¥). In 2018, 6.88 Chinese yuan equaled 1 United States dollar. The direct medical cost was derived from multiplying medical resource use and the unit cost, retrieved from the electronic medical records. The medical resource included medicine, non-pharmacological treatment, diagnosis, disposable equipment, and medical service (e.g., consultation, nursing, oxygen therapy, bed, heating) incurred at each outpatient visit or hospital admission episode.
Drug costs were derived from multiplying the total amount of prescribed medicines and the unit cost of each drug, which was retrieved from the Hospital's Drug Information System. The drug costing calculation included Western medication for managing PD, its complications (i.e., antidepressants, hypnotics, and dementia medications), and chronic comorbidities (e.g., diabetes, hypertension, cerebral infarction, and so on) and traditional Chinese medicine (TCM; Chinese patent and herbal medicine) prescribed to patients with PD at each outpatient visit or hospital admission episode.
A total of 8 drugs (of 6 categories) are available for managing PD at the PUTH, including levodopa/benserazide, selegiline, pramipexole, piribedil, carbidopa/levodopa, amantadine, entacapone, and trihexyphenidyl. As the prescribed dose varied in different patients, the total amount of prescribed medicines was further quantified by the defined daily dose (DDD) and levodopa equivalent dose (LED).
The number of DDDs was derived from dividing the total amount of prescribed doses by the DDD. The DDD as “the assumed average maintenance dose per day for a drug used for its main indication in adults” was obtained from the World Health Organization Collaborating Centre for Drug Statistics Methodology website (13). The LED of medications for managing PD was derived from dividing the number of prescribed doses by conversion factors to the daily equivalent dose of levodopa (14).
The defined daily dose cost (DDDc) and levodopa equivalent dose cost (LEDc) were then calculated by dividing the drug cost by DDD and LED as the following formulae:
| [1] |
| [2] |
| [3] |
| [4] |
Likewise, the number of DDD and DDDc were calculated for medicines indicated for managing complications of PD and chronic comorbidities.
Statistical analysis
Total medical and drug costs were analyzed separately for outpatient visits and inpatient admission episodes and presented as the average cost per year, per visit, and admission episode. The average annual cost was calculated by dividing the total cost by 2.625 years (i.e., entire study period, 31.5 months). The average cost per visit or admission episode was derived from dividing the total medical or drug cost by the number of outpatient visits or inpatient admission episodes when the corresponding medical resource use was incurred. The same methods were also applied to repeatedly analyze the average cost per year, per visit, and admission episode in each calendar year.
The results were presented by descriptive statistics. For quantitative data following a normal distribution, data were expressed as mean with standard deviation (SD); for non-normally distributed data, as median with interquartile range (IQR); for qualitative data, as frequency and constituent ratio, and the chi-squared test was used to compare the differences between groups. Total and average direct medical costs per year, visit, or admission were presented in tables and figures and stratified by categories of medical resource use or type of drugs. The statistical analyses were performed with the software SPSS 25.0 (IBM Corp., Armonk, NY, USA) and Microsoft Office Excel 2019 (Microsoft Corp., Redmond, WA, USA).
Results
Characteristics of patients
During the study period, there were 2,640 and 330 patients with PD recruited from outpatient and inpatient settings without missing data, and the median age was 71.0 (IQR: 61.0 to 79.0) and 73.5 (IQR: 66.0 to 80.0) years, and males accounted for 54.20% and 62.73%, respectively. More than 60% of patients were elderly (>70 years), and the proportion was significantly higher in patients with PD admitted to the hospital than those who visited the outpatient clinics (79.40% vs. 63.52%, P<0.001). On the contrary, a significantly higher proportion of outpatients with PD had health insurance coverage than the inpatients (64.24% vs. 50.00%, P<0.001). In total, 18,158 outpatient visits and 366 inpatient hospitalization episodes were identified, and the median number of visits or admission was 65 (IQR: 27 to 143) and 2 (IQR: 1 to 5) during the study period, respectively. The median length of hospital stay was 10 days (IQR: 6 to 19) of 366 hospitalization episodes (Table 1).
Table 1. Characteristics of patients with Parkinson’s disease who visited or were admitted to Peking University Third Hospital during the study period.
| Characteristics | Outpatients (n=2,640) | Inpatients (n=330) | P value |
|---|---|---|---|
| Gender | 0.003 | ||
| Male | 1,431 (54.20%) | 207 (62.73%) | |
| Female | 1,209 (45.80%) | 123 (37.37%) | |
| Age (median, IQR) | 71.0 (61.0, 79.0) | 73.5 (66.0, 80.0) | <0.001 |
| <65 | 963 (36.48%) | 68 (20.61%) | <0.001 |
| ≥65, <75 | 591 (22.38%) | 108 (32.73%) | |
| ≥75 | 1,086 (41.14%) | 154 (46.67%) | |
| Type of health insurance | <0.001 | ||
| Health insurance | 1,696 (64.24%) | 165 (50.00%) | |
| No health insurancea | 944 (35.76%) | 165 (50.00%) | |
| Number of visitsb (median, IQR) | 65 [27, 143] | 2 [1, 5] | <0.001 |
| Length of hospital stayc (median, IQR) | Not applicable | 10 [6, 19] |
a, patients funded by public health services and at their own expenses; b, accumulative number of visits for each patient in Peking University Third Hospital; c, duration (days) of each hospitalization episode. IQR, interquartile range.
Direct medical costs
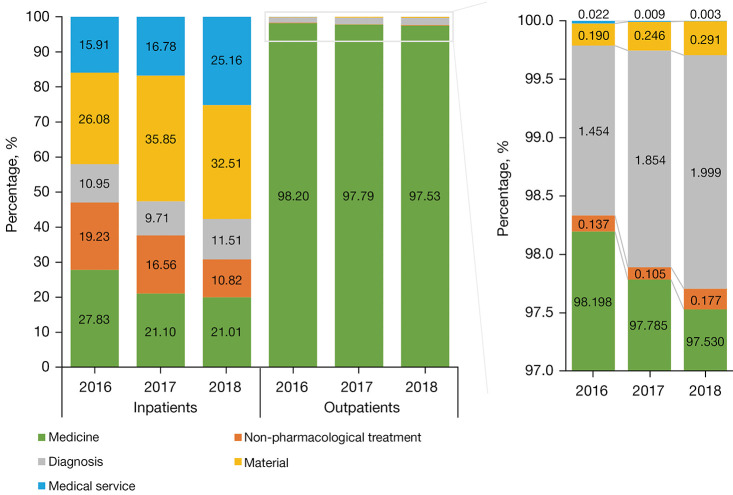
Over the study period, drug costs (¥10,044,433.66) accounted for a significant part (97.82%) of the average annual direct medical costs (¥10,263,118.09) of outpatient visits. Of the average annual outpatient drug cost, medications for managing PD and its complications weighted 60.48% and 11.38%, respectively (Table 2). On the contrary, of the average annual inpatient direct medical costs (¥6,437,186.31), the cost of disposable equipment weighted the highest (31.42%); in comparison, drug cost (¥1,523,360.71) only accounted for 23.33%. Of the average annual inpatient drug cost, medications for managing PD and its complications accounted for 2.70% and 0.70%, respectively (Table 2). The weight of drug cost over the annual direct medical cost slightly decreased from 2016 to 2018 in both outpatient visits (98.20% to 97.53%) and inpatient admissions (27.83% to 20.01%) (Figure 1).
Table 2. Direct medical costs and drug costs of outpatients and inpatients with Parkinson’s disease.
| Item | Outpatient visits | Inpatient admission episodes | |||
|---|---|---|---|---|---|
| Cost per year (%) | Cost per visita | Cost per year (%) | Cost per episodeb | ||
| Total medical cost | 10,263,118.09 (100.00) | 1,483.68 | 6,437,186.31 (100.00) | 46,168.34 | |
| Medicine | 10,044,433.66 (97.82) | 1,472.09 | 1,523,360.71 (23.33) | 10,985.77 | |
| Non-pharmacological treatment | 14,038.13 (0.14) | 80.81 | 1,074,869.49 (16.17) | 7,646.43 | |
| Diagnosis | 178,990.10 (1.78) | 556.69 | 676,691.39 (10.60) | 4,879.99 | |
| Disposable equipment | 24,387.24 (0.25) | 83.46 | 2,015,620.30 (31.42) | 18,896.44 | |
| Medical service | 1,268.96 (0.01) | 23.96 | 1,146,644.42 (18.48) | 6,270.71 | |
| Total drug cost | 10,044,433.66 (100.00) | 1,523,360.71 (100.00) | |||
| Western medicine | |||||
| For managing PD | 6,074,519.85 (60.48) | 952.50 | 41,104.23 (2.70) | 564.90 | |
| For PD-related complications | 1,143,266.90 (11.38) | 723.70 | 10,685.45 (0.70) | 326.20 | |
| For managing comorbidities | 2,437,353.83 (24.27) | 517.70 | 1,462,990.06 (96.04) | 7,994.48 | |
| Subtotal | 9,655,140.57 (96.12) | 1,420.20 | 1,514,779.73 (99.44) | 14,303.20 | |
| Traditional Chinese medicine | |||||
| Chinese patent medicines | 388,000.37 (3.86) | 293.94 | 8,190.59 (0.54) | 158.09 | |
| Chinese herbal medicines | 1,292.73 (0.01) | 55.63 | 390.38 (0.03) | 204.95 | |
| Subtotal | 389,293.09 (3.88) | 293.10 | 8,580.95 (0.56) | 229.80 | |
All the cost was presented in Chinese yuan (in 2018, 6.88 Chinese yuan equaled 1 United States dollar). a, cost each outpatient visit; b, cost per hospital admission episode. PD, Parkinson’s disease.
Figure 1.
Composition of direct medical costs for outpatients and inpatients with Parkinson’s disease in each calendar year.
Similarly, the drug cost constituted a significant part of the medical cost per outpatient visit (¥1,472.09 vs. ¥1,483.68) and less so per inpatient admission episode (¥10,985.77 vs. ¥46,168.34). The cost of medicines for managing PD was ¥952.50 and ¥564.90 per outpatient visit and inpatient admission episode, respectively, when prescribed (Table 2).
Utilization and costs of medications for managing PD
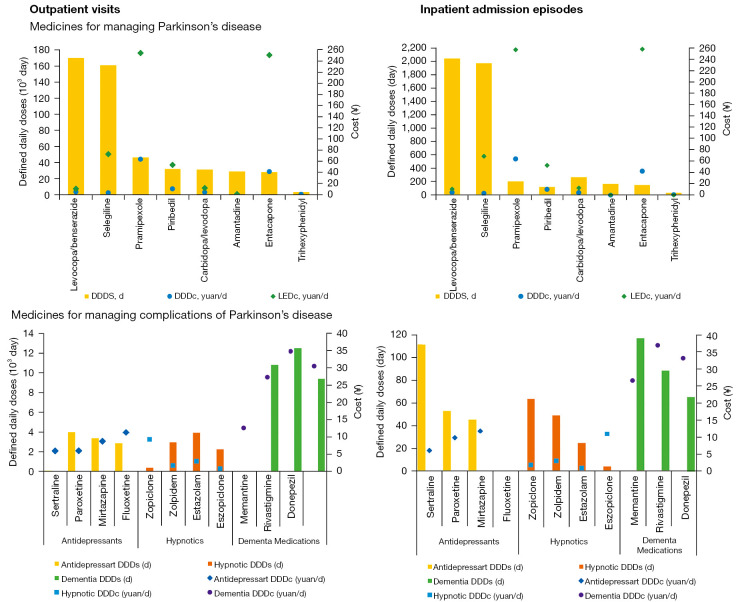
Among the 8 drugs for managing PD, levodopa/benserazide and selegiline had the highest utilization, with the average annual number of DDDs of 170,184.15 and 161,104.76 in outpatient visits and 2,052.84 and 1,980.95 in inpatient admission episodes, respectively. On the contrary, trihexyphenidyl was used least for both outpatients and inpatients with PD (Figure 2). Although not on the top rank of utilization, the medicine with the highest cost in either outpatient visits or inpatient admission episodes was the dopamine agonist, pramipexole, with DDDc of ¥63.70 and ¥64.66 and LEDc of ¥254.78 and ¥209.14, respectively. The catechol-O-methyl-transferase inhibitor, entacapone, incurred the second-highest cost with ¥41.43 and ¥42.75 of DDDc and ¥251.07 and ¥259.07 of LEDc, respectively (Figure 2).
Figure 2.
Average drug utilization and cost of medications for managing Parkinson’s disease and its related complications. The DDDc of fluoxetine in inpatients was unavailable as the total dose was 0. The LEDc of trihexyphenidyl was unavailable due to the lack of a corresponding conversion factor. DDDs, defined daily doses; DDDc, defined daily dose cost; LEDc, levodopa equivalent dose cost; ¥, Chinese yuan; d, day.
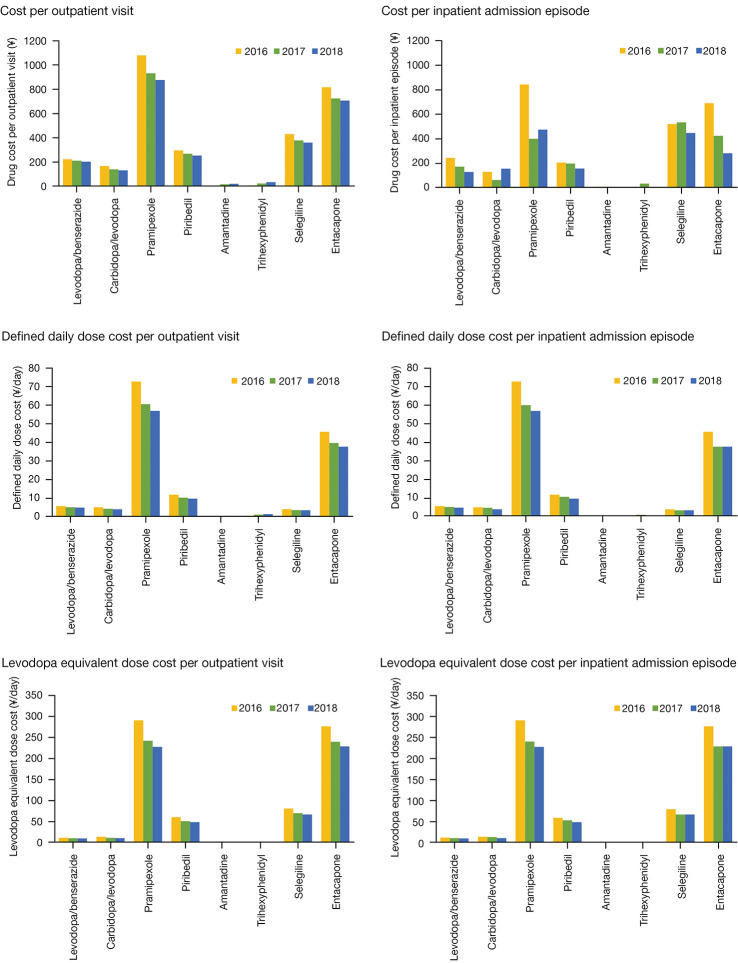
Similarly, the highest average cost per visit or admission episode in each calendar year was incurred by pramipexole for both outpatients (¥1,078.02 to ¥876.05) and inpatients (¥842.93 to ¥473.92) from 2016 to 2018. It was followed by entacapone (¥816.70 to ¥706.87 for outpatients; ¥690.07 to ¥282.94 for inpatients) and selegiline (¥430.79 to ¥361.71 for outpatients; ¥520.73 to ¥447.19 for inpatients). On the contrary, the lowest cost was incurred by amantadine and trihexyphenidyl (Figure 3). The DDDc and LEDc per visit or admission episode in each calendar year of most medications for managing PD declined gradually, particularly from 2016 to 2017. However, the DDDc per outpatient visit of amantadine (¥0.10, ¥0.30, ¥0.40) and trihexyphenidyl (¥0.50, ¥1.10, ¥1.40) slightly increased from 2016 to 2018 over sequential years (Figure 3).
Figure 3.
Average cost per visit or admission episode of medications for managing Parkinson’s disease in each calendar year. LEDc of trihexyphenidyl was not applicable because of lack of a corresponding conversion formula; DDDc of amantadine in 2018 and trihexyphenidyl in 2016 and 2018 for inpatients were not applicable because the total annual dose was 0. DDDc, defined daily dose cost; LEDc, levodopa equivalent dose cost; ¥, Chinese yuan.
Cost of drugs for managing complications of PD
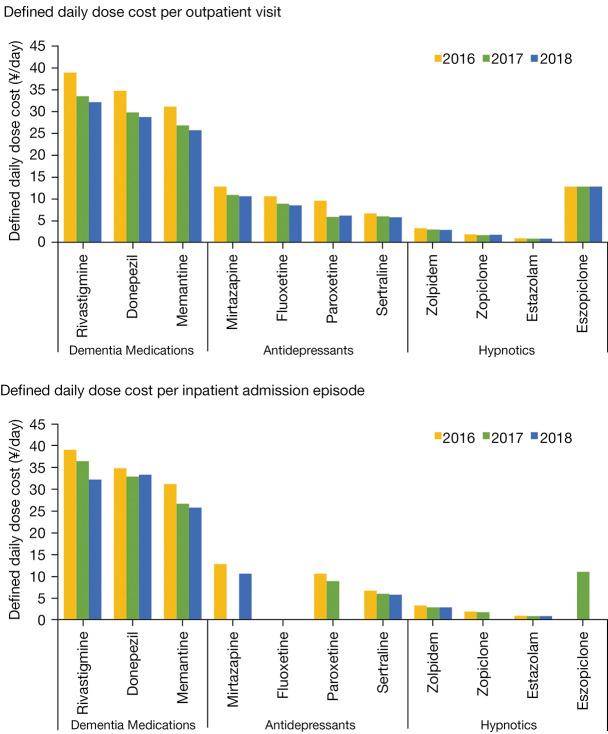
The ratio between the number of prescriptions for drugs managing PD and its complications was about 4:1. Drug costs for managing complications of PD (¥1,143,266.90 and ¥10,685.45) accounted for 11.38% and 0.70% of total inpatient and outpatient drug costs, respectively (Table 2). Of the 11 medications for PD complications, the highest DDDc per outpatient visit or inpatient admission episode in each calendar year was incurred by dementia medications [i.e., rivastigmine (¥38.90 to ¥32.10), donepezil (¥34.70 to ¥28.70), and memantine (¥31.10 to ¥25.70)] (Figure 4). In contrast, the lowest cost was incurred hypnotics, including estazolam (¥1.00 to ¥0.90), zopiclone (¥1.90 to ¥1.70), and zolpidem (¥3.30 to ¥2.90). The average cost per visit or admission episode for managing complications of PD in each calendar year decreased gradually during the study period, especially for the drugs sertraline, memantine, and rivastigmine (Figure 4).
Figure 4.
Average DDDc per visit or admission episode of drugs for managing complications of Parkinson’s disease in each calendar year. The DDDc of eszopiclone in 2016 and 2018, mirtazapine in 2017, fluoxetine in 2016, 2017, and 2018, paroxetine in 2018, zopiclone in 2018 were not applicable because the total annual dose was 0. DDDc, defined daily dose costs; ¥, Chinese yuan.
Discussion
Drug costs accounted for 97.82% and 23.33% of the total direct medical expenditure for outpatients and inpatients at PUTH, respectively. Drugs for managing PD accounted for 60.48% and 2.70% for outpatient and inpatient drug costs, respectively, while medications for managing complications of PD weighted a smaller proportion. Of the drugs for managing PD, levodopa/benserazide and selegiline incurred the highest utilization (DDDs). In contrast, pramipexole and entacapone incurred the highest average daily costs (DDDc and LEDc) and average costs per outpatient visit or inpatient admission episode. A slightly and gradually decreasing trend was found in the drug cost per visit or admission episode over the calendar years.
In contrast, previous literature has reported that medicine costs account for 27.34% of total expenses for PD outpatients and inpatients enrolled in the U.S. Medicare population (10). However, the differences of drug-related economic burden for PD patients between the U.S. and China need more research. In a recent study, Li et al. [2021] found that the weight of drug costs (75.83%), diagnosis (21.84%), and medical service (1.42%) for medical cost of managing early PD patients (Hoehn-Yahr 1-2) differed from the weight of diagnosis (97.08%) and medical services (0.28%) for the medical cost of managing advanced PD patients (Hoehn-Yahr 3-5) in China (8). In our study, diagnostic costs accounted for a relatively smaller proportion, while drug costs were higher. Besides, drug costs accounted for 97.82% of the total costs for outpatients and 23.33% for inpatients, which are higher than the results of studies from Singapore and Metro Manila (11,15). This may be due to the relatively low acquisition cost for other treatment services in China during the study period.
Consistent with previous research (16), we found that levodopa/benserazide was the most frequently prescribed drug of the eight medicines for PD. The Chinese Guidelines for Anti-Parkinson’s Disease (third edition) and the latest edition (fourth edition) both recommend levodopa as the priority for early PD patients (Hoehn-Yahr 1-2.5) (2,17). Besides, McIntosh et al. found that initial treatment with levodopa is highly cost-effective compared with levodopa-sparing therapies, which is similar to our results (18). Drugs with higher costs per visit or admission episode, such as pramipexole, entacapone, and selegiline, may be attributed to their higher unit price.
Congruently, medications with lower prices, such as amantadine and trihexyphenidyl, cost less. However, the DDDc and LEDc of pramipexole and entacapone were first and second for both outpatients and inpatients. It is worth mentioning that the DDDc of selegiline were lower than those of levodopa/benserazide, piribedil, and carbidopa/levodopa, while the LEDc and average costs per visit or admission episode were higher. The higher DDDc indicated a higher cost of defined daily dose for pramipexole and entacapone used in the main indication in adults, while higher LEDc indicated potentially less cost-effectiveness. Piribedil has an economic advantage among the dopamine receptor agonists compared to pramipexole, which needs further verification by cost-effective analysis. The higher costs of dopamine agonists pramipexole was consistent with a previous Chinese study (8).
The severity of PD may affect the complications, including anxiety and depression, sleep disorders, mental disorders, cognitive impairment or dementia, constipation, and so on, which require more medications and result in increased costs. In our study, medications for PD complications accounted for a lower percentage of medication treatment costs than that reported by Li et al. (11.38% vs. 26.69%) (8). This may be because early and advanced PD were included in our study, while the higher percentage was for advanced PD patients (Hoehn-Yahr 3-5), supported by Weir et al. (19). In addition, patients in our study also had comorbidities, which may have increased their economic burden. The top 2 medications with the highest DDDc were acetylcholinesterase inhibitors for dementia, rivastigmine and donepezil, similar to Li et al.’s finding that dementia medications were more costly than antidepressants and hypnotics due to higher unit price (8).
Direct medical costs, especially drug costs, decline gradually as it is widely recognized that drug policies impact direct medical costs. Owing to the elimination of the additional drug mark-up in public hospitals called zero mark-up drug policy (ZMDP), implemented on 1 April 2017 in Beijing, the price of most medicines has dropped, especially for high-priced medications. However, because of their low price and shortages, trihexyphenidyl and amantadine have had their prices rise to guarantee their supply 1 to 2 months after ZMDP. Several studies have indicated that ZMDP reduces direct medical costs of patients, especially drug costs (20-23). Nevertheless, Yan et al. [2020] drew inconsistent conclusions (24) due to differences in geographical areas, medical institution levels, and diseases among studies. Thus, it is necessary to continuously monitor the impact of policies, dynamically adjust the prices of medical services, and implement the corresponding policy interventions (25).
This study had several strengths. Firstly, from the perspective of medical service providers, combined with the work experience of hospital pharmacists, our study was even closer to the reality of clinical practice. Secondly, the data source was the electronic medical record of PUTH, which might have improved the accuracy of results and connected diagnosis and medications well. Thirdly, we compared daily dose costs, levodopa equivalent dose costs, and average costs of 8 commonly used drugs for PD, providing a basis for research on prescribing patterns and pharmacoeconomics.
Our study also had several limitations that need to be addressed in further studies. Firstly, as a single-center study, the study sample may have selection bias. Therefore, the sample’s representativeness and the credibility of this study’s conclusions need to be fully validated. Further multi-center research will be carried out to evaluate the costs of PD in a broader population. Secondly, this study was conducted retrospectively, in which information of the stages of PD was not available when complication costs were related to the severity of PD. Thus, cohort studies in which patients will be followed up are suggested to enhance understanding of disease burden in different stages. Thirdly, the portions covered by the medical insurance differed among PD patients. Medical cost only accounts for a small portion of the total cost for chronic neuropsychiatric conditions, while caregiving such as nursing workers’ fee is costly. However, the data was not available in the electronic medical records from the hospital. Thus, we took the healthcare provider’s perspective in which the portions covered by the medical insurance and costs for caregiving were not usually considered. It is recommended that further research collect the information about health insurance and caregiving costs more comprehensively to improve the research.
Conclusions
Drug costs are an essential part of direct medical costs for PD patients, and long-term medication produces a heavy economic burden. Among medications for PD, levodopa/benserazide and selegiline topped the list of DDDs. Consistently, the top 2 drugs with higher DDDc, LEDc and average cost per outpatient visit or admission episode were pramipexole and entacapone. Amantadine, levodopa/benserazide, and carbidopa/levodopa cost less than other medications for PD and achieve a similar effect. Further cost-effectiveness analysis is required to verify this finding and provide suggestions for medication selection. It is also necessary to continuously monitor the impact of policies and carry out effective policy interventions.
Supplementary
The article’s supplementary files as
Acknowledgments
We extend special thanks to Yayong Wang from the Department of Pharmacy, The Second Affiliated Hospital, Xinjiang Medical University, Wenting Li from the Department of Pharmacy, the Eighth Affiliated Hospital, Sun Yat-Sen University, Qian Cai from School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester for help with editing.
Funding: This study was funded by the National Natural Science Foundation of China (NSFC) (No. 72104003).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Peking University Third Hospital (No. IRB00006761-M2018228). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1014/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1014/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1014/coif). The authors have no conflicts of interest to declare.
References
- 1.Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020;323:548-60. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- 2.Parkinson's Disease and Movement Disorders Group, Neurology Branch of Chinese Medical Association, Parkinson's Disease and Movement Disorders Group, Neurologist Branch of Chinese Medical Doctor Association. Guidelines for the Treatment of Parkinson's Disease in China (Fourth Edition). Chinese Journal of Neurology 2020;53:973-86. [Google Scholar]
- 3.Li G, Ma J, Cui S, et al. Parkinson's disease in China: a forty-year growing track of bedside work. Transl Neurodegener 2019;8:22. 10.1186/s40035-019-0162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:939-53. 10.1016/S1474-4422(18)30295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet 2019;394:1192-204. 10.1016/S0140-6736(19)32136-1 [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis 2020;6:15. 10.1038/s41531-020-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bie RMA, Clarke CE, Espay AJ, et al. Initiation of pharmacological therapy in Parkinson's disease: when, why, and how. Lancet Neurol 2020;19:452-61. 10.1016/S1474-4422(20)30036-3 [DOI] [PubMed] [Google Scholar]
- 8.Li X, Jiang D, Chen JY, et al. Estimation on Direct Medical Cost of Patients with Parkinson's Disease in China from the Perspective of Suppliers. Health Economics Research 2021;38:26-8. [Google Scholar]
- 9.Liu YX, Yin BL, Liu ZH. Investigation on the economic burden and related factors of Parkinson's disease in China. China Journal of Modern Medicine 2016;26:105-8. [Google Scholar]
- 10.Dahodwala N, Li P, Jahnke J, et al. Burden of Parkinson's Disease by Severity: Health Care Costs in the U.S. Medicare Population. Mov Disord 2021;36:133-42. 10.1002/mds.28265 [DOI] [PubMed] [Google Scholar]
- 11.Zhao YJ, Tan LC, Au WL, et al. Estimating the lifetime economic burden of Parkinson's disease in Singapore. Eur J Neurol 2013;20:368-74. 10.1111/j.1468-1331.2012.03868.x [DOI] [PubMed] [Google Scholar]
- 12.Yi ZM, Willis S, Zhang Y, et al. Impact of a Collaborative Pharmaceutical Care Service for Patients With Parkinson's Disease. Front Pharmacol 2022;12:793361. 10.3389/fphar.2021.793361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Collaborating Centre for Drug Statistics Methodology . Available online: https://www.whocc.no/atc_ddd_methodology/who_collaborating_centre/
- 14.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649-53. 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 15.Prado M, Jr, Jamora RD. Cost of Parkinson's disease among Filipino patients seen at a public tertiary hospital in Metro Manila. J Clin Neurosci 2020;74:41-6. 10.1016/j.jocn.2020.01.057 [DOI] [PubMed] [Google Scholar]
- 16.Orayj K, Lane E. Patterns and Determinants of Prescribing for Parkinson's Disease: A Systematic Literature Review. Parkinsons Dis 2019;2019:9237181. 10.1155/2019/9237181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkinson's Disease and Movement Disorders Group, Neurology Branch of Chinese Medical Association. Guidelines for the Treatment of Parkinson's Disease in China (Third Edition). Chinese Journal of Neurology 2014:428-33. [Google Scholar]
- 18.McIntosh E, Kent S, Gray A, et al. Cost-Effectiveness of Dopamine Agonists and Monoamine Oxidase B Inhibitors in Early Parkinson's Disease. Mov Disord 2021;36:2136-43. 10.1002/mds.28623 [DOI] [PubMed] [Google Scholar]
- 19.Weir S, Samnaliev M, Kuo TC, et al. Short- and long-term cost and utilization of health care resources in Parkinson's disease in the UK. Mov Disord 2018;33:974-81. 10.1002/mds.27302 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Li P, Wen J. Impacts of the zero mark-up drug policy on hospitalization expenses of COPD inpatients in Sichuan province, western China: an interrupted time series analysis. BMC Health Serv Res 2020;20:519. 10.1186/s12913-020-05378-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng W, Fang Y, Fan D, et al. The effect of implementing "medicines zero mark-up policy" in Beijing community health facilities. South Med Rev 2012;5:53-6. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang WY, Li YR, Li YJ, et al. A cross-sectional analysis of prescription and stakeholder surveys following essential medicine reform in Guangdong Province, China. BMC Health Serv Res 2015;15:98. 10.1186/s12913-015-0778-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao J, Gu J, Zhang H, et al. The Impact of the National Essential Medicines Policy on Rational Drug Use in Primary Care Institutions in Jiangsu Province of China. Iran J Public Health 2018;47:24-32. [PMC free article] [PubMed] [Google Scholar]
- 24.Yan K, Yang C, Zhang H, et al. Impact of the zero-mark-up drug policy on drug-related expenditures and use in public hospitals, 2016-2018: an interrupted time series study in Shaanxi. BMJ Open 2020;10:e037034. 10.1136/bmjopen-2020-037034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Jia M, Lin Q, et al. Effects of Chinese medical pricing reform on the structure of hospital revenue and healthcare expenditure in county hospital: an interrupted time series analysis. BMC Health Serv Res 2021;21:385. 10.1186/s12913-021-06388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as