Abstract
Background
Biomarkers are a key tool in early detection, prognostication, survival, and predicting treatment response of colorectal cancer (CRC). However, little is known about biomarker testing for CRC patients in real-life clinical practice in China. This study aimed to address the usage of biomarker testing and analyze factors related to its acceptance among Chinese patients with advanced CRC.
Methods
A multicenter, cross-sectional, hospital-based clinical epidemiology study was conducted from March 2020 to March 2021. Nineteen hospitals were selected in seven geographical regions of China using stratified, multistage, nonrandomized cluster sampling. Data on demographics and clinical characteristics of each eligible CRC patient in stage III or IV diseases were recorded based on the patients’ self-reporting and/or medical records. In addition, information on whether biomarker testing [RAS, BRAF, and microsatellite instability (MSI)] was performed, the results and timing for performing biomarker testing, and the reasons for refusing biomarker testing were also recorded. Univariate and multivariate logistic regression were conducted to explore the potential factors of biomarker testing.
Results
A total of 4,526 patients were enrolled in the study, of whom 41.4%, 36.1%, and 28.2% underwent RAS, BRAF, and MSI testing, respectively. RAS, BRAF, and high-level MSI (MSI-high) mutation rates in Chinese patients with advanced CRC were 37.0%, 9.9%, and 8.1%, respectively. The logistic regression analysis revealed that the treating hospital, age at diagnosis, education, family income, tumor site, history of chemotherapy and radiotherapy, and metastases were dependent factors affecting the utilization of biomarker testing in advanced CRC in China (P<0.005).
Conclusions
The biomarker testing rate, especially MSI testing, is less prevalent in clinical practice for patients with advanced CRC in China. Our findings may guide the formulation of biomarker testing of CRC strategies in China and other low-income countries.
Keywords: Colorectal cancer (CRC), biomarker, RAS, BRAF, microsatellite instability (MSI)
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy worldwide, with an estimated 1.9 million new cases and 935,173 deaths occurring in 2020 (1). Although CRC incidence and mortality rates have been steady or declining in Western countries for the past two to three decades, both rates have been significantly growing in many developing countries, such as China and Brazil, due to westernization (2). According to China’s most recent national cancer registry, approximately 408,000 new cases of CRC and 195,600 CRC-related deaths occurred in 2016 (3). Even though the age-standardized 5-year survival rate has increased from 47.2% in 2003–2005 to 56.9% in 2012–2015 (4), it is still approximately 10% lower than that in the United States (5). Of more concern, by the time of initial diagnosis, more than half (51.4%) of Chinese CRC patients had progressed to an advanced stage, which dramatically reduced their survival (6).
In China, the current therapeutic options for CRC vary, with therapy allocation largely dependent on available resources, the patient’s financial situation, and the tumor burden (7). With the development of next-generation sequencing (NGS) and medicine design techniques in recent decades, several of the following gene-based biomarkers have helped clinicians make optimal treatment decisions: NRAS, KRAS, and BRAF mutations, and microsatellite instability (MSI) or mismatch repair (MMR). They all play an increasingly critical role in the early diagnosis, targeted drug identification, and drug reactions of patients with CRC.
Monoclonal antibodies against epidermal growth factor receptors (EGFRs), such as cetuximab and panitumumab, have been proven to significantly improve overall survival (OS) in patients with advanced CRC (8,9). RAS mutations have been recognized as a predictor of EGFR-targeted therapy resistance, as confirmed by the OPUS and CCRYSTAL trials (10,11). In addition, BRAF mutations are well-recognized poor prognostic factors for CRC (12,13). Furthermore, recent research has found that CRC patients with varying MSI levels have diverse drug reactivity and sensitivity, hinting that MSI status could be a predictive marker for clinical therapy (14,15).
Although previous studies have been conducted to estimate biomarker prevalence in China, most of them have only focused on a single hospital with a relatively small sample size (16,17). Here, for the first time, our study aims to estimate the percentage of advanced CRC patients in China who have undergone biomarker testing, the mutation prevalence of different molecular subtypes, and identify the barriers limiting testing. We hypothesized that these investigations might yield recommendations to update treatment guidelines and develop tailored treatment strategies for patients with advanced CRC in China and other low- and middle-income countries. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-988/rc).
Methods
This study was approved by the independent review board of Henan Cancer Hospital (No. 2019273), and all patients provided signed informed consent form. The study was conducted in accordance with the Declaration of Helsinki Declaration (as revised in 2013).
Study design
We conducted a multicenter, cross-sectional, hospital-based clinical epidemiology study of advanced CRC in mainland China using stratified, multistage, nonrandomized cluster sampling.
Selection of hospitals
In brief, China was stratified into seven geographic regions according to the traditional administrative district definition: North, Northeast, Northwest, Central, Eastern, Southern, and Southwest. These regions encompass most of the geographic area of China’s mainland and represent different levels of CRC burden (18). Two cities in each geographic region were selected through convenience sampling to ensure the national survey was representative. In each city, one or two leading tertiary cancer hospitals and one or two tertiary general hospitals that were considered representative of the regional resources available to patients were chosen based on their ability to provide comprehensive CRC therapy. Ultimately, nineteen tertiary hospitals (10 cancer hospitals and 9 general hospitals) were involved in the study.
Patient selection and pathologic diagnostic criteria
All eligible CRC patients in the selected hospitals were invited by the interviewer to participate current study. The inclusion criteria of the study were as follows: (I) patients were aged ≥18 years; (II) patients were able to consent to participate in the study; (III) patients had a pathologically confirmed diagnosis of stage III or IV CRC. Patients were excluded if they had severe physical, cognitive, and/or verbal impairments that interfered with the completion of the questionnaire. CRC staging was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system (19).
Data collection and quality control
A designed case report form (CRF) was used to collect information on the enrolled patients, including demographic and medical history, medical experience with CRC diagnosis and treatment, and clinicopathological features (including pathological reports and biomarker status). The detailed information included: (I) basic demographic, such as age, gender, occupational situation, marital status, education, and annual household income of patients; (II) clinical information, such as cancer types (colon cancer, rectal cancer, and both), stage, and metastasis status at diagnosis; and (III) biomarker testing status, such as whether biomarker testing (RAS, BRAF, and MSI) was performed, the results and timing for performing biomarker testing, and the reasons for refusing biomarker testing. All completed questionnaires were checked immediately by the clerks to avoid any missing or invalid data.
Trained interviewers extracted the above information from the patients’ self-report and/or medical records. Then, two data input clerks were recruited in each hospital to independently double-enter the data from the paper questionnaire into an electronic database. The completed databases were sent for validation by running EpiData, version 3.1 (EpiData Association, Odense, Denmark) software. Inconsistencies between the two databases were reported to the local interviewers for adjudication until the databases agreed.
Statistical analysis
All statistical analyses were performed utilizing SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). The demographic and clinical characteristics of the patients were described by the mean and standard deviation (SD) of continuous variables or the percentage and proportion of categorical variables. The chi-square test was employed when comparing differences in the use of biomarker testing and mutation status between colon and rectal cancer. Univariate and multivariate logistic regressions were conducted on the data of patients with complete information to explore predictor variables associated with biomarker testing acceptability. Variables with a P value <0.10 in the univariate model were entered into the multivariate model, and only those variables with a P value <0.05 were retained in the final multivariate model. Odds ratios (ORs) and adjusted ORs (AORs) with 95% confidence intervals (CIs) were calculated using Wald chi-square statistics. All tests were two-tailed tests with a significance level of 0.05, and all the missing data were excluded.
Results
A total of 4,589 advanced CRC patients across seven geographic regions of China’s mainland were included in the study from March 2020 to March 2021. Sixty-three cases were excluded due to a lack of biomarker testing information. Ultimately, 4,526 cases (60.1±11.6 years) with complete information were included in the analysis. Of all cases, 2,059 (45.5%) were colon cancer, and 2,080 (46.0%) were recruited from cancer hospitals (Table 1).
Table 1. Demographic and clinical characteristics of advanced CRC patients.
| Variables | All cases | Colon cancer | Rectal cancer | P value |
|---|---|---|---|---|
| All patients, n | 4,526 | 2,059 | 2,467 | |
| Type of treating hospital, n (%) | 0.091 | |||
| Cancer hospitals | 2,080 (46.0) | 918 (44.6) | 1,162 (47.1) | |
| General hospitals | 2,446 (54.0) | 1,141 (55.4) | 1,305 (52.9) | |
| Gender, n (%) | 0.327 | |||
| Males | 2,693 (59.5) | 1,209 (58.7) | 1,484 (60.2) | |
| Females | 1,833 (40.5) | 850 (41.3) | 983 (39.8) | |
| Age at current therapy | 0.062 | |||
| Mean ± SD, years | 60.1±11.6 | 59.7±11.8 | 60.4±11.4 | |
| <50 years, n (%) | 749 (16.5) | 364 (17.7) | 385 (15.6) | |
| ≥50 years, n (%) | 3,777 (83.5) | 1,695 (82.3) | 2,082 (84.4) | |
| Age at initial diagnosis | 0.075 | |||
| Mean ± SD, years | 58.7±11.8 | 58.3±12.0 | 59.0±11.6 | |
| <50 years, n (%) | 921 (20.3) | 443 (21.5) | 478 (19.4) | |
| ≥50 years, n (%) | 3,605 (79.7) | 1,616 (78.5) | 1,989 (80.6) | |
| Educational level, n (%)† | <0.001 | |||
| ≤6 years | 1,312 (29.0) | 538 (26.2) | 774 (31.4) | |
| 7–12 years | 2,491 (55.1) | 1,155 (56.2) | 1,336 (54.2) | |
| >12 years | 720 (15.9) | 363 (17.7) | 357 (14.5) | |
| Family income per year, n (%) | <0.001 | |||
| <50,000 CNY | 2,601 (57.5) | 1,088 (52.8) | 1,513 (61.3) | |
| 50,000–99,999 CNY | 1,278 (28.2) | 654 (31.8) | 624 (25.3) | |
| ≥100,000 CNY | 647 (14.3) | 317 (15.4) | 330 (13.4) | |
| Stage at diagnosis, n (%) | <0.001 | |||
| Stage I | 109 (2.4) | 43 (2.1) | 66 (2.7) | |
| Stage II | 760 (16.8) | 361 (17.5) | 399 (16.2) | |
| Stage III | 1,947 (43.0) | 753 (36.6) | 1,194 (48.4) | |
| Stage IV | 1,534 (33.9) | 833 (40.5) | 701 (28.4) | |
| Unknown | 176 (3.9) | 69 (3.4) | 107 (4.3) | |
| Metastases, n (%) | <0.001 | |||
| Localized | 2,813 (62.2) | 1,147 (55.7) | 1,666 (67.5) | |
| Liver only | 635 (14.0) | 362 (17.6) | 273 (11.1) | |
| Lung only | 178 (3.9) | 70 (3.4) | 108 (4.4) | |
| Liver and lung combination | 191 (4.2) | 98 (4.8) | 93 (3.8) | |
| Others or unknown | 709 (15.7) | 382 (18.6) | 327 (13.3) |
†, the total number varies due to missing values. CRC, colorectal cancer; SD, standard deviation; CNY, Chinese yuan.
Patient characteristics
Overall, the majority of patients were male (59.5%), received 7–12 years of schooling (55.1%), and had a lower family income [<50,000 Chinese yuan (CNY) per year] (57.5%). The mean age at first diagnosis was 58.7±11.8 years, 76.9% were diagnosed with late-stage (stage III or IV) cancer, and 62.2% had no metastases at the time of diagnosis (Table 1).
Biomarker testing in patients with advanced CRC
Clinicians recommended biomarker testing to 67.7% of all advanced CRC patients. However, less than half (46.2%) underwent at least one molecular test (RAS, BRAF, or MSI). Regarding tumor sites, we found that doctors advised biomarker testing to more colon cancer patients, and more colon cancer patients completed the test (P<0.001). Overall, RAS and BRAF testing was performed in approximately 41.4% and 36.1% of advanced CRC patients in China, respectively. The rate of MSI testing was only 28.2% in patients with advanced CRC, with a statistically significant difference between colon (30.4%) and rectal cancer patients (25.3%) (P=0.037) (Table 2).
Table 2. Percentage of advanced CRC patients tested for RAS, BRAF, and MSI and mutant biomarker status.
| Variables | Total | Colon cancer | Rectal cancer | P value |
|---|---|---|---|---|
| Doctor recommend testing, n (%)† | <0.001 | |||
| Yes | 3,061 (67.7) | 1,472 (71.6) | 1,589 (64.5) | |
| No | 1,460 (32.3) | 585 (28.4) | 875 (35.5) | |
| Complete any genetic testing, n (%) | <0.001 | |||
| Yes | 2,092 (46.2) | 1,068 (51.9) | 1,024 (41.5) | |
| No | 2,434 (53.8) | 991 (48.1) | 1,443 (58.5) | |
| RAS tests, n/N (%) | 1,873/4,526 (41.4) | 953/2,059 (46.3) | 920/2,467 (37.3) | 0.167 |
| RAS wild-type, n (%) | 1,035 (63.0) | 545 (64.7) | 490 (61.3) | |
| RAS mutation, n (%) | 607 (37.0) | 298 (35.3) | 309 (38.7) | |
| Unknown, n | 231 | 110 | 121 | |
| BRAF tests, n/N (%) | 1,632/4,526 (36.1) | 836/2,059 (40.6) | 796/2,467 (32.3) | 0.262 |
| BRAF wild-type, n (%) | 1,275 (90.1) | 649 (88.9) | 626 (91.4) | |
| BRAF mutation, n (%) | 140 (9.9) | 81 (11.1) | 59 (8.6) | |
| Unknown, n | 217 | 106 | 111 | |
| MSI tests, n/N (%) | 1,276/4,526 (28.2) | 625/2,059 (30.4) | 623/2,467 (25.3) | 0.037 |
| MSS, n (%) | 910 (87.9) | 436 (85.3) | 448 (90.3) | |
| MSI-L, n (%) | 41 (4.0) | 21 (4.1) | 20 (4.0) | |
| MSI-H, n (%) | 84 (8.1) | 54 (10.6) | 28 (5.6) | |
| Unknown, n | 241 | 114 | 127 |
†, the total number varies due to missing values. CRC, colorectal cancer; MSI, microsatellite instability; MSS, microsatellite stability; MSI-L, low-level MSI; MSI-H, high-level MSI.
Mutation of RAS, BRAF, and MSI in patients with advanced CRC
Of the 1,873 patients who underwent RAS testing, 607 (37.0%) had a positive RAS mutation status. A positive BRAF mutation status was found in 140 (9.9%) of the 1,632 patients who received BRAF testing. High-level MSI (MSI-H) and low-level MSI (MSI-L) tumors were found in 8.1% and 4.0% of advanced CRC patients, respectively (Table 2).
Factors affecting decision-making regarding the use of biomarker testing
We performed a logistic regression analysis of factors that might affect decision-making regarding the use of biomarker testing for advanced CRC patients. The findings revealed that biomarker testing was more common in patients who were treated at cancer hospitals (P<0.001), diagnosed at a younger age (<50 years old) (P=0.026), received higher education (more than 6 years) (P=0.005 and P<0.001), had a higher family income (≥100,000 CNY per year) (P=0.001), had a diagnosis of colon cancer (P=0.003), had a history of chemotherapy (P=0.001), had no history of radiotherapy (P=0.009), and had metastases (P<0.001). Sex, locality, and age at the time of the survey, TNM stage, and surgical history had no significant effect on decision-making regarding the use of biomarkers (P>0.05) (Table 3).
Table 3. Factors associated with the use of biomarker testing in advanced CRC patients in China.
| Variables | Had biomarker testing (n=2,092), n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | AOR (95% CI) | P value | |||
| Treating hospital | ||||||
| Cancer hospitals | 1,190 (57.2) | Ref | Ref | |||
| General hospitals | 902 (36.9) | 0.44 (0.38–0.49) | <0.001 | 0.39 (0.34–0.46) | <0.001 | |
| Age at survey day | ||||||
| <50 years | 426 (56.9) | Ref | – | |||
| ≥50 years | 1,666 (44.1) | 0.60 (0.51–0.70) | <0.001 | – | – | |
| Age at diagnosis | ||||||
| <50 years | 520 (56.5) | Ref | Ref | |||
| ≥50 years | 1,572 (43.6) | 0.60 (0.51–0.68) | <0.001 | 0.80 (0.66–0.97) | 0.026 | |
| Geographic† | ||||||
| Less developed areas | 955 (47.4) | Ref | – | |||
| Developed areas | 1,137 (45.2) | 0.92 (0.81–1.02) | 0.141 | – | – | |
| Gender | ||||||
| Males | 1,253 (46.5) | Ref | – | |||
| Females | 839 (45.8) | 0.97 (0.86–1.09) | 0.617 | – | – | |
| Educational level | ||||||
| ≤6 years | 471 (35.9) | Ref | Ref | |||
| 6–12 years | 1,175 (47.2) | 1.59 (1.38–1.82) | <0.001 | 1.30 (1.08–1.56) | 0.005 | |
| >12 years | 445 (61.8) | 2.89 (2.39–3.48) | <0.001 | 2.12 (1.61–2.80) | <0.001 | |
| Family income per year | ||||||
| <50,000 CNY | 1,125 (43.3) | Ref | Ref | |||
| 50,000–99,999 CNY | 583 (45.6) | 1.10 (0.96–1.25) | 0.163 | 0.99 (0.82–1.18) | 0.872 | |
| ≥100,000 CNY | 384 (59.4) | 1.92 (1.60–2.28) | <0.001 | 1.52 (1.19–1.94) | 0.001 | |
| Doctor recommend | ||||||
| No | 107 (7.3) | Ref | Ref | |||
| Yes | 1,982 (64.8) | 23.22 (18.80–28.60) | <0.001 | 23.61 (18.78–29.70) | <0.001 | |
| Tumor sites | ||||||
| Colon | 1,068 (51.9) | Ref | Ref | |||
| Rectal | 1,024 (41.5) | 0.66 (0.58–0.74) | <0.001 | 0.79 (0.67–0.92) | 0.003 | |
| Surgery | ||||||
| No | 399 (54.2) | Ref | – | |||
| Yes | 1,692 (44.7) | 0.68 (0.58–0.80) | <0.001 | – | – | |
| Adjuvant chemotherapy | ||||||
| No | 161 (26.5) | Ref | Ref | |||
| Yes | 1,930 (49.4) | 2.71 (2.23–3.27) | <0.001 | 1.54 (1.21–1.96) | 0.001 | |
| Adjuvant radiotherapy | ||||||
| No | 1,676 (47.7) | Ref | Ref | |||
| Yes | 415 (41.5) | 0.78 (0.67–0.80) | <0.001 | 0.78 (0.64–0.94) | 0.009 | |
| Stage at diagnosis | ||||||
| I & II | 291 (33.5) | Ref | – | |||
| III & IV | 1,737 (49.9) | 1.98 (1.69–2.31) | <0.001 | – | – | |
| Metastases | ||||||
| No | 1,016 (36.1) | Ref | Ref | |||
| Yes | 1,066 (63.0) | 3.01 (2.65–3.41) | <0.001 | 2.13 (1.82–2.49) | <0.001 | |
†, less developed areas: Central, Northwest, Southwest, and Northeast; developed areas: East, South, Northern. CRC, colorectal cancer; OR, odds ratio; AOR, adjusted odds ratio; CI, confidence interval; CNY, Chinese yuan.
The time frame for biomarker testing
The time frame for biomarker detection varied considerably among Chinese patients with advanced CRC. Of the 2,092 patients who underwent biomarker testing, nearly one-third (32.6%) received testing after surgery, 24.5% after diagnosis, 19.2% before initial treatment, 18.3% after recurrence, and 5.4% before changing treatment regimens (Figure 1).
Figure 1.
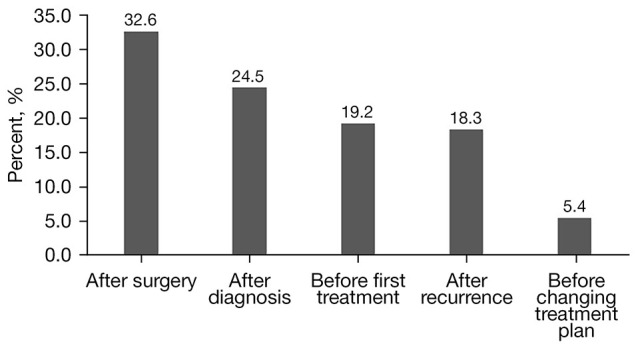
Time frame for performing biomarker testing in Chinese advanced colorectal cancer patients.
Reasons for refusing biomarker testing
Barriers to biomarker testing included the unaffordable price of the testing (23.7%), concerns about the effectiveness of targeted therapy (16.1%), and eagerness to start treatment (5.6%). Surprisingly, most patients (42.5%) did not participate in biomarker testing because they were not being informed of the availability of biomarker testing (Figure 2).
Figure 2.
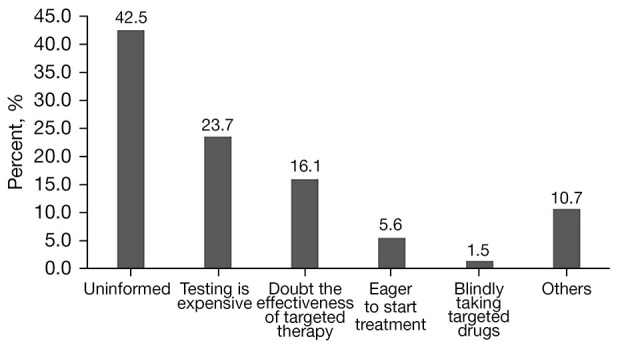
Reasons for refusing biomarker testing in Chinese advanced colorectal cancer patients.
Discussion
This study presents the results of 4,526 Chinese patients with advanced CRC. To the best of our knowledge, this is the first study at a national level in China to analyze biomarkers in patients with advanced CRC. We obtained information on critical markers in CRC patients and analyzed potential influencing factors. These real-world practice data will assist health providers in identifying personalized treatment plans for patients and ultimately reduce the disease burden of CRC.
Some leading guideline bodies, such as the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) (20-22), have recommended important markers for selecting personalized therapies for patients with CRC. In our analysis, less than half (41.4% for RAS, 36.1% for BRAF, and 28.2% for MSI) of patients with advanced CRC had biomarker testing, which was much lower than European rates (>90% for RAS, 66.9% for BRAF, and 41.4% for MSI) (23). There are two possible explanations for the low uptake of biomarker testing: one reason is that referring physicians disagree with the guideline recommendations and are unaware of the utility of biomarkers. Only 67.7% of physicians in this study recommended biomarker testing to patients. Of more concern, 18.3% of these patients only received biomarker evaluation after acquiring recurrent metastases, and 5.4% had biomarker testing on changing treatment regimens, contrary to existing clinical standards. Another reason for patients’ hesitation in undergoing biomarker testing was related to financial concerns. Despite their physicians’ advice, 23.7% of patients did not comply with treatment recommendations due to expense. Furthermore, this study revealed that patients in better financial situations received more biomarker testing than those in worse financial situations. These findings suggest that physicians at all levels need to be better educated on the most updated clinical guidelines. Moreover, policymakers should consider reducing the cost of key biomarker tests or incorporating them into insurance plans.
Gene mutations in the EGFR signaling pathway have emerged as a significant aspect of CRC treatment and prognosis, as their alterations may determine the therapeutic response to anti-EGFR receptor therapy. Several studies have demonstrated that patients harboring RAS alterations have a much shorter survival than patients with wild-type tumors (24,25). The 37.0% RAS mutation frequency observed in this study is similar to that in other Chinese (40.15%) and Asian studies (17,26,27). However, it is lower than that in several Western countries (~50%) (28) but higher than that in patients from the Middle East (19.5%) (29). The discrepancy may be attributed to different diagnostic techniques, ethnicities, genetic factors, multiple sample sizes, and regional diversity. More in-depth investigations are needed to further understand the mechanisms and pathways that might affect mutations in RAS genes.
BRAF mutation status is considered a biomarker of poor prognosis in patients with advanced CRC. Previous analyses have proven that CRC patients with BRAF mutations have a lower survival and shorter progression-free survival (PFS) than BRAF wild-type patients (30,31). In the current study, BRAF-mutated tumors accounted for 9.9% of all patients suffering from advanced CRC, consistent with studies conducted in other countries (8–12%) (32-35). This suggests that from the perspective of BRAF genes, the prognosis of patients with CRC in China is similar to that of other countries.
MSI is the only predictive biomarker approved by the FDA for immune checkpoint blockade inhibitor therapy, such as pembrolizumab and nivolumab (36). In this investigation, MSI testing was performed in 30.4% of colon cancer patients and 25.3% of rectal cancer patients, far from the recommendation of the Chinese Society of Clinical Oncology (CSCO) guidelines. Notably, MSI-H mutations were more frequent in the colon (10.6%) than in the rectum (5.6%), which is consistent with previous research findings (37). The reasons affecting MSI-H incidence vary. First, MSI-H prevalence varies by population; for example, in CRC, MSI-H is most common among Egyptians (37%) and African Americans (20–45%) but least common among Europeans and Americans (8–20%). In comparison to MSI-H, the incidence in Chinese patients is approximately 4.5–15%. Second, MSI-H incidence varies among different stages. MSI-H is more common in stage II CRC (20%) than stage III (12%), and it is rare in stage IV (4%) (38). Finally, the incidence was obtained using several approaches or panels composed of varying microsatellite markers. According to a meta-analysis of 17 articles, the incidence of MSI-H in Chinese CRC patients ranged from 7.7% to 13.5% when utilizing various diagnostic methodologies or panels (39).
Some potential limitations need to be addressed in this study. Firstly, most Chinese people preferred to attend tertiary hospitals for CRC treatment since these facilities have the ability and equipment to treat cancer patients holistically. As a result, the catchment of CRC patients was performed at tertiary hospitals in this study. However, a smaller proportion of patients were being treated at lower-grade hospitals. Thus, the current study results cannot be generalized to lower-grade hospitals in China. Secondly, the data quality was partly dependent on the thoroughness of the clinician’s records, and it is possible that some biomarker testing information was not included. For example, patients were sometimes treated in multiple hospitals, and it is possible that information concerning biomarker testing was missed.
Conclusions
In conclusion, biomarker testing in advanced CRC patients has become routine in clinical practice in China. However, the rate of biomarker testing was relatively low. The findings of this study underscore the need to eliminate discrepancies in CRC biomarker testing and highlight the limited application of the Chinese guidelines, particularly in regard to MSI testing.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank the Beijing Love Book Cancer Foundation for originating this clinical epidemiology study of CRC. The authors also thank the local investigators in different cities across China’s mainland for the data collection and assistance.
Funding: This research was funded by the Beijing Love Book Cancer Foundation and Merck Serono Co., Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the independent review board of Henan Cancer Hospital (No. 2019273), and all patients provided signed informed consent form. The study was conducted in accordance with the Declaration of Helsinki Declaration (as revised in 2013).
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-988/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-988/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-988/coif). All authors report funding from the Beijing Love Book Cancer Foundation and Merck Serono Co., Ltd. The authors have no other conflicts of interest to declare.
(English Language Editor: D. Fitzgerald)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 3.Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022. doi: 10.1016/j.jncc.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute. Surveillance, Epidemiology, and End Results: SEER stat fact sheets-all sites. Available online: https://seer.cancer.gov/explorer/application.html (Accessed 2021 Oct 18).
- 6.Shi JF, Wang L, Ran JC, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: Overall design and results from a multicenter retrospective epidemiologic survey. Cancer 2021;127:1880-93. 10.1002/cncr.33445 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen Z, Li J. The current status of treatment for colorectal cancer in China: A systematic review. Medicine (Baltimore) 2017;96:e8242. 10.1097/MD.0000000000008242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017;317:2392-401. 10.1001/jama.2017.7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426-34. Erratum in: Lancet Oncol 2016;17:e420. Erratum in: Lancet Oncol 2016;17:e479. 10.1016/S1470-2045(16)30269-8 [DOI] [PubMed] [Google Scholar]
- 10.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535-46. 10.1093/annonc/mdq632 [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 12.Taieb J, Lapeyre-Prost A, Laurent Puig P, et al. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br J Cancer 2019;121:434-42. 10.1038/s41416-019-0526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen R, Cervera P, Svrcek M, et al. BRAF-Mutated Colorectal Cancer: What Is the Optimal Strategy for Treatment? Curr Treat Options Oncol 2017;18:9. 10.1007/s11864-017-0453-5 [DOI] [PubMed] [Google Scholar]
- 14.De' Angelis GL , Bottarelli L, Azzoni C, et al. Microsatellite instability in colorectal cancer. Acta Biomed 2018;89:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishore C, Bhadra P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol 2021;893:173819. 10.1016/j.ejphar.2020.173819 [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Ran W, Wu J, et al. Deficient mismatch repair and RAS mutation in colorectal carcinoma patients: a retrospective study in Eastern China. PeerJ 2018;6:e4341. 10.7717/peerj.4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Wang L, Ran W, et al. Effect of Tumor Location on Clinicopathological and Molecular Markers in Colorectal Cancer in Eastern China Patients: An Analysis of 2,356 Cases. Front Genet 2020;11:96. 10.3389/fgene.2020.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Han Z, Li X, et al. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res 2020;32:729-41. 10.21147/j.issn.1000-9604.2020.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol 2018;25:1454-5. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Nordlinger B, Cervantes A, et al. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 2010;21 Suppl 5:v93-7. 10.1093/annonc/mdq222 [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:329-59. 10.6004/jnccn.2021.0012 [DOI] [PubMed] [Google Scholar]
- 22.Levine RA, Chawla B, Bergeron S, et al. Multidisciplinary management of colorectal cancer enhances access to multimodal therapy and compliance with National Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal Dis 2012;27:1531-8. 10.1007/s00384-012-1501-z [DOI] [PubMed] [Google Scholar]
- 23.Kafatos G, Banks V, Burdon P, et al. Biomarker testing and mutation prevalence in metastatic colorectal cancer patients in five European countries using a large oncology database. Future Oncol 2021;17:1483-94. 10.2217/fon-2020-0975 [DOI] [PubMed] [Google Scholar]
- 24.Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13-21. 10.1093/annonc/mdu378 [DOI] [PubMed] [Google Scholar]
- 25.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. 10.1056/NEJMoa0804385 [DOI] [PubMed] [Google Scholar]
- 26.Yokota T, Shibata N, Ura T, et al. Cycleave polymerase chain reaction method is practically applicable for V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)/V-raf murine sarcoma viral oncogene homolog B1 (BRAF) genotyping in colorectal cancer. Transl Res 2010;156:98-105. 10.1016/j.trsl.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Chaiyapan W, Duangpakdee P, Boonpipattanapong T, et al. Somatic mutations of K-ras and BRAF in Thai colorectal cancer and their prognostic value. Asian Pac J Cancer Prev 2013;14:329-32. 10.7314/APJCP.2013.14.1.329 [DOI] [PubMed] [Google Scholar]
- 28.Serebriiskii IG, Connelly C, Frampton G, et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun 2019;10:3722. 10.1038/s41467-019-11530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchoudi N, Amrani Hassani Joutei H, Jouali F, et al. Distribution of KRAS and BRAF mutations in Moroccan patients with advanced colorectal cancer. Pathol Biol (Paris) 2013;61:273-6. 10.1016/j.patbio.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 31.Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322-30. 10.1158/1078-0432.CCR-14-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. 10.1016/S0140-6736(11)60613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 2011;128:2075-84. 10.1002/ijc.25555 [DOI] [PubMed] [Google Scholar]
- 34.Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 2011;104:856-62. 10.1038/bjc.2011.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AACR Project GENIE Consortium . AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov 2017;7:818-31. 10.1158/2159-8290.CD-17-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.André T, Shiu KK, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020;383:2207-18. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 37.Carethers JM, Jung BH. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015;149:1177-90.e3. 10.1053/j.gastro.2015.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol 2010;7:153-62. 10.1038/nrclinonc.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Ding H, Sun S, et al. Incidence and detection of high microsatellite instability in colorectal cancer in a Chinese population: a meta-analysis. J Gastrointest Oncol 2020;11:1155-63. 10.21037/jgo-20-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


