Abstract
Background
Patients with epidermal growth factor receptor (EGFR)-sensitive mutations have great opportunity to benefit from EGFR-tyrosine kinase inhibitors (TKI) in non-small cell lung cancer (NSCLC). Although the presence of Kirsten rat sarcoma virus (KRAS) mutations is predictive of lack of benefit from EGFR-tyrosine kinase inhibitor (TKI) therapy for NSCLC, patients with KRAS mutations could be more sensitive to programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors. However, the application of immunotherapy in EGFR mutated NSCLC patients is still controversial.
Case Description
In this study, we reported the case of a 56-year-old NSCLC patient who harbored the mutations of EGFR L858R and KRAS G12D, with a high tumor mutational burden value and positive PD-L1 expression. Considering the EGFR sensitive mutation, gefitinib combined pemetrexed was administered; however, the disease progressed soon after. The patient then underwent combined treatment of bevacizumab (400 mg), camrelizumab (200 mg), and pemetrexed (0.8 mg), and partial response was observed after 4 months. When chemotherapy was removed from the combined treatment, liver metastasis was detected. Interestingly, the disease was well controlled when the combined treatment of bevacizumab, camrelizumab, and pemetrexed was resumed. Overall, the patient benefits lasted more than 17 months.
Conclusions
Our results indicated that immunotherapy may be a potential choice in NSCLC with EGFR and KRAS mutations, and combined chemotherapy may effectively increase therapeutic efficiency during combined immunotherapy.
Keywords: Non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), programmed death-ligand 1 (PD-L1), immunotherapy, case report
Introduction
Lung cancer is a disease with high rates of incidence and mortality worldwide (1). Most patients are diagnosed at stages III and IV. The factors related to the staging and prognosis of non-small cell lung cancer (NSCLC) include age, overall health, lifestyle such as smoking and drinking, immune and genetic markers of cancer, family history, and so on. Traditional treatments for lung cancer include surgery, chemotherapy, and radiotherapy (2). In the past few years, with the continuous progress of molecular tissue detection technology, the development of targeted therapy has been greatly promoted. The survival of advanced NSCLC patients with sensitive epidermal growth factor receptor (EGFR) mutation has been significantly improved (3). However, the majority of NSCLC patients with EGFR-tyrosine kinase inhibitor (TKI) mutations have no response or early resistance to EGFR-TKI (4,5). The mutation of EGFR T790M is a common resistance mechanism of TKI. Bypass activation such as MET and HER2 activation, downstream pathway including BRAF and PI3K, and histological exchange such as NSCLC transformation to small cell lung cancer, are all potential resistance mechanisms (6,7).
Programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) immunotherapy is another effective treatment option for lung cancer patients, and the combination of chemotherapy, radiotherapy and immunotherapy can effectively improve the efficiency and clinical prognosis of various solid tumors, including lung cancer (8). In a study, only 20% of the unselected patients responded to immunotherapy (9). The expression of PD-L1 and tumor mutational burden (TMB) are biomarkers for the prediction of response to immunosuppressants (10). The heterogeneity of expression of PD-L1 existed in patients with NSCLC. Among them, 33.7% of patients with PD-L1 positive expression ≥1% and 10.8% of patients with PD-L1 positive expression ≥50% (11). Previous study also showed that the expression of PD-L1 is associated with type of tumor sample, resection versus biopsy samples, and biopsies of primary versus metastatic cancers (12). Clinical trials have shown that pembrolizumab or nivolumab combination therapy can significantly improve patient prognosis (13). However, the efficacy of anti-PD-1/PD-L1 immunotherapy is limited in patients with EGFR-sensitive mutations (14). Although the combination of atelizumab, chemotherapy, and bevacizumab is effective in EGFR positive patients (15), there have been few reports of camrelizumab in EGFR positive patients. Especially in the treatment of patients with EGFR and KRAS double mutations, there is still controversy about which is more suitable for targeted therapy or chemotherapy (16-18). Immunotherapy in patients with EGFR and KRAS double mutations is also rarely reported. In this case report, we detailed the case of a male patient with EGFR sensitive mutation who benefited from combined immunotherapy. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-403/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
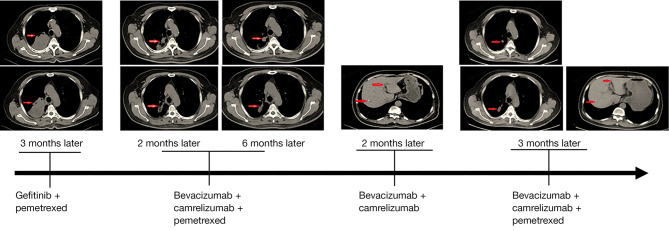
In October 2019, a male patient was diagnosed with NSCLC and invasive lung adenocarcinoma, with tumor stage of IVA (cT3N2M1b). Next generation sequencing-based genomic detection was performed and the results showed that the patient harbored the mutations of EGFR L858R and KRAS G12D (Table 1). Meanwhile, the TMB was evaluated as 12.7 muts/Mb, which was considered TMB-high, and the expression of PD-L1 was also evaluated as positive (22C3 TPS: positive, 50%; 28-8 TPS: positive, 80%). According to the EGFR L858R mutation, gefitinib combined with pemetrexed was used for 4 months, but the tumor progressed. The diameter of the tumor was 77 mm × 56 mm. On 20 March 2020, considering the level of TMB and PD-L1 expression, the combined treatment of bevacizumab (400 mg), camrelizumab (200 mg), and pemetrexed (0.8 mg) was administered. After 2 months of this treatment, the adrenal nodule found at the beginning of admission had disappeared, and the diameter of the tumor had decreased to 56 mm × 25 mm (Figure 1). The treatment duration was 4 months, the diameter of the tumor continued decreased to 49 mm × 25 mm, and the curative effect was evaluated as partial response (PR). After 6 cycles of chemotherapy, the combined treatment of bevacizumab (400 mg) and camrelizumab (200 mg) was continued for 2 months. On 4 November 2020, liver metastasis was detected upon reexamination (Figure 1). We attempted to continue the combined treatment of bevacizumab (400 mg), camrelizumab (200 mg), and pemetrexed (0.8 mg), and interestingly, we found that both the primary and metastatic tumors were diminished for 3 months. To date, the patient has continued to benefit from the combination of the 3 drugs for more than 17 months (the last follow-up time was August 2021).
Table 1. Patient genomic variations information.
| Gene name | Amino acid variation | Variation site | Variation type |
|---|---|---|---|
| EGFR | L858R | Exon 21 | SNV |
| KRAS | G12D | Exon 2 | SNV |
| NOTCH1 | M771I, T772A | Exon 14 | SNV |
| K814* | Exon 15 | SNV | |
| CTNNA2 | K873N | Exon 18 | SNV |
| DNMT3A | A572S | Exon 15 | SNV |
| FAM1358 | E515A | Exon 13 | SNV |
| GRIN2A | P1053S | Exon 14 | SNV |
| LRP1B | H42Q | Exon 2 | SNV |
| G3378V | Exon 64 | SNV | |
| MTAP | – | – | Deletion |
| PIK3CG | E415Q | Exon 2 | SNV |
| RBM10 | L454Sfs*31 | Exon 13 | InDel |
| TNFRSF19 | E82* | Exon 4 | SNV |
| TRIO | S1948F | Exon 38 | SNV |
*, Stop codon. SNV, single nucleotide variant; InDel, short insertion/deletion.
Figure 1.
Details of the pathological images and treatments during the course of the disease. Red arrows indicate tumor lesions or nodules.
Discussion
With the development of next generation sequencing technology, the targeted therapies based on oncogenic mutations greatly increase the selection of treatment of advanced NSCLC. The EGFR activation mutation, which is approximately almost 50% in the Asian population and nearly 15% in Caucasians, is a hot spot mutation widely used in targeted therapy (4,5,19). The most common mutations are exon 19 deletion and exon 21 L858R point mutation, accounting for nearly 85% of EGFR mutated NSCLC (20). KRAS is another commonly mutated gene and is approximately 8-12% in Chinese NSCLC patients (21,22). Previous study showed that EGFR and KRAS mutations are mutually exclusive in NSCLC, however, the KRAS mutations are probably a resistance mechanism of first-generation EGFR-TKI (23). Jia et al. also reported that KRAS mutation is a negative predictive factor in advanced NSCLC patients (24). Although studies have shown that KRAS mutations do not affect the responses of EGFR-TKI (16,17,25), low efficiency was observed, with the short disease-free survival (DFS) (26). Considering the mutation of EGFR L858R, we tried to administer gefitinib combined with chemotherapy, however, the patient was not sensitive to gefitinib, which may have been the cause of KRAS mutation. Studies have shown that different driving gene mutations have different effects on the efficacy of immunotherapy (27,28). When treated with immunotherapy, patients with EGFR sensitive mutations have a poor prognosis, while those with KRAS mutations may have a longer progression-free survival (PFS) (29,30). In this study, the patients carried both EGFR sensitive mutation and KRAS mutation, and high TMB and high PD-L1 expression (≥50%) were also detected. Patients with PD-L1 expression higher than 50% showed improved objective response rate (31). These results support the possibility of benefit from immunotherapy. Although there has been a precedent of TKI combined with immunotherapy (32), considering the rapid progress of the patient while receiving gefitinib, treatment was switched to combined immunotherapy, anti-vascular inhibitor, chemotherapy, and they continued to benefit for 4 months. It is suggested that immunotherapy can potentially be selected for patients with both KRAS and EGFR mutations.
Generally, the TMB of patients with general EGFR mutation in lung adenocarcinoma is low, which may be related to the microenvironment of lung adenocarcinoma, and leads to poor prognosis of immunotherapy (14). Low TMB represents a lack of killer T cells. When the dominant tumor cells are inhibited and other non-dominant tumor cells are enriched, TMB may be increased and promote the infiltration of killer T cells, so as to increase the opportunity of immunotherapy. Chemotherapy may play a key role in this process. So far, the treatment of NSCLC patients with EGFR and KRAS mutations is still under exploration. Zhuang et al. concluded that patients harboring co-alterations tend to benefit more from TKI therapy than from chemotherapy (16). Lee et al. also showed that NSCLC patients with EGFR and KRAS mutations achieved partial responses after receiving targeted therapy with EGFR TKI gefitinib and erlotinib, but showed disease progression less than 20 months (17). In contrast, another report suggested that a subgroup of EGFR mutated tumors with concomitant driver mutations affected the activity of first-line EGFR TKIs (18). In this case report, chemotherapy may have played a key role in the treatment process, which was manifested as stopping chemotherapy and metastasis occurring, and upon resumption of the combination of 3 drugs, the metastatic and primary lesions effectively responded. The possible mechanism is as follows: first, chemotherapy can kill tumor cells and cause aseptic inflammation, which changes tumor microenvironment and may promote the infiltration of killer T cells; second, chemotherapy has the opportunity to kill immunosuppressive cells, so that immune cells can play a better role in killing tumor cells; and third, chemotherapy can kill tumor cells and release a large number of immune antigens to promote the anti-tumor immunity of the immune system. However, the specific mechanism remains to be further studied.
In conclusion, our patient continued to benefit for 17 months through combined treatment of immunotherapy, anti-vascular inhibitor, and chemotherapy, suggesting that next-generation sequencing based genomic alteration detection is necessary and immunotherapy can be attempted in advanced NSCLC patients with EGFR and KRAS mutations. Combined chemotherapy may effectively increase efficiency during immunotherapy treatment.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-403/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-403/coif). XS and SZ are from Shanghai OrigiMed Co., Ltd. The other authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Luo X, Cheng C, et al. A gene expression-based immune signature for lung adenocarcinoma prognosis. Cancer Immunol Immunother 2020;69:1881-90. 10.1007/s00262-020-02595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen RL, Sun LL, Cao Y, et al. Adjuvant EGFR-TKIs for Patients With Resected EGFR-Mutant Non-Small Cell Lung Cancer: A Meta-Analysis of 1,283 Patients. Front Oncol 2021;11:629394. 10.3389/fonc.2021.629394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong RF, Zhu ML, Liu MM, et al. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol Res 2021;167:105583. 10.1016/j.phrs.2021.105583 [DOI] [PubMed] [Google Scholar]
- 5.Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. 10.1038/nm.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Chen D, Chen S, et al. Transcriptional activation of cyclin D1 via HER2/HER3 contributes to EGFR-TKI resistance in lung cancer. Biochem Pharmacol 2020;178:114095. 10.1016/j.bcp.2020.114095 [DOI] [PubMed] [Google Scholar]
- 7.Kenessey I, Kramer Z, István L, et al. Inhibition of EGFR improves antitumor efficacy of vemurafenib in BRAF-mutant human melanoma in preclinical model. Melanoma Res 2018;28:536-46. 10.1097/CMR.0000000000000488 [DOI] [PubMed] [Google Scholar]
- 8.Kong X, Lu P, Liu C, et al. A combination of PD 1/PD L1 inhibitors: The prospect of overcoming the weakness of tumor immunotherapy (Review). Mol Med Rep 2021;23:362. 10.3892/mmr.2021.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol 2018;52:269-77. 10.1016/j.semcancer.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020;126:260-70. 10.1002/cncr.32468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Fu YY, Yue QN, et al. Distribution of PD-L1 expression and its relationship with clinicopathological variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol 2019;12:774-786. [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Shen X, Pan Y, et al. Correlation between PD-L1 expression and clinicopathological characteristics of non-small cell lung cancer: A real-world study of a large Chinese cohort. J Thorac Dis 2019;11:4591-601. 10.21037/jtd.2019.10.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama E, Togashi Y, Takeuchi Y, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in -mutated non-small cell lung cancer. Sci Immunol 2020;5:eaav3937. 10.1126/sciimmunol.aav3937 [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 16.Zhuang X, Zhao C, Li J, et al. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med 2019;8:2858-66. 10.1002/cam4.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee T, Lee B, Choi YL, et al. Non-small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Transl Med. 2016;50:197-203. 10.4132/jptm.2016.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachiglio AM, Fenizia F, Piccirillo MC, et al. The presence of concomitant mutations affects the activity of EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer (NSCLC) patients. Cancers (Basel). 2019;11:341. 10.3390/cancers11030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. 10.1097/JTO.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 2011;29:3307-15. 10.1200/JCO.2010.34.0570 [DOI] [PubMed] [Google Scholar]
- 21.Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110:2812-20. 10.1038/bjc.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng S, Wang X, Fu Y, et al. Targeted next-generation sequencing for cancer-associated gene mutation and copy number detection in 206 patients with non-small-cell lung cancer. Bioengineered. 2021;12:791-802. 10.1080/21655979.2021.1890382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Re M, Tiseo M, Bordi P, et al. Contribution of KRAS mutations and c.2369C > T (p.T790M) EGFR to acquired resistance to EGFR-TKIs in EGFR mutant NSCLC: a study on circulating tumor DNA. Oncotarget 2017;8:13611-9. 10.18632/oncotarget.6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Y, Jiang T, Li X, et al. Characterization of distinct types of KRAS mutation and its impact on first-line platinum-based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Oncol Lett. 2017;14:6525-32. 10.3892/ol.2017.7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts P. J, Stinchcombe T.E. KRAS mutation: should we test for it or does it matter?. J Clin Oncol 2013;31:1112-21. 10.1200/JCO.2012.43.0454 [DOI] [PubMed] [Google Scholar]
- 26.Benesova L, Minarik M, Jancarikova D, et al. Multiplicity of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC) patients treated with tyrosine kinase inhibitors. Anticancer Res 2010;30:1667-71. [PubMed] [Google Scholar]
- 27.Seegobin K, Majeed U, Wiest N, et al. Immunotherapy in Non-Small Cell Lung Cancer With Actionable Mutations Other Than EGFR. Front Oncol 2021;11:750657. 10.3389/fonc.2021.750657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin R, Liu C, Zheng S, et al. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol Med 2020;17:768-81. 10.20892/j.issn.2095-3941.2020.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinausero M, Laprovitera N, De Maglio G, et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol 2019;11:1758835919885540. 10.1177/1758835919885540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Wan B, Chen X, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res 2019;8:413-28. 10.21037/tlcr.2019.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CK, Kao SJ, Lai HC. Targeted Therapy and Immunotherapy Lead to Rapid Regression of Advanced Non-Small Cell Lung Cancer with Multiple Driver Mutations. J Thorac Oncol 2018;13:e103-5. 10.1016/j.jtho.2018.01.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as