Abstract
Background and Objective
Rheumatoid arthritis (RA) is a chronic autoimmune disease affected by genetics and the environment factors. Its early diagnosis and treatment are difficult, and the infection risk is serious. The treatment effects for most patients were not significant, which has become a difficult challenge to overcome. Cell signals play an important role in regulating basic cellular activities such as immunity. Notch signaling is a near secretory signal that can affects many processes of cell normal morphogenesis, including the differentiation of pluripotent progenitor cells, apoptosis, cell proliferation and the formation of cell boundary. In addition, the expression and activation of Notch signaling are increased in the synovial cells and vascular endothelial cells of RA patients. The purpose of this review was to elucidate the related mechanisms of Notch signaling in RA progression, as well as the potential therapeutic value of Notch signaling in a variety of autoimmune diseases.
Methods
Literatures about Notch signaling and RA were extensively reviewed to analyze and discuss.
Key Content and Findings
This article briefly reviews the role of Notch signaling in RA. It also summarizes the functional role of Notch signaling in the treatment of RA, with the goal to provide a new treatment option for RA patients.
Conclusions
In this review, the approach we discussed focuses on Notch signaling as a potential therapeutic target against RA, enriching therapeutic strategies for inflammatory diseases including RA.
Keywords: Rheumatoid arthritis (RA), Notch signaling, cytokines, T helper cells (Th cells), macrophages
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease affected by heredity and environmental factors and is mainly chronic (1). The main clinical features of RA are synovial hyperplasia and pannus formation, resulting in irreversible joint injury and severe disability. Patients usually present with symmetrical polyarthritis of small joints in the limbs, accompanied by morning stiffness and occasionally systemic symptoms (2,3). The main pathological features of RA are macrophage and lymphocyte infiltration, synovial fibroblast proliferation and joint destruction (4). The incidence rate of RA is approximately 0.5–1% (5), while the incidence rate in mainland China is 0.42%, and the total number of patients living with RA is approximately 5 million (6). The peak age of occurrence for RA is 20–45 years old and is mostly in women, with the prevalence rate of men to women of approximately 1:4 (7). Thus far, the pathogenesis of RA is unclear. At present, it is generally accepted that genetic and environmental factors play a key role in the prevalence of RA, and the risk of RA in patients with a family history increases 3–5 times (8). The influence of the environment on RA is divided into internal and external factors. Similar to other autoimmune diseases, the impact of intestinal microbiota on disease risk and progression in RA also plays a very important role (9). External factors such as eating habits and lifestyle are also associated with RA. A higher incidence of RA occurs on a low-vitamin, high-carbohydrate, meat, high-protein, and high-speed rail diet. Smoking is considered to be one of the most serious external risk factors for RA development. The current study highlights the pathophysiology of RA patients associated with smoking, such as the occurrence of oxidative stress, the release of inflammatory cytokines and the altered epigenetics (10). Epidemiological and animal model studies confirmed the association between smoking and RA (11). If the treatment is not sufficient, it will bring irreversible lifelong disability to the patient. Unfortunately, the diagnosis and treatment effect of some RA patients is not ideal, and biotherapy also increases the risk of infection. Therefore, it is very important to understand the pathogenesis of RA and find new treatment strategies.
Notch signaling, a near secretory signal that mediates intercellular signal transmission through receptor-ligand interactions between adjacent cells (12), is a highly conserved regulatory signaling pathway present in all mammalian cells (13). Notch signaling is involved in proliferation, survival and differentiation during embryonic development and survival (14,15). Previous studies have focused on the activation of Notch signaling in synovial cells (16), blood vessel endothelial cells (17) and peripheral blood lymphocytes (18) of RA patients. In addition, the Notch-1 intracellular domain (N1ICD) and Notch-3 intracellular domain (N3ICD) were upregulated in the synovium of RA patients and collagen-induced arthritis (CIA) rats, and the Notch-4 signaling pathway is involved in RA synovial fibroblasts (19). In this review, we focus on the different conditions and interactions of Notch signaling within RA pathogenesis, and we also discuss using Notch signaling as a targeting challenge against RA. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-142/rc)
Methods
The information used to write this paper was collected from the sources listed in Table 1.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 25, October 2021 |
| Databases and other sources searched | PubMed, Web of Science, and EMBASE databases |
| Search terms used (including MeSH and free text search terms and filters) (note: please use an independent supplement table to present detailed search strategy of one database as an example) | “Rheumatoid arthritis”, “Notch signaling pathway”, “macrophages”, and “Inflammation/infection” |
| Timeframe | Up to December 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | None |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Yue Zhuang and Wenke Lu conducted the selection independently; consensus was obtained by all researchers’ discussion |
| Any additional considerations, if applicable | None |
Discussion
Notch signal pathway
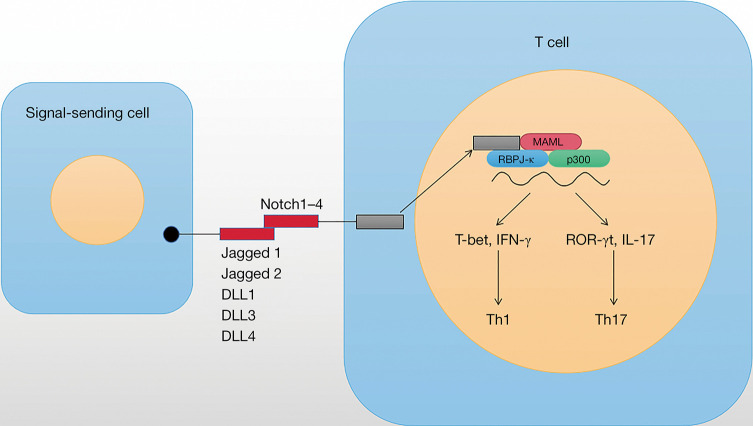
The Notch family contains four transmembrane receptors (Notch1–4), which interact with Notch ligands. Classical or typical Notch ligands are single pass membrane proteins Jagged1 and jagged2 and Delta-like (DLL)1, DLL3, and DLL4 (20,21). Once the Notch ligand binds to the Notch receptor on adjacent cells, triggering intracellular Notch signaling cascade (Figure 1), including: (I) the cleavage of extracellular a disintegrin and metalloprotease (ADAM) family protease; (II) the cytoplasmic cleavage of Notch intracellular domain (NICD) by the γ-secretase; (III) NICD translocates into the nucleus, which interacts with the CBF1/Su(H)/LAG1 (CSL) family of transcription factors to activate its downstream targets, such as Hes family (22). Many Notch target genes have recently been identified in different cell types, which has been considered to be the main way of Notch activation.
Figure 1.
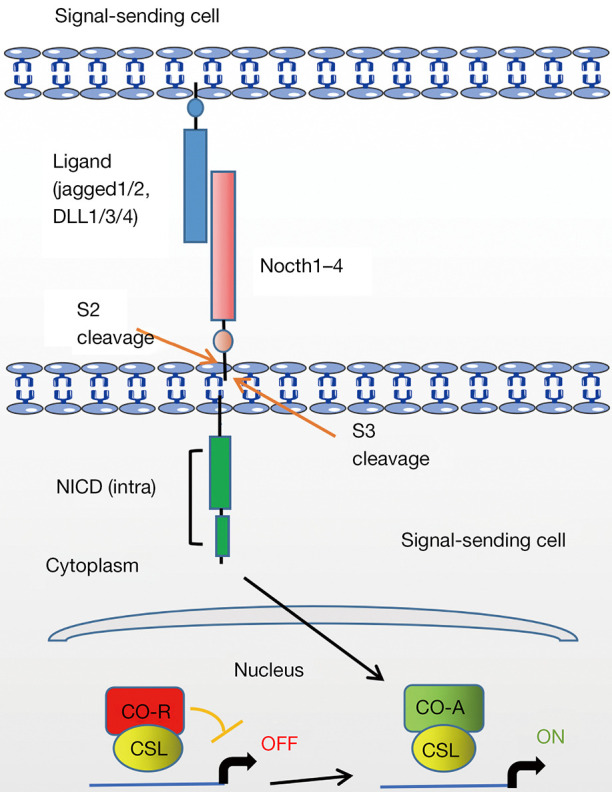
Basic cascade of intracellular Notch signaling. DLL, Delta-like; NICD, Notch intracellular domain; CSL, CBF1/Su(H)/LAG1; CO-R, co-repressor; CO-A, co-activator.
Mechanisms of Notch signaling in the RA progression
Effect of Notch signaling on cytokine secretion in RA
Cytokines are signaling molecules that coordinate the systemic or local immune response of the body. Unfortunately, dysregulation of cytokine release and signaling mediates pathogenic effects (23). Notch signaling regulates embryonic development, differentiation processes and tissue homeostasis in multiple organ systems (14). Thus, dysregulation of Notch signaling is associated with inflammatory disease processes, including RA (24), atherosclerosis (25), systemic lupus erythematosus (26), systemic sclerosis (27), experimental autoimmune encephalomyelitis (28), and infectious diseases (29,30). Recent studies have shown that Notch signal expression and activation can stimulate synovial cells, thereby accelerating the production of proinflammatory cytokines in RA (16,19,24,31,32). Also, overexpression of N1ICD in mouse macrophages increases the content of the proinflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin (IL)-6 (33). Notch signaling has also been shown to mediate TNF-α-induced IL-6 production in fibroblast-like synovial cells in RA, exacerbating RA (34). On the other hand, in primary human and mouse macrophages, interferon γ (IFN-γ, the most potent macrophage activator) strongly enhances jagged-1 expression and reduces DLL1 and DLL4 expression (35). These results indicate that Notch signal activation promotes cytokine secretion and aggravates RA.
The relationship between Notch signal and oxygen in RA
Changes in oxygen content and inflammogenesis in the synovium of arthritis have been fully shown to play critical roles in the progression of RA (36). In addition, it has been reported that the synovial tissues of RA patients are relatively anoxic compared to the noninflammatory synovial tissues of non-RA patients (37). In inflamed joints, oxygen levels were negatively correlated with vascular distribution, oxidative damage, and synovial inflammation levels (38,39). Hypoxia regulates hypoxia-inducible factors (HIFs) and vascular endothelial growth factor (VEGF). VEGF and other key genes induce angiogenesis and promote cell growth (40). For instance, hypoxia promotes cell proliferation of RA-fibroblast-like synoviocytes (FLSs) and RA angiogenesis by increasing glucose-6-phosphate isomerase (G6PI) (41). It has also been reported that hypoxia can induce autophagy (42). Hypoxia stress is mediated by HIF-1α and is a strong signal for the initiation of autophagy (43). Low PO2 levels in synovial tissues were inversely correlated with NICD expression in vivo, and Notch-1/HIF-1α interactions mediated hypoxia-induced angiogenesis and invasion of RA (44). Meanwhile, Notch-1 and Notch-3 are involved in hypoxia-induced activation of RA synovial fibroblasts (45). Furthermore, the contents of HIF-1α, N1ICD and N3ICD are highly expressed in synovial tissues of RA patients. In vitro, loss of Notch-1 and Notch-3 inhibited hypoxia-induced invasion and angiogenesis in rheumatoid arthritis synovial fibroblasts (RASFs). The overexpression of N1ICD and N3ICD promotes this process. In addition, Notch-1 mediates RASF migration and epithelial-mesenchymal transformation (EMT) under hypoxia conditions, while Notch-3 mediates antiapoptotic and autophagy processes. Furthermore, animal models of CIA have shown that N1ICD and LY411575 (N3ICD inhibitor) have therapeutic effects in improving symptoms and disease severity (46). The above studies indicate that hypoxia promotes the incidence of RA, while hypoxia also activates Notch signaling, so Notch signaling plays an important role in the pathogenesis of RA.
The relationship between Notch signaling and T cell differentiation
Notch signaling not only regulates cell fate during development but also affects the growth and survival of progenitor cells. In the immune system, Notch plays a crucial role in maintaining hematopoietic stem cells, guiding lymphocyte development and regulating T cell differentiation and function (14,47-49). In addition to regulating lymphocyte development, Notch receptor and ligand interactions can also serve as antigen-presenting cells to promote T cell differentiation. Peripheral T cell activation mediated by T cell receptor (TCR) is a routine process of the adaptive immune system. Ulteriorly, the activation of CD4+ T cells is achieved by binding antigen to TCR delivered by major histocompatibility complex (MHC) class II molecules (50). Costimulatory signals between B7 (CD80/CD86) and CD28 leads to the occurrence of several downstream signaling molecular events, such as T cell activation and proliferation (51). Notch stimulates T cell effector function by kinase-dependent signaling of phosphatidylinositol 3 downstream of TCR and CD28 by promoting Akt kinase and rapamycin activation to enhancing function and survival in response to lower antigen doses (52,53). Notch signaling is mediated by enhancing protein kinase PKCθ, which is involved in TCR and CD28 signaling, as well as the regulation of actin cytoskeleton (54). In addition, Notch signaling mediates effector T cell differentiation, such as CD4+ T helper cells (Th1, 2, 9 and 17) and CD8+ T cells (55). In terms of function, Th1 cells play a crucial role in clearing intracellular pathogens and viruses and mediating autoimmune diseases. Th1 cells regulate the genealogy-specific transcription factor T-bet and secrete the IFN-γ. Th2 cells play a crucial role in mediating the immunity of helminth parasites and allergic reactions. Differentiation of Th2 cells is induced by the cytokine IL-4 and requires the GATA3 to release IL-4, 5 and 13. Th17 cells play an important role in fighting extracellular bacterial and fungal infections and controlling body autoimmunity. IL-6 and TGF-β produce Th17 cells, which secrete IL-17 and IL-23 and regulate retinoic acid-related orphan receptor γt (ROR-γt) (56) (Figure 2).
Figure 2.
The role of Notch signaling in T cell differentiation. DLL, Delta-like; IL, interleukin; IFN-γ, interferon γ; ROR-γt, retinoic acid-related orphan receptor γt.
Effect of Notch signaling on Th1 cell differentiation in RA
The characteristic Th1 genes, TBX21 and IFNG, were confirmed as the direct targets of Notch (55,57). Depletion of IFN-γ secreted by Th1 cells in in vivo Leishmania mice infected with Notch-1/Notch-2 dual gene defects has been shown, but Th1 cell function has not been reported in DN-MAML transgenic or conditional RBPJκ knockout in mice (58-60). Thus, signaling that regulates Th1 differentiation is involved in Notch RBPJκ independence. Deletion of the Jagged1 or Mindbomb1 genes was found to be essential for the expression of functional Notch ligands without affecting Th1 cell differentiation in vitro (61,62). The ability of DLL1/DLL4 to promote Th1 cell differentiation has been demonstrated in several experiments, such as the reduction of anti-DLL4 antibodies in vivo affecting T cell secretion of IFN-γ and TNF-α (63,64). DLL1 blockade also reduced the number of Th1 cells in allograft models (65). Th cells in RA patients exhibit altered Notch receptor expression profiles and enhanced Notch signal activation compared with healthy controls (18). In addition, Notch signaling is involved in specific Th1 and Th17 types of amplification, involving Notch-3 and DLL1, so selective inhibition of Notch-3 or DLL1-mediated Notch signaling provides novel strategies for the treatment of RA (66).
Effect of Notch signaling on Th17 cell differentiation in RA
In recent years, many researchers have conducted in-depth studies on the regulation of Th17 differentiation in naive CD4+ T cells. Studies have shown that Th17 cells and proinflammatory cytokines produced by Th17 cells, such as IL-17A, IL-17F, IL-21, and IL-22, are associated with various autoimmune and inflammatory diseases (67,68). The pathogenic role of the cytokines IL-17 and Th17 cells has been found in several autoimmune diseases, including RA (69), primary sclerosis (70), psoriasis (71), Crohn’s disease (72), and systemic lupus erythematosus (73). IL-17 expression is increased in both the serum and articular fluid of patients with active RA, which in turn promotes the secretion of various cytokines by synovial cells, enhances osteoclast activity, inhibits chondrocyte synthesis, and ultimately leads to bone erosion (74). Notch signaling is enhanced in RA patients, with altered expression patterns in helper T cells (18). Notch signaling regulates Th17 cell function by integrating signals supplied by cytokine such as IL-6 or TGF-β. In addition, TGF-β is a cytokine active in the gut that binds with IL-6 to promote Th17 cells (75). Studies have shown that the ROR-γT, IL-17, and IL-23R gene promoters are direct targets of Notch; thus, when Notch signaling is blocked, Th17 cell differentiation is impaired (68,76,77). Notch-1 binds directly to the ROR-γT and IL-17 promoters to regulate Th17 differentiation (78). Notch-3 presents a critical role in antigen-specific T cell differentiation, and Notch-3 blocking inhibits the activation of Th1 and Th17 cell in CIA mice (66).
The effect of Notch signaling on macrophage in RA
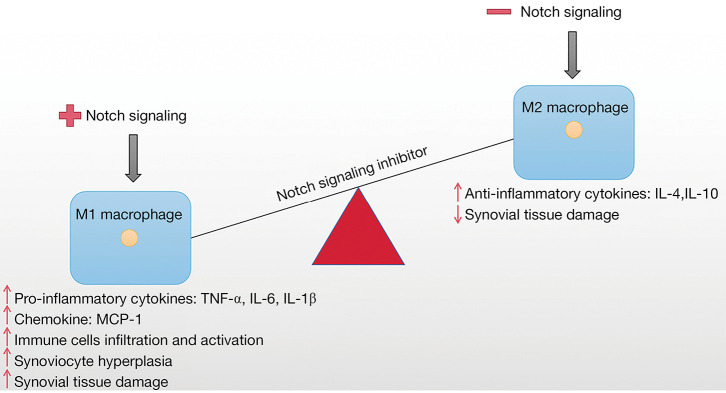
Macrophages are key immune cells in the presence of host tissues that play a critical role in organismal defense and inflammation to endogenous and exogenous stimuli (79-81). Macrophages present a key role in the pathophysiology of RA. It has been reported that the number of macrophages in synovial tissue is evidently associated with disease severity when activated (82). Meanwhile, macrophages not only produce a variety of proinflammatory cytokines and chemokines but also promote cartilage and bone destruction in RA through a variety of mechanisms, showing a wide range of proinflammatory, destructive and remodeling properties, which have a significant impact on the acute and chronic stages of RA pathogenesis (4,83,84). In addition, macrophages are influenced by RA or intercellular contact produced by innate immune cells, T cells, and fibroblasts (85). It is worth noting that the activation of aromatic hydrocarbon receptor (AHR) is related to the pathogenesis of RA. AHR agonists inhibit the expression of proinflammatory cytokines in macrophages, which are key cells in the pathogenesis of RA, suggesting specific circuits that regulate the AHR pathway in RA macrophages. However, the high expression of miR-223 in bone marrow cells prevented the inhibition of IL-β, IL-6, and TNF-α by the AHR. This connection of the miR-223/AHR pathway provides conceptual evidence for miR-223 antagonist-based RA therapies. What’s more, notch-regulated miR-223 has been shown to target AHR pathways and increase cytokine production in macrophages of patients with RA (86,87). In addition, our study has shown that the imbalance between M1 and M2 macrophages is considered to be one of the main causes of RA (Figure 3). M1 macrophages were detected mainly in patients with severe RA, while M2 macrophages were detected in patients with mild or clinical remission of RA (88,89). M1 macrophages produce proinflammatory cytokines such as TNF-α and IL-1β, which are highly expressed in RA patients, whereas M2 macrophages produce anti-inflammatory cytokines such as IL-4 and IL-10, which are under expressed in RA patients (90). Interestingly, the interrelationship between cytokines in macrophages and Notch signaling further highlighting the key role of Notch signaling in modulating macrophage immune responses (23). Notch signaling guides the proinflammatory response of macrophages during inflammation and infection, DLL1 and Notch target genes were upregulated following Gram-positive/negative bacterial infection in human monocytes (91,92), seems to indicate that Notch signaling appears to negatively regulate immune defense mechanisms in macrophages. In RAW264.7 macrophages, miR-146 promoted the polarization of M2 macrophages and reduced the polarization of M1 macrophages by targeting Notch-1 signaling (93). These evidences clearly indicates an important role for Notch signaling in the pathogenesis of RA. Together, targeted Notch signals in the myeloid system may be a novel potential target in the pathogenesis of RA by controlling the polarized balance of M1 and M2 macrophages and reconstructing the homeostasis immune system.
Figure 3.
Notch signaling coordination of macrophage polarization in the RA synovial junctions. IL, interleukin; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemoattractant protein-1; RA, rheumatoid arthritis.
The therapeutic role of Notch signaling in RA
Targeting Notch signaling was found to reduce inflammation and minimize associated tissue damage (24,94). Some enzymatic reactions mediate the regulation and activation of Notch signaling, providing potential adversarial targets for disease treatment. For example, γ-secretase inhibitors prevent Notch receptor cleavage, and specific antibodies block either Notch receptors or ligands. It is important that crosstalk between Notch and other signaling provides an opportunity for a combination therapy that can target many pathways simultaneously, which may increase therapeutic effectiveness (95). Notch-activated M1 macrophages reduce joint tissue injury in mice with inflammatory arthritis (96). The Notch signaling inhibitor LY411575, which inhibits both Notch-1 and Notch-3, was used to treat CIA in rats (97). In patients with RA, lncRNA MALAT1 is reduced and hsa-miR155-3p is elevated, coregulating changes in the Notch signaling pathway (98). Survivin alleviates RA by activating the Notch signaling pathway to promote RA fibroblast-like synovial cell proliferation and the expression of angiogenic-related proteins and inhibit apoptosis (99). Unfortunately, no single clinical product to date can safely and efficiently target Notch patients. Therefore, further studies are needed to elucidate the biological significance of Notch signaling. We need to discover new strategies (e.g., new targets, new antagonists) to provide a promising treatment for immune diseases such as RA.
Conclusions
Notch signaling is an evolutionarily highly conserved intercellular signaling pathway, and an increasing number of studies have confirmed that Notch signaling is a promising target for pathological therapy by regulating the development and function of immune cells. Therefore, it is important to understand the dynamic balance of beneficial and harmful activation of Notch signals, especially for the study of the mechanism. A large body of evidence supports the role of Notch signaling in the inflammatory response of macrophages and helper T cells to various stimuli. Due to its ease of pharmacological application, treatment targeting Notch signaling in multiple pathological conditions may provide a promising strategy to improve inflammation. Evidence shows that targeting Notch signaling can reduce tissue inflammation and minimize tissue injury under many pathological conditions, including RA. Unfortunately, the use of Notch signal targeting remains many challenges against immune diseases. More in-depth research is needed to clarify the mechanism of various Notch receptors and ligands in RA, which will make it easier to understand how Notch signal drives the body’s immune response and contribute novel therapeutic options for inflammatory diseases. In conclusion, Notch signaling in RA and other autoimmune diseases is a promising therapeutic target, which will help us to better develop effective therapeutic strategies for related inflammatory diseases in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Medical Science and Technology Project of the Health Commission of Sichuan Province (No. 21PJ085) and was supported by Sichuan Science and Technology Program (No. 2022JDRC0069).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-142/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-142/coif). The authors have no conflicts of interest to declare.
References
- 1.Croia C, Bursi R, Sutera D, et al. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2019;37:347-57. [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023-38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 3.Ngian GS. Rheumatoid arthritis. Aust Fam Physician 2010;39:626-8. [PubMed] [Google Scholar]
- 4.Ma Y, Pope RM. The role of macrophages in rheumatoid arthritis. Curr Pharm Des 2005;11:569-80. 10.2174/1381612053381927 [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1-26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 6.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res 2002;4 Suppl 3:S265-72. 10.1186/ar578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favalli EG, Biggioggero M, Crotti C, et al. Sex and Management of Rheumatoid Arthritis. Clin Rev Allergy Immunol 2019;56:333-45. 10.1007/s12016-018-8672-5 [DOI] [PubMed] [Google Scholar]
- 8.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006;36:182-8. 10.1016/j.semarthrit.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Scher JU, Littman DR, Abramson SB. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol 2016;68:35-45. 10.1002/art.39259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K, Yang SM, Kim SH, et al. Smoking and rheumatoid arthritis. Int J Mol Sci 2014;15:22279-95. 10.3390/ijms151222279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa Y, Terao C. The Impact of Cigarette Smoking on Risk of Rheumatoid Arthritis: A Narrative Review. Cells 2020;9:475. 10.3390/cells9020475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai EC. Notch signaling: control of cell communication and cell fate. Development 2004;131:965-73. 10.1242/dev.01074 [DOI] [PubMed] [Google Scholar]
- 13.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol 2000;228:151-65. 10.1006/dbio.2000.9960 [DOI] [PubMed] [Google Scholar]
- 14.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity 2010;32:14-27. 10.1016/j.immuni.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Sato C, Zhao G, Ilagan MX. An overview of notch signaling in adult tissue renewal and maintenance. Curr Alzheimer Res 2012;9:227-40. 10.2174/156720512799361600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazawa M, Ishii H, Aono H, et al. Role of Notch-1 intracellular domain in activation of rheumatoid synoviocytes. Arthritis Rheum 2001;44:1545-54. [DOI] [PubMed] [Google Scholar]
- 17.Gao W, Sweeney C, Walsh C, et al. Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann Rheum Dis 2013;72:1080-8. 10.1136/annrheumdis-2012-201978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao Z, Wang W, Guo M, et al. Expression analysis of Notch-related molecules in peripheral blood T helper cells of patients with rheumatoid arthritis. Scand J Rheumatol 2010;39:26-32. 10.3109/03009740903124424 [DOI] [PubMed] [Google Scholar]
- 19.Ando K, Kanazawa S, Tetsuka T, et al. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene 2003;22:7796-803. 10.1038/sj.onc.1206965 [DOI] [PubMed] [Google Scholar]
- 20.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 2003;26:565-97. 10.1146/annurev.neuro.26.041002.131334 [DOI] [PubMed] [Google Scholar]
- 21.Lai EC. Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Rep 2002;3:840-5. 10.1093/embo-reports/kvf170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Zhang Y, Xiang X, et al. Notch signaling contributes to the expression of inflammatory cytokines induced by highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection in porcine alveolar macrophages. Dev Comp Immunol 2020;108:103690. 10.1016/j.dci.2020.103690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014;5:491. 10.3389/fimmu.2014.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Kim SH, Kim K, et al. Inhibition of notch signalling ameliorates experimental inflammatory arthritis. Ann Rheum Dis 2015;74:267-74. 10.1136/annrheumdis-2013-203467 [DOI] [PubMed] [Google Scholar]
- 25.Fukuda D, Aikawa E, Swirski FK, et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A 2012;109:E1868-77. 10.1073/pnas.1116889109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol 2010;184:6465-78. 10.4049/jimmunol.0904016 [DOI] [PubMed] [Google Scholar]
- 27.Harrach S, Barz V, Pap T, et al. Notch Signaling Activity Determines Uptake and Biological Effect of Imatinib in Systemic Sclerosis Dermal Fibroblasts. J Invest Dermatol 2019;139:439-47. 10.1016/j.jid.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 28.Sandy AR, Stoolman J, Malott K, et al. Notch signaling regulates T cell accumulation and function in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol 2013;191:1606-13. 10.4049/jimmunol.1301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Zhang H, Yu L, et al. Down-regulation of Notch signaling pathway reverses the Th1/Th2 imbalance in tuberculosis patients. Int Immunopharmacol 2018;54:24-32. 10.1016/j.intimp.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 30.Narayana Y, Balaji KN. NOTCH1 up-regulation and signaling involved in Mycobacterium bovis BCG-induced SOCS3 expression in macrophages. J Biol Chem 2008;283:12501-11. 10.1074/jbc.M709960200 [DOI] [PubMed] [Google Scholar]
- 31.Khan IM, Palmer EA, Archer CW. Fibroblast growth factor-2 induced chondrocyte cluster formation in experimentally wounded articular cartilage is blocked by soluble Jagged-1. Osteoarthritis Cartilage 2010;18:208-19. 10.1016/j.joca.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 32.Ishii H, Nakazawa M, Yoshino S, et al. Expression of notch homologues in the synovium of rheumatoid arthritis and osteoarthritis patients. Rheumatol Int 2001;21:10-4. 10.1007/s002960100119 [DOI] [PubMed] [Google Scholar]
- 33.Fung E, Tang SM, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation 2007;115:2948-56. 10.1161/CIRCULATIONAHA.106.675462 [DOI] [PubMed] [Google Scholar]
- 34.Jiao Z, Wang W, Ma J, et al. Notch signaling mediates TNF-α-induced IL-6 production in cultured fibroblast-like synoviocytes from rheumatoid arthritis. Clin Dev Immunol 2012;2012:350209. 10.1155/2012/350209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foldi J, Chung AY, Xu H, et al. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. J Immunol 2010;185:5023-31. 10.4049/jimmunol.1001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum 1970;13:769-76. 10.1002/art.1780130606 [DOI] [PubMed] [Google Scholar]
- 37.Muz B, Khan MN, Kiriakidis S, et al. Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res Ther 2009;11:201. 10.1186/ar2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biniecka M, Connolly M, Gao W, et al. Redox-mediated angiogenesis in the hypoxic joint of inflammatory arthritis. Arthritis Rheumatol 2014;66:3300-10. 10.1002/art.38822 [DOI] [PubMed] [Google Scholar]
- 39.Akhavani MA, Madden L, Buysschaert I, et al. Hypoxia upregulates angiogenesis and synovial cell migration in rheumatoid arthritis. Arthritis Res Ther 2009;11:R64. 10.1186/ar2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol 2012;8:153-62. 10.1038/nrrheum.2011.205 [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Yu SS, Zong M, et al. Glucose-6-Phosphate Isomerase (G6PI) Mediates Hypoxia-Induced Angiogenesis in Rheumatoid Arthritis. Sci Rep 2017;7:40274. 10.1038/srep40274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin JJG, Lozano E, Perez MJ. Lack of mitochondrial DNA impairs chemical hypoxia-induced autophagy in liver tumor cells through ROS-AMPK-ULK1 signaling dysregulation independently of HIF-1α. Free Radic Biol Med 2016;101:71-84. 10.1016/j.freeradbiomed.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Yang M, Wang Y, et al. Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biol Int 2016;40:671-85. 10.1002/cbin.10604 [DOI] [PubMed] [Google Scholar]
- 44.Gao W, Sweeney C, Connolly M, et al. Notch-1 mediates hypoxia-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum 2012;64:2104-13. 10.1002/art.34397 [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Sun A, Li J, et al. Reply. Arthritis Rheumatol 2021;73:2350-1. 10.1002/art.41904 [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Cheng W, Li J, et al. Notch-1 and Notch-3 Mediate Hypoxia-Induced Activation of Synovial Fibroblasts in Rheumatoid Arthritis. Arthritis Rheumatol 2021;73:1810-9. 10.1002/art.41748 [DOI] [PubMed] [Google Scholar]
- 47.Hoyne GF. Notch signaling in the immune system. J Leukoc Biol 2003;74:971-81. 10.1189/jlb.0303089 [DOI] [PubMed] [Google Scholar]
- 48.Tanigaki K, Honjo T. Regulation of lymphocyte development by Notch signaling. Nat Immunol 2007;8:451-6. 10.1038/ni1453 [DOI] [PubMed] [Google Scholar]
- 49.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol 2005;23:945-74. 10.1146/annurev.immunol.23.021704.115747 [DOI] [PubMed] [Google Scholar]
- 50.Shevach EM. Biological functions of regulatory T cells. Adv Immunol 2011;112:137-76. 10.1016/B978-0-12-387827-4.00004-8 [DOI] [PubMed] [Google Scholar]
- 51.Dongre A, Surampudi L, Lawlor RG, et al. Non-Canonical Notch Signaling Drives Activation and Differentiation of Peripheral CD4(+) T Cells. Front Immunol 2014;5:54. 10.3389/fimmu.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laky K, Evans S, Perez-Diez A, et al. Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity 2015;42:80-94. 10.1016/j.immuni.2014.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci U S A 2010;107:6882-7. 10.1073/pnas.0910060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Britton GJ, Ambler R, Clark DJ, et al. PKCθ links proximal T cell and Notch signaling through localized regulation of the actin cytoskeleton. Elife 2017;6:20003. 10.7554/eLife.20003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amsen D, Helbig C, Backer RA. Notch in T Cell Differentiation: All Things Considered. Trends Immunol 2015;36:802-14. 10.1016/j.it.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 56.Bailis W, Yashiro-Ohtani Y, Fang TC, et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity 2013;39:148-59. 10.1016/j.immuni.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukherjee S, Rasky AJ, Lundy PA, et al. STAT5-induced lunatic fringe during Th2 development alters delta-like 4-mediated Th2 cytokine production in respiratory syncytial virus-exacerbated airway allergic disease. J Immunol 2014;192:996-1003. 10.4049/jimmunol.1301991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tindemans I, Lukkes M, de Bruijn MJW, et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J Allergy Clin Immunol 2017;140:1079-89. 10.1016/j.jaci.2016.11.046 [DOI] [PubMed] [Google Scholar]
- 59.Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 2007;27:89-99. 10.1016/j.immuni.2007.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auderset F, Schuster S, Coutaz M, et al. Redundant Notch1 and Notch2 signaling is necessary for IFNγ secretion by T helper 1 cells during infection with Leishmania major. PLoS Pathog 2012;8:e1002560. 10.1371/journal.ppat.1002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto M, Matsuda H, Joetham A, et al. Jagged1 on dendritic cells and Notch on CD4+ T cells initiate lung allergic responsiveness by inducing IL-4 production. J Immunol 2009;183:2995-3003. 10.4049/jimmunol.0900692 [DOI] [PubMed] [Google Scholar]
- 62.Jeong HW, Kim JH, Kim JY, et al. Mind bomb-1 in dendritic cells is specifically required for Notch-mediated T helper type 2 differentiation. PLoS One 2012;7:e36359. 10.1371/journal.pone.0036359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bassil R, Zhu B, Lahoud Y, et al. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J Immunol 2011;187:2322-8. 10.4049/jimmunol.1100725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eixarch H, Mansilla MJ, Costa C, et al. Inhibition of delta-like ligand 4 decreases Th1/Th17 response in a mouse model of multiple sclerosis. Neurosci Lett 2013;541:161-6. 10.1016/j.neulet.2013.02.038 [DOI] [PubMed] [Google Scholar]
- 65.Riella LV, Ueno T, Batal I, et al. Blockade of Notch ligand δ1 promotes allograft survival by inhibiting alloreactive Th1 cells and cytotoxic T cell generation. J Immunol 2011;187:4629-38. 10.4049/jimmunol.1004076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiao Z, Wang W, Xu H, et al. Engagement of activated Notch signalling in collagen II-specific T helper type 1 (Th1)- and Th17-type expansion involving Notch3 and Delta-like1. Clin Exp Immunol 2011;164:66-71. 10.1111/j.1365-2249.2010.04310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821-52. 10.1146/annurev.immunol.25.022106.141557 [DOI] [PubMed] [Google Scholar]
- 68.Keerthivasan S, Suleiman R, Lawlor R, et al. Notch signaling regulates mouse and human Th17 differentiation. J Immunol 2011;187:692-701. 10.4049/jimmunol.1003658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benedetti G, Miossec P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur J Immunol 2014;44:339-47. 10.1002/eji.201344184 [DOI] [PubMed] [Google Scholar]
- 70.Lückel C, Picard F, Raifer H, et al. IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat Commun 2019;10:5722. 10.1038/s41467-019-13731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blauvelt A, Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol 2018;55:379-90. 10.1007/s12016-018-8702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Müller A, Lamprecht P. Interleukin-17 in chronic inflammatory and autoimmune diseases: rheumatoid arthritis, Crohn's disease and Wegener's granulomatosis. Z Rheumatol 2008;67:72-4. [DOI] [PubMed] [Google Scholar]
- 73.Li H, Adamopoulos IE, Moulton VR, et al. Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun 2020;11:2859. 10.1038/s41467-020-16636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agonia I, Couras J, Cunha A, et al. IL-17, IL-21 and IL-22 polymorphisms in rheumatoid arthritis: A systematic review and meta-analysis. Cytokine 2020;125:154813. 10.1016/j.cyto.2019.154813 [DOI] [PubMed] [Google Scholar]
- 75.Okuda Y, Sakoda S, Bernard CC, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol 1998;10:703-8. 10.1093/intimm/10.5.703 [DOI] [PubMed] [Google Scholar]
- 76.Meyer Zu Horste G, Wu C, Wang C, et al. RBPJ Controls Development of Pathogenic Th17 Cells by Regulating IL-23 Receptor Expression. Cell Rep 2016;16:392-404. 10.1016/j.celrep.2016.05.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang W, Zhang X, Sheng A, et al. γ-Secretase Inhibitor Alleviates Acute Airway Inflammation of Allergic Asthma in Mice by Downregulating Th17 Cell Differentiation. Mediators Inflamm 2015;2015:258168. 10.1155/2015/258168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma L, Xue H, Gao T, et al. Notch1 Signaling Regulates the Th17/Treg Immune Imbalance in Patients with Psoriasis Vulgaris. Mediators Inflamm 2018;2018:3069521. 10.1155/2018/3069521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723-37. 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol 2008;214:161-78. 10.1002/path.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirayama D, Iida T, Nakase H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int J Mol Sci 2017;19:92. 10.3390/ijms19010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 1996;39:115-24. 10.1002/art.1780390116 [DOI] [PubMed] [Google Scholar]
- 83.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol 2007;19:289-95. 10.1097/BOR.0b013e32805e87ae [DOI] [PubMed] [Google Scholar]
- 84.Kinne RW, Bräuer R, Stuhlmüller B, et al. Macrophages in rheumatoid arthritis. Arthritis Res 2000;2:189-202. 10.1186/ar86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 2016;12:472-85. 10.1038/nrrheum.2016.91 [DOI] [PubMed] [Google Scholar]
- 86.Li YT, Chen SY, Wang CR, et al. Brief report: amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum 2012;64:3240-5. 10.1002/art.34550 [DOI] [PubMed] [Google Scholar]
- 87.Ogando J, Tardáguila M, Díaz-Alderete A, et al. Notch-regulated miR-223 targets the aryl hydrocarbon receptor pathway and increases cytokine production in macrophages from rheumatoid arthritis patients. Sci Rep 2016;6:20223. 10.1038/srep20223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210-21. 10.1002/art.24505 [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Han CC, Cui D, et al. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol 2017;50:345-52. 10.1016/j.intimp.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 90.Kennedy A, Fearon U, Veale DJ, et al. Macrophages in synovial inflammation. Front Immunol 2011;2:52. 10.3389/fimmu.2011.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ito T, Allen RM, Carson WF, 4th, et al. The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog 2011;7:e1002341. 10.1371/journal.ppat.1002341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito T, Schaller M, Hogaboam CM, et al. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest 2009;119:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C, Liu XJ, QunZhou, et al. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int Immunopharmacol 2016;32:46-54. 10.1016/j.intimp.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 94.Bai X, Zhang J, Cao M, et al. MicroRNA-146a protects against LPS-induced organ damage by inhibiting Notch1 in macrophage. Int Immunopharmacol 2018;63:220-6. 10.1016/j.intimp.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 95.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov 2014;13:357-78. 10.1038/nrd4252 [DOI] [PubMed] [Google Scholar]
- 96.Sun W, Zhang H, Wang H, et al. Targeting Notch-Activated M1 Macrophages Attenuates Joint Tissue Damage in a Mouse Model of Inflammatory Arthritis. J Bone Miner Res 2017;32:1469-80. 10.1002/jbmr.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Li J, Chen J, et al. Treatment of collagen-induced arthritis rat model by using Notch signalling inhibitor. J Orthop Translat 2021;28:100-7. 10.1016/j.jot.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan L, Liu J, Huang C, et al. Decreased long-chain non-coding RNA MALAT1 expression and increased hsa-miR155-3p expression involved in Notch signaling pathway regulation in rheumatoid arthritis patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2020;36:535-41. [PubMed] [Google Scholar]
- 99.Ma S, Wang J, Lin J, et al. Survivin promotes rheumatoid arthritis fibroblast-like synoviocyte cell proliferation, and the expression of angiogenesis-related proteins by activating the NOTCH pathway. Int J Rheum Dis 2021;24:922-9.v [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as