Abstract
Background
Evidence on the effects of regular physical activity (PA) on asthmatic adults is rather weak and inconsistent since previous studies were conducted based on the limited studies, various populations groups and single outcome. A systematic review (SR) and meta-analysis have a high level of evidence to comprehensively evaluate the effects of PA for adults with asthma based on the available data. Our study aims to provide an SR of available evidence regarding the effect of regular PA on asthma in adults.
Methods
A comprehensive search strategy was conducted using the PubMed, EMBASE, Cochrane Library, and Web of Science databases from their inception to October 2021. We identifying the eligible studies based on the PIOS principles, namely, populations (adults with asthma), interventions (regular PA), outcomes (quality of life or relapse-related outcomes), study design [randomized controlled trials (RCTs)]. A Bayesian-based meta-analysis was performed to pool available evidence. Quality assessment for individual studies was performed with the Cochrane risk of bias tool and the Newcastle-Ottawa Scale (NOS).
Results
A total of 22 publications (18 RCTs and 4 longitudinal studies) were identified, comprised of 85,392 individuals aged between 18 and 75 years old. Overall quality of the included studies was rated as low-to-moderated quality. We found that PA was effective in improving quality of life (QOL) [health related quality of life, (HRQoL) & Asthma Control Questionnaire, (ACQ): standard mean difference (SMD) =−0.80, 95% credible interval (CrI): −1.30 to −0.31; I2=86.9%, Pheterogeneity<0.001], pulmonary function (FEV1/pred) [weighted mean difference (MD) =0.47, 95% CrI: 0.03 to 0.90; I2=74.9%, Pheterogeneity<0.001], and maximal oxygen consumption (VO2max) (MD =1.18, 95% CrI: 0.87 to 1.48; I2=17.0%, Pheterogeneity=0.31). Based on the longitudinal studies, the long-term high-level PA group had a lower risk of developing asthma compared with the low-level PA group [odds ratio (OR) =0.87, 95% CrI: 0.78 to 0.95; I2=27.4%, Pheterogeneity=0.22)].
Discussion
Adults with asthma need to carried out regular PA in accordance with the recommendations, which will improve their QOL and pulmonary function, and moreover, long-term PA appears to be more beneficial for asthmatic patients. The quality and quantity of included studies might affect the interpretation.
Keywords: Asthma, physical activity (PA), adults, Bayesian meta-analysis
Introduction
As one of the most common chronic respiratory inflammatory diseases, asthma is characterized by variable symptoms of wheeze, breathlessness, shortness of breath, chest tightness, or cough, as well as variable expiratory airflow limitation, which occurs due to impaired epithelial barrier function and a dysregulated immune response. The prevalence of asthma has risen sharply over the past decades, reaching 8.5–11.2% in adults and influencing 1–18% of the population in different areas (1). It places a significant burden on families and the medical system, and poses a significant socioeconomic burden, thereby becoming a serious public health issue.
Reliever or controller medications are used among asthmatics for reducing exacerbations. Nevertheless, long-term use of medications may cause minor and serious adverse events (2-4). Moreover, most asthmatics on prescribed controller therapies do not adhere to them for several reasons such as forgetfulness and fear of side effects and costs (5). Considerable attention has recently been devoted to non-pharmacological treatments as alternative strategies for improving asthmatics’ quality of life (QOL) and reducing disease risk (6).
Physical activity (PA) is defined by the World Health Organization as “any bodily movement produced by skeletal muscles that results in energy expenditure”, and involves 4 core elements, namely frequency, intensity, duration, and modality (7). Regular moderate PA has been recommended as an important non-pharmacological intervention for reducing cardiovascular risk and improving QOL among people with asthma (8). Substantial evidence has indicated that PA exhibits a beneficial effect on asthma symptom control and lung function, particularly aerobic exercise (AE) (9,10). In particular, those with low active and high sedentary typology compared with their peers appeared to have a significant improvement in their asthma symptoms, cardiopulmonary fitness, and maximum oxygen uptake, as well as improved QOL without adverse effects (11-13). Several researchers have hypothesized the etiological relationship between low PA levels and incident asthma. For instance, PA has protective properties against asthma by reducing airway inflammation or positively affecting bronchiole patency (14), and this positive impact was also demonstrated in a mouse model of allergic asthma (15).
A prior study revealed that aerobic training could significantly reduce medication for asthmatic patients (16). Despite the recognized benefits of PA, controversies persist, and existing evidence remains equivocal (17,18). First, previous reviews regarding PA and asthma have demonstrated that some PA had beneficial effects on asthma patients while others showed no significant differences (17-19). For instance, a publication focusing on AE with limited included studies (11 publications) and outcomes (asthma control, lung function and airway inflammation) found a statistically significant improvement regarding asthma control [SMD: −0.48 (95% CI: −0.81 to −0.16)] (9), contradicting another review on the same outcome that demonstrated no statistically significant difference [SMD: −0.25 (95% CI: −0.51 to 0.02)] between the PA group and control group (CG) (6). This probably due to an inconsistent difference of study design, such as search sources, outcome measurement and definition of PA. Furthermore, numerous recent studies focusing on PA and asthma have been published with significantly larger datasets, requiring updated reliable evidence summaries (20,21). Second, although the Global Initiative for Asthma (GINA) has issued guidelines to encourage people with asthma to engage in regular PA for general health benefits, further specific details on using regular PA in treating asthmatic symptoms per se remain to be elucidated (8). In addition, PA is a diverse behavior that requires participants to engage in a wide range of alternative activities in their daily lives, whereas previous studies disregarded this point and included populations with varying phenotypes, which may result in different responses to PA, or focused exclusively on asthma control outcomes (1,12). Lastly, whether long-term regular PA is an effective asthma treatment for reducing symptoms and exacerbations remains to be determined, as asthma is an irreversible airway obstruction disease, and evidence from follow-up longitudinal studies examining the long-term effects of PA on asthma are required. A meta-analysis is a method for summarizing the findings of similar studies, which can extend the sample size and increase the statistical effectiveness, especially when the findings of previous studies are inconsistent, meta-analysis has the ability to obtain the results based on a comprehensive analysis which would closer to the real life. Compared with the frequentist approach, the high sensitivity of Bayesian method can provide a flexibly modeling framework and outputting a more accurate results for the traditional meta-analysis. Therefore, we analyzed available evidence using a Bayesian modeling approach, which proposes more precise results than traditional frequency methods with its simple flexible prior assumptions (22), to examine the long-term effects of regular PA on QOL and other health outcomes in asthmatics patients based on trials and observational studies. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1170/rc) (23).
Methods
This meta-analysis was performed following the Cochrane Collaboration Handbook recommendations (24). No ethical approval or patient consent was required because all analyses were conducted based on previously published studies.
Search strategies and study selection
Without any language and publication date restrictions, we performed an exhaustive strategic literature search to identify relevant publications concerning the association between regular PA and QOL, as well as other health outcomes in asthmatic adults using the following databases: PubMed (via Medline), EMBASE, the Cochrane Library, Web of Science, PsycINFO (via Ovid), CINAHL, SPORTDiscus, and clinicaltrials.gov (search strategy performed for all databases were from their inception to October 2021). The search strategy was divided into 3 parts to meet the aims of the study using medical subject heading (MeSH) terms associated with keywords combined with Boolean operators. It included the following items: “asthma”, “asthmatic”, “wheeze”, “adults”, “physical activity”, “aerobic exercise”, “resistance training”, “muscle-strengthening activities”, “skeletal-strengthening activities”, “balance activities”, and “randomized controlled trials”. All database-specific search queries can be found in Appendix 1.
We also conducted a recursive search to identify relevant publications by manually screening bibliography lists of similar reviews and large-scale specialized conferences. Two independent investigators performed study selection, and any discrepancies which emerged during the process were discussed or resolved by consulting a third expert. Automatic deletion of duplicates was performed first, and irrelevant studies were removed based on their titles and abstracts. Subsequently, we analyzed the full texts of potentially eligible studies. Two independent investigators cross-checked selected citations to ensure their integrity and accuracy. All citations were downloaded and managed in EndNote X9 Software (Thompson ISI ResearchSoft, Clarivate Analytics, Philadelphia, USA).
Inclusion and exclusion criteria and data abstraction
The studies were included when they met the eligibility criteria as follows.
Populations
Participants were adults above 18 years old with mild-to-moderate or severe chronic asthma, regardless of whether they were diagnosed by a physician or self-reported using standard diagnostic interviews or exceeded a predefined threshold for asthma using a validated severity measurement.
Interventions
We included studies with PA interventions in adults with asthma. Acceptable exercise training included PA interventions performed at least twice weekly for at least 8 weeks, as defined by the American College of Sports Medicine Guidelines (25). The interventions included any type of PA, such as AE comprising jogging, swimming, cycling, and aerobic dance; anaerobic exercise (ANE), including weight lifting, sprinting, throwing, long jump, and tug-of-war; resistance training (RT), comprising push-ups, squats, sit-ups, dumbbells, and sandbags; or other PA modalities such as muscle-strengthening activities (MSA), skeletal-strengthening activities (SSA), and balance activities (BA). The above-mentioned PA was conducted with supervised and unsupervised interventions.
Comparators
Comparators included the CG along with diet intervention, education, and placebo groups.
Outcomes
The primary outcome was any measurement that assessed QOL for asthmatic patients including various clinical measurement approaches which can evaluate the change in QOL (self or assessor-rated) from baseline to endpoint. The secondary outcome measurements were asthma control, pulmonary function, and other indicators for assessing asthma symptom severity.
Study design
Our study included any type of randomized controlled trials (RCTs) and longitudinal population-based studies, regardless of whether they were prospective or retrospective cohort studies, and regardless of race, country, or publication date. For detecting whether asthmatic adults would benefit from long-term PA, we additional included the observational studies. Studies were excluded if they met the following conditions: (I) participants were less than 18 years old or studies which could not distinguish participants 18 years old and below, and if most enrolled patients were substance withdrawal; (II) PAs whether combinatorial or multicomponent, were eliminated since they introduced confounding variables into our analyses; the definition of PA in study is vague, (III) full-texts or data could not be obtained for our analyses; (IV) studies were excluded if they were protocols or review studies; (V) outcomes were reported with biological indicators or other measures that could not produce the intersecting endpoint for our analyses, placebo or control group in any combination with any intervention.
Outcome measurement and quality assessment
The following key information of the included studies was extracted into a pre-designed comprehensive Excel form: first author, publication year, origin, intervention duration/follow-up time, and study design, as well as the characteristics of participants such as age, sample size, and sex ratio. If the essential data were not reported in the original studies, they were obtained by contacting corresponding authors of potential studies.
Two reviewers independently evaluated the quality of the included RCTs based on the Cochrane risk of bias tool (24). RCTs were evaluated by the Cochrane risk of bias tool, which included 7 items, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases, and items were judged as “high risk”, “low risk”, and “unclear risk”.
The quality assessment of observational studies was performed using the Newcastle-Ottawa Scale (NOS), independently judged by 2 assessors. Three major separate items contributed to the overall NOS quality assessment tool: patient selection, comparability of the treatment and observation groups, and outcome assessment. Included studies with scores over or equal to 6 were considered to be of high quality, whereas those with a score below 7 were considered to be of low quality (26,27).
Statistical analyses
First, we conducted a traditional pairwise meta-analysis of included trials for each existing comparison (28). For outcomes presented as dichotomous variables, the odds ratio (OR) was applied to calculate the pooled effect sizes (ESs) for each study. For numerical variables, we extracted their mean difference and standard deviation (SD) of the change from baseline or transformed them into a standard format to ensure that our analyses were conducted smoothly. As the presence of ESs indicates a continuous outcome, weighted mean difference (WMD) or standard mean difference (SMD) were calculated for each comparison using group (relevant) means and SD from individual studies with a random effect model. The 95% credible interval (CrI) was calculated along with the above combined ESs as a measure of estimated uncertainty (29). The quantitative pooled analyses were conducted based on the fixed effect model (inverse variance), and the random effect model (I–V heterogeneity approach) was applied if heterogeneity was present (28). I2 statistics were used to evaluate statistical heterogeneity, with estimated values of 25%, 50%, and 75% indicating mild, moderate, and high heterogeneity, respectively (30). The Bayesian statistical approach has a strong advantage over the traditional frequency approach in that the former approach not only provides a flexible and efficient modeling instrument by estimating posterior probability, but also controls various biases generated during the iteration process compared with the frequency method (22,31). Additionally, potential bias in small studies was evaluated using a comparison-adjusted funnel plot, which serves as an intuitive visual instrument for detecting the presence of any dominant types of potential bias, such as publication bias, selective reporting, or other biases. Quantitative Egger’s test was performed to determine whether P values were less than 0.05 (32). A sequence of subgroup analyses was conducted to explain any observed heterogeneity or to explore statistically significant differences between studies. The variables of interest are as follows: quality of included studies (low and high), publication year (≥2010 and <2010), region (Europe, America, and other regions), indoor PA or outdoor PA (indoor and outdoor), whether under self-supervision (supervised and unsupervised), intervention duration (≥12 weeks and <12 weeks), total sample size (≥50 and <50), male-to-female ratio (≥1 and <1), and participant diagnosis (self-report and diagnosed with asthma by physician). All the above sequences of analyses were performed in STATA, version 14.0, using the code “winbugsfromststa” to debug WinBUGS software (Stata, Corp, College Station, TX, USA).
Results
Study selection and characteristics of the included studies
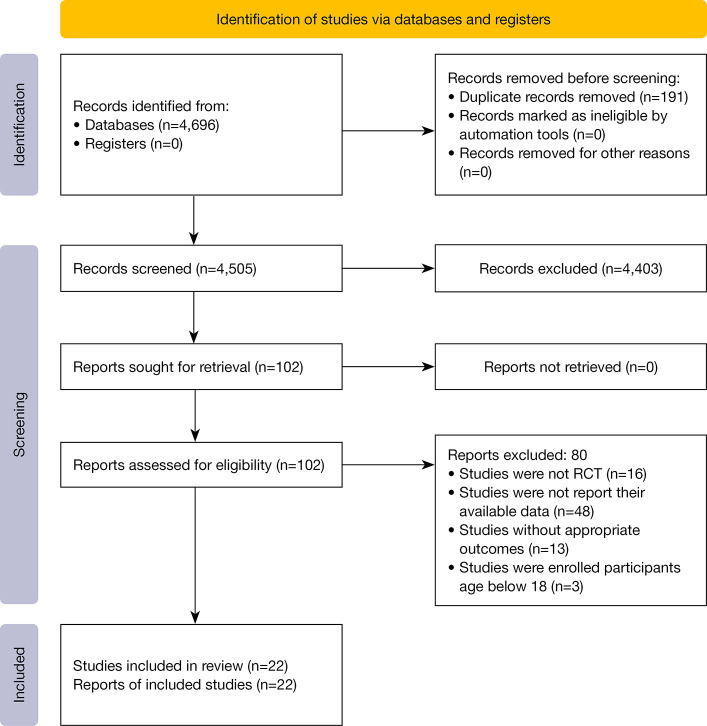
We obtained 4,696 studies from the database searches (12 publications were identified through other sources), of which 191 duplicates were first excluded, while 4,403 were removed based on their titles and abstracts. Subsequently, the remaining 102 studies were eligible for full-text screening. A total of 80 publications were excluded for various reasons, as follows: 16 studies were presented as protocols, 48 did not provide their available data for our analyses, 13 had irrelevant outcomes, and 3 enrolled participants below 18 years old. Finally, 22 studies were included in our analyses, comprising 18 RCTs (33-50) and 4 longitudinal studies (51-54), and details of the study selection process are depicted in Figure 1.
Figure 1.
Literature review flowchart. RCT, randomized controlled trial.
All 22 included studies enrolled 85,392 individuals aged between 18 and 75 years, with 1,073 and 84,319 participants in RCTs and observational studies, respectively. There was a significantly lower proportion of male participants than female participants [males in RCTs: 173 (32.15%), males in longitudinal studies: 4,449 (5.28%)]. The intervention duration of trials ranged from 8 weeks to 12 months, with a median follow-up time of 9 years across the 4 included longitudinal studies. Participants were recruited primarily in South America (N=8, 44.4%) and Europe (N=4, 22.2%). All subjects enrolled in the included studies received the appropriate basic treatment and the majority of them had moderate to severe asthma. Ten of the 18 RCTs gave a clear description of the daily doses of inhaled corticosteroids (ICS) administered to the subjects. Table 1 summarizes the demographic characteristics of the included studies.
Table 1. Demographic characteristics of included studies.
| Sources | Study design | PA type | Participants sample | Proportion of female (%) | Age (years), mean ± SD/range | Whether supervision | Therapeutic course/length of follow-up | Region | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | CG | PA | CG | ||||||||
| Cochrane, 1990 | RCT | Varied aerobic exercises, including cycling, jogging, and “aerobics” | 18 | 18 | 61.10 | 28±7 | 28±8 | Some sessions | 3 months | UK | FEV1, FEV1/pred, VO2max |
| Farid, 2005 | RCT | A course of aerobic exercise | 18 | 18 | 55.60 | 27 | 29 | Unclear | 8 weeks | Iran | FEV1, FVC/pred, FEV1/FVC |
| Gonalves, 2008 | RCT | Treadmill training, education and breathing exercises | 10 | 10 | 65.00 | 34.6 (21.0−47.0) | 34.6 (21.0−47.0) | Yes | 12 weeks | Brazil | HRQoL, HRQoL (4 sub-items), ASFD, FeNO |
| Mendes, 2010 | RCT | Unspecified aerobic exercise training, breathing exercises and educational programme | 50 | 51 | 73.30 | 39 (22−47.9) | 39.5 (23.5−47.0) | Yes | 3 months | Brazil | HRQoL, HRQoL (4 sub-items), ASFD, FEV1, FEV1/pred, FVC, FVC/pred, FEV1/FVC |
| Mendes, 2011 | RCT | Unspecified aerobic exercise training, breathing exercises and educational programme | 27 | 24 | 41.20 | 37.9 (25.7−47.3) | 36.0 (22.0−47.5) | Yes | 3 months | Brazil | FEV1, FEV1/pred, FVC, FVC/pred, FEV1/FVC, FeNO, VO2max |
| Boyd, 2012 | RCT | Walking | 6 | 8 | 92.90 | 53 [38−62] | 54 [33−78] | No | 12 weeks | UK | ACQ, FEV1/pred |
| Scott, 2013 | RCT | Gym and intermittent personal training sessions and a diet intervention | 13 | 15 | 53.60 | 33.9±11.5 | 44.7±14.7 | Once a week | 12 weeks | Australian | AQLQ, ACQ, FEV1, FVC, FEV1/FVC, FeNO |
| França-Pinto, 2015 | RCT | Treadmill training and breathing exercises | 22 | 21 | 48.80 | 40±11 | 44±99 | Yes | 12 weeks | Brazil | AQLQ, AQLQ (4 sub-items), ASFD, ACQ, FEV1, FEV1/pred, FeNO, VO2max |
| Toennesen, 2018 | RCT | Indoor cycling | 29 | 34 | 76.20 | 43.7±13.9 | 38.2±12.7 | Yes | 8 weeks | Denmark | AQLQ, ACQ, FEV1pred, FVC/pred, FeNO, VO2max |
| Freitas, 2017 | RCT | Unspecified aerobic exercise training and hypocaloric diet | 26 | 25 | 98.00 | 45.9±7.7 | 48.5±9.6 | No | 3 months | Brazil | AQLQ (4 sub-items), ACQ, FEV1, FVC, FeNO |
| Refaat, 2015 | RCT | Exercise training | 38 | 30 | 45.60 | 35.8±1.7 | 38±5.3 | Yes | 3 months | Kuwait | AQLQ, AQLQ (4 sub-items), FEV1, FVC |
| Turner, 2011 | RCT | Exercise training | 19 | 15 | 55.90 | 65.3±10.8 | 71.0±9.7 | Yes | 3 months | Australia | AQLQ (4 sub-items), ACQ |
| Shaw, 2011 | RCT | Aerobic exercise | 22 | 22 | 63.60 | 21.95±3.84 | 21.90±3.89 | Yes | 8 weeks | South Africa | FEV1, FVC, FEV1/FVC |
| Coelho, 2018 | RCT | Physical activity + usual care | 20 | 17 | 86.50 | 45±19 | 47±14 | No | 3 months | Brazil | AQLQ, ACQ, FEV1/pred, FVC/pred, FEV1/FVC |
| Freitas, 2018 | RCT | A treadmill interspersed with either bike or elliptical machine workouts | 26 | 26 | 96.20 | 45.9±7.7 | 48.5±9.6 | The exercise program included two sessions of supervised aerobic and resistance exercises | 3 months | Brazil | ASFD |
| Ma, 2015 | RCT | Moderate-intensity physical activity | 165 | 165 | 70.60 | 47.5±12.6 | 47.7±12.1 | Ongoing supervision | 12 months | US | AQLQ, AQLQ (4 sub-items), ACQ, FEV1, FVC, FEV1/FVC |
| Meyer, 2015 | RCT | Walking, endurance and circuit training, cardiopulmonary exercise | 13 | 8 | 61.90 | 54±11 | 59±9 | Once weekly supervised sessions | 12 months | Germany | AQLQ, AQLQ (4 sub-items) |
| Shaw, 2010 | RCT | Weekly supervised aerobic exercise | 22 | 22 | 63.60 | 21.95±3.87 | 21.90±3.89 | three times weekly supervised | 8 weeks | Republic of South Africa | FEV1, FVC, VO2max |
| Beckett, 2001 | LS | MPA | 4,547 | 54.90 | 18−30 | Unclear | 10 years | US | Incidence | ||
| Benet, 2011 | LS | Recreational physical activity habits (walking, cycling, gardening, home do-it-yourself activities, sports, and climbing stairs) | 51,080 | Women | 40−65 | Unclear | 9 years | France | Incidence | ||
| Huovinen, 2003 | LS | A series of PA | 9,671 | 54.00 | 25−52 | Unclear | 9 years | Finland | Incidence | ||
| Lucke, 2007 | LS | A series of PA | 1,9021 | Women | 18−75 | Unclear | 5–7 years | Australia | Incidence | ||
ACQ, Asthma Control Questionnaire; AQLQ, Asthma-related quality of life questionnaire; AQLQ (4 sub-items), AQLQ-Activity limitation domain, AQLQ-Symptoms domain, AQLQ-Emotional function domain, AQLQ-Environmental stimuli domain; ASFD, asthma symptom-free days; CG, control group; FEV1, forced expiratory volume in one second (L); FEV1/pred, FEV1 as a percentage of the predicted value; FeNO, fractional exhaled nitric oxide; FVC, forced vital capacity (L); FVC/pred, FVC as a percentage of the predicted value; HRQoL, health related quality of life; HRQoL (4 sub-items), HRQoL-Physical limitation, HRQoL-Symptom frequency, HRQoL-Socioeconomic, HRQoL-Psychosocial; LS, longitudinal study; MPA, moderate PA; PA, physical activity; RCT, randomized controlled trial; UK, the United Kingdom; US, the United States of America; VO2max, maximal oxygen uptake.
Quality of the included studies
All 18 RCTs were evaluated as having a low risk of bias regarding random sequence generation. Thirteen out of 17 trials were rated as having a low risk of selection bias. Six studies were judged as having a low risk of performance bias, whereas 13 of the 18 studies were rated as having a high risk of detection bias. Regarding attrition bias, only 1 study was rated as having a high risk of bias, and of the remaining studies, 16 were rated as having a low risk of bias while 1 study had an unclear risk of bias. Concerning reporting bias, most studies (11/17, 64.71%) were judged as high quality with a low risk of bias. Figures S1,S2 provide detailed justifications for the risk of bias assessments of the included RCTs. For longitudinal studies, 3 studies were rated as high quality, scoring 7 points according to the NOS criteria. Figure S3 displays the detailed descriptions of the NOS assessment process.
Primary outcomes
QOL
Two trials used health-related quality of life (HRQOL) (121 participants) (45,46) while 8 trials used the asthma control questionnaire (ACQ) (600 participants) (33,36,40,41,44,48-50) as one of their endpoints to assess QOL among asthma patients. The results revealed that participants receiving PA were more likely to improve their QOL (SMD =−0.80, 95% CrI: −1.30 to −0.31; I2=86.9%, Pheterogeneity<0.001) (Table 2). There were significant differences between the PA group and CG for the 4 domains of HRQOL [(physical limitation: SMD=−2.73, 95% CrI: −3.32 to −2.13; I2=11.0%, Pheterogeneity=0.29), (symptom frequency: SMD =−2.20, 95% CrI: −2.65 to −1.74; I2=0.0%, Pheterogeneity=0.94), (socioeconomic: SMD =−1.01, 95% CrI: −2.02 to −0.01; I2=76.2%, Pheterogeneity<0.05), (psychosocial: SMD =−2.32, 95% CrI: −2.79 to −1.86; I2=0.0%, Pheterogeneity=0.81)] (Table 2). The funnel plot was not symmetrical, suggesting the existence of potential publication bias (PEgger’s test=0.648) (Figure S4).
Table 2. Primary results based on various outcomes and subgroup analyses.
| Meta-analyses outcomes | Meta-analyses variables | No. of studies | No. of patients | Pool effect size | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| PA | CG | I2 (%) | P | ||||||
| RCTsa | 18 | 544 | 529 | ||||||
| Primary outcomes | |||||||||
| Quality of life | HRQoL & ACQ | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| HRQoL-Physical limitation | 2 | 60 | 61 | −2.73 (−3.32 to −2.13) | 11.00 | 0.29 | |||
| HRQoL-Symptom frequency | 2 | 60 | 61 | −2.20 (−2.65 to −1.74) | 0.00 | 0.94 | |||
| HRQoL-Socioeconomic | 2 | 60 | 61 | −1.01 (−2.02 to −0.01) | 76.20 | <0.05 | |||
| HRQoL-Psychosocial | 2 | 60 | 61 | −2.32 (−2.79 to −1.86) | 0.00 | 0.81 | |||
| AQLQ | 7 | 300 | 290 | −0.03 (−0.61 to 0.54) | 87.90 | <0.001 | |||
| AQLQ-Activity limitation domain | 6 | 283 | 264 | 0.16 (−0.67 to 1.00) | 93.30 | <0.001 | |||
| AQLQ-Symptoms domain | 6 | 283 | 264 | 0.39 (−0.37 to 1.14) | 91.90 | <0.001 | |||
| AQLQ-Emotional function domain | 6 | 283 | 264 | 0.44 (−0.16 to 1.03) | 87.20 | <0.001 | |||
| AQLQ-Environmental stimuli domain | 6 | 283 | 264 | 0.55 (−0.49 to 1.59) | 95.60 | <0.001 | |||
| Secondary outcomes | |||||||||
| Asthma control | Asthma symptom free-day | 4 | 108 | 108 | 0.93 (−0.39 to 2.25) | 94.20 | <0.001 | ||
| Pulmonary function | FEV1 | 10 | 403 | 393 | 0.24 (−0.13 to 0.60) | 81.40 | <0.001 | ||
| FEV1/pred | 8 | 190 | 191 | 0.47 (0.03 to 0.90) | 74.90 | <0.001 | |||
| FVC | 8 | 363 | 354 | −0.02 (−0.70 to 0.65) | 93.50 | <0.05 | |||
| FVC/pred | 5 | 144 | 144 | 0.39 (−0.05 to 0.82) | 67.90 | <0.001 | |||
| FEV1/FVC | 7 | 315 | 312 | −0.03 (−0.41 to 0.35) | 76.20 | <0.001 | |||
| Airway inflammation | FeNO | 6 | 127 | 129 | 0.58 (−0.90 to 2.06) | 96.00 | <0.001 | ||
| Exercise tolerance | VO2Max | 5 | 118 | 119 | 1.18 (0.87 to 1.48) | 17.00 | 0.31 | ||
| Longitudinal studyb | |||||||||
| High level PA vs. low level PA | 7 | 30,470 | 34,260 | 0.87 (0.77 to 0.98) | 27.40% | 0.22 | |||
| Subgroup analysis based on the primary outcome of quality of lifec | |||||||||
| Quality of included studies | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Low bias | 9 | 354 | 353 | −0.91 (−1.07 to −0.75) | 88.20 | <0.001 | |||
| High bias | 1 | 6 | 8 | −0.41 (−1.48 to 0.66) | – | – | |||
| Region | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Europe and America | 3 | 200 | 207 | −0.95 (−1.15 to −0.74) | 0.00 | 0.47 | |||
| Other regions | 7 | 160 | 154 | −0.83 (−1.08 to −0.58) | 91.00 | <0.001 | |||
| Publication year | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Above and equal 2010 | 1 | 10 | 10 | −2.89 (−4.18 to −1.61) | – | – | |||
| Below 2010 | 9 | 350 | 351 | −0.87 (−1.03 to −0.71) | 86.60 | <0.001 | |||
| Total sample size | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | ||||
| Above or equal 50 | 6 | 90 | 86 | −0.23 (−0.54 to 0.07) | 74.10 | <0.001 | |||
| Below 50 | 4 | 270 | 275 | −1.14 (−1.32 to −0.95) | 88.00 | <0.001 | |||
| Male to female ratio | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Above or equal 1 | 10 | 360 | 361 | −0.90 (−1.06 to −0.74) | – | – | |||
| Below 1 | 0 | – | – | − | – | – | |||
| Indoor or outdoor | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Indoor | 8 | 189 | 188 | −0.82 (−1.04 to −0.60) | 89.50 | <0.001 | |||
| Outdoor | 2 | 171 | 173 | −0.97 (−1.20 to −0.75) | 9.90 | 0.29 | |||
| Self-supervision | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Yes | 6 | 295 | 296 | −1.02 (−1.20 to −0.84) | 90.80 | <0.001 | |||
| No or session | 4 | 65 | 65 | −0.41 (−0.76 to 0.06) | 40.70 | 0.17 | |||
| Intervention durations | Overall | 10 | 360 | 361 | −0.80 (−1.30 to −0.31) | 86.90 | <0.001 | ||
| Above or equal 12 weeks | 9 | 331 | 327 | −0.91 (−1.07 to −0.74) | 88.40 | <0.001 | |||
| Below 12 weeks | 1 | 29 | 34 | −0.79 (−1.31 to −0.28) | 86.90 | <0.001 | |||
If studies reported with more than two categories of different PA levels, they were converted into two groups, namely high level PA and low level PA while the later group was used as the reference category. Pool effect size: a, pooled WMDs/SMDs (95% CrI); b, pooled ORs (95% CrI); c, pooled SMDs (95% CrI). ACQ, Asthma Control Questionnaire; AQLQ, asthma-related quality of life questionnaire; AQLQ (4 sub-items), AQLQ-Activity limitation domain, AQLQ-Symptoms domain, AQLQ-Emotional function domain, AQLQ-Environmental stimuli domain; ASFD, asthma symptom-free days; CI, confidence interval; CG, control group; FEV1, forced expiratory volume in one second (L); FEV1/pred, FEV1 as a percentage of the predicted value; FeNO, fractional exhaled nitric oxide; FVC, Forced vital capacity (L); FVC/pred, FVC as a percentage of the predicted value; HRQoL, Health related quality of life; HRQoL (4 sub-items), HRQoL-Physical limitation, HRQoL-Symptom frequency, HRQoL-Socioeconomic, HRQoL-Psychosocial; LS, longitudinal study; MPA, moderate PA; OR, odds ratio; PA, physical activity; RCT, randomized controlled trial; SMD, standard mean differences; UK, the United Kingdom; US, the United States of America; VO2max, Maximal oxygen uptake; WMD, weighted mean differences.
Seven studies (590 participants) (33,40,41,43,44,47,48) reported their outcomes based on the asthma-related quality of life questionnaire (AQLQ), and the pooled results failed to reach statistical significance between the PA group and CG (MD =−0.03, 95% CrI: −0.61 to 0.54; I2=87.9%, Pheterogeneity<0.001) (Table 2). No symmetry was observed in the funnel plot, indicating publication bias (PEgger’s test=0.783) (Figure S5). The same results were also found for the 4 domains of the AQLQ [(activity limitation: MD =0.16, 95% CrI: −0.67 to 1.00; I2=93.3%, Pheterogeneity<0.001), (symptom: MD =0.39, 95% CrI: −0.37 to 1.14; I2=91.9%, Pheterogeneity<0.001), (emotional function: MD =0.44, 95% CrI: −0.16 to 1.03; I2=87.2%, Pheterogeneity<0.001), (environmental stimuli: MD =0.55, 95% CrI: −0.49 to 1.59; I2=95.6%, Pheterogeneity<0.001)] (Table 2).
Secondary outcomes
Asthma control
Four studies (216 participants) (33,38,45,46) investigated the effectiveness of PA on asthma symptom free-days, without observing a significant difference (MD =0.93, 95% CrI: −0.39 to 2.25; I2=94.2%, Pheterogeneity<0.001) (Table 2). The asymmetry of the funnel plot for asthma symptom free-day indicated publication bias (PEgger’s test=0.867) (Figure S6).
Pulmonary function
Forced expiratory volume in one second (FEV1)
Ten trials (796 participants) (33-35,37,39,43-46,48,49) considered FEV1 in liters as their primary outcome (MD=0.24, 95% CrI: −0.13 to 0.60; I2=81.4%, Pheterogeneity<0.001), whereas 8 trials (381 participants) considered FEV1 as a percentage of the predicted value (FEV1/pred) (MD =0.47, 95% CrI: 0.03 to 0.90; I2=74.9%, Pheterogeneity<0.001) (33,34,37,40-42,45,50) (Table 2). Significant publication bias was identified based on the above 2 outcomes as the funnel plots showed asymmetry [FEV1 in liters: PEgger’s test=0.802, FEV1/pred: PEgger’s test=0.741] (Figures S7,S8).
Forced vital capacity (FVC)
A random effect model was performed to estimate the pooled effect of FVC in liters (8 studies, 717 participants; MD =−0.02, 95% CrI: −0.70 to 0.65; I2=93.5%, Pheterogeneity<0.001) (35,37,39,43-45,48,49) and FVC as a percentage of the predicted value (FVC/pred) (5 studies, 288 participants; MD =0.39, 95% CrI: −0.05 to 0.82; I2=67.9%, Pheterogeneity<0.001) (37,40-42,45) (Table 2). The funnel plot for the above outcomes appeared asymmetrical, indicating publication bias [FVC in liters: PEgger’s test=0.842, FVC/pred: PEgger’s test=0.141] (Figures S9,S10).
FEV1/FVC
Seven studies (627 participants) examined the outcome of FEV1/FVC, and no significant change was found between the 2 groups (MD =−0.03, 95% CrI: −0.41 to 0.35; I2=76.2%, Pheterogeneity<0.001) (37,39,40,42,44,45,48) (Table 2). Bilateral asymmetry on the funnel plot was observed (PEgger’s test=0.013) (Figure S11).
Airway inflammation
Fraction of exhaled nitric oxide (FeNO)
Six studies comprising 256 participants reported on the FeNO. There were no significant differences in FeNO (MD =0.58, 95% CrI: −0.90 to 2.06; I2=96.0%, Pheterogeneity<0.001) (33,37,41,44,46,49) (Table 2). The funnel plot and Egger’s test were employed to evaluate publication bias with the relevant estimates (PEgger’s test=0.013) (Figure S12).
Exercise tolerance
Maximal oxygen consumption (VO2max)
Five studies (237 participants) examined the effects of PA on VO2max and discovered a significant improvement between the 2 groups (MD =1.18, 95% CrI: 0.87 to 1.48; I2=17.0%, Pheterogeneity=0.31) (33-35,37,41) (Table 2). A highly symmetrical funnel plot was obtained, indicating the absence of publication bias, and Egger’s test (P=0.117) was consistent with the funnel plot result (Figure S13).
Observational studies
If studies reported more than 2 categories of different PA levels, they were converted into 2 groups, namely high level PA and low level PA, and the latter group was used as the reference category. Four longitudinal studies (84,319 participants) were splitted into 7 studies (64,730 participants) for presenting their PA level with more than 2 groups (48-51). The pooled ES (OR) was 0.87 (95% CrI: 0.77 to 0.98), with mild heterogeneity (I2=27.4%, P=0.22) (Table 2). Symmetry of the funnel plot was observed (PEgger’s test=0.992) (Figure S14).
Subgroup analyses
Based on the primary outcome of QOL, the subgroup analyses were conducted with different variables of interest, and most of them were yielded consistency that without significantly statistical differences between subgroup items. Nevertheless, when the items of total sample size and self-supervision were taken into consideration, the total participants below 50 (MD =−1.14, 95% CrI: −1.32 to −0.95) saw a notable improvement compared with the CG (MD =−0.23, 95% CrI: −0.54 to 0.07). The same result was found for participants under self-supervision (MD =−1.02, 95% CrI: −1.20 to −0.84) compared with group without or with part of supervision (MD =−0.41, 95% CrI: −0.76 to 0.06) (Table 2).
Discussion
To the best of our knowledge, this study is the first meta-analysis to provide an exhaustive overview of the effectiveness of PA in adults with asthma by simultaneously incorporating both trials and observational studies. Based on the 22 studies, comprising 18 RCTs and 4 longitudinal studies, our findings confirmed that regular PA could improve the QOL and other health outcomes among adult patients, and has also been linked to maintaining long-term PA.
There is poor evidence to endorse one form of PA over another, even from the latest guideline issued in 2021 (8). Our findings establish a link between increased regular PA and favorable outcomes, including higher QOL (HRQOL and ACQ), better lung function (FEV1/pred), improved VO2max, and long-term effects. As a critical index for characterizing individuals and assessing the effectiveness of interventions, the HRQOL scale is a widely used tool consisting of 4 domains, namely physical limitation, symptom frequency, socioeconomic conditions, and psychosocial health. It can evaluate QOL among asthmatic patients that can be captured by biological or clinical indicators. When SMD was pooled with HRQOL and ACQ, we detected significant differences between the PA group and CG (SMD =−0.80, 95% CrI: −1.30 to −0.31). The same results were also obtained for the 4 sub-items of HRQOL, although only 2 included studies assessed these items. While a substantial body of evidence already demonstrated that asthmatic patients could benefit from regular PA, the same efficacy was also observed in patients with exercise-induced asthma (55-57), as asthmatics’ variable symptoms such as wheeze and shortness of breath are regularly triggered by exercise (58). Furthermore, asthmatics were shown to develop anxiety about exercise-induced bronchoconstriction (EIB) (59-61), which is why some often intuitively or purposely adopt a sedentary lifestyle over an active one. Although exercise is an important cause of airway obstruction, which can eventually develop into asthma, the maintenance of medications such as ICS-formoterol can generally be reduced to maintain symptom control in EIB (62). Additionally, a short warm-up pre-exercise can be introduced for controlling exercise-related symptoms, or a short-acting beta agonist (SABA) reliever (62) can be taken whenever a low dose of ICS (63) is taken before or during exercise.
Our study also found that when asthmatic individuals performed PA, their pulmonary function outcome significantly improved (FEV1/pred). This outcome is critical when evaluating health outcomes for asthmatics patients, as peripheral airway involvement and functional status are closely related to pathophysiology, disease progression, and clinical manifestations of asthma (64), although they are not the gold-standard airflow limitation measurements (65). Long-term beneficial effects of high-level PA have also been shown (OR: 0.87, 95% CrI: 0.77 to 0.98), with a lower risk of developing asthma than the low-level PA group. Although the number of longitudinal studies we identified was small (N=4), the total accrued number of subjects was considerable (N=84,319). A reverse causal relationship should also be considered to explain why asthmatic patients have lower PA rates. Several factors contribute to this, including poor education, fear for asthma symptoms, or inadequately regulated asthma (66).
Any PA performed during daily life such as household activities, recreation, transportation, sports, and other leisure-time activities should be encouraged, as they have been shown to benefit not only asthma-related outcomes but also other dimensions of health-related endpoints (cardiorespiratory and muscular fitness) in asthmatic patients (67,68), particularly severe asthma and specific asthma populations (69,70). Notably, PA combined with pharmacological interventions was more effective than PA alone, and this requires further investigation. Other remaining issues should also be investigated, including the treatment’s side effects and patient acceptability of asthma self-management (71,72). In particular, for obese asthmatic patients, a weight-reduction program incorporating aerobic and strength exercises is more effective for symptom control than a weight reduction program alone (49). Further research should focus on objectively investigating assessment technology for measuring PA, such as an accelerometer, which is a wearable device that enables continuous and precise monitoring of the multiaxial accelerations of body movement in patients, offering a greater degree of reliability than traditional self-report questionnaires (73).
Strengths and limitations
To summarize, our study contributes to the latest evidence by providing an informative summary of the evidence regarding how regular PA may help asthmatic patients to improve their QOL and control asthma. This guide may assist policymakers, clinicians, or caregivers in making choices and navigates the direction of clinical decision making, thereby facilitating future research and clinical application. As far as we know, this is the first attempt to summarize intervention trials and observational follow-up studies to evaluate the relationship between regular PA and asthma-related outcomes among adults. Potential studies were retrieved and further retrieved through screening the references of similar studies and by manual searches during the study selection process in case of the omission of relevant studies.
This study has several limitations that must be acknowledged. First, all results were based on a relatively limited number of included studies, leading to insufficient evidence in our analysis. Additionally, the methodological shortcomings of the 18 RCTs may jeopardize the study's overall quality. The issues of heterogeneity, imprecision, inconsistency between included studies, and further risk of bias of individual studies are mainly reflected in outcome assessment blinding and selective bias items. Moreover, a language bias was generated due to non-English language databased were search. We also included both RCTs and cohort studies while such two different type studies can generate heterogenous or even conflicting results which may cause possible bias to our findings. Finally, several included studies enrolled asthmatic patients with comorbidities such as obesity, which may introduce bias to our findings (37,52). Considering the above limitations, the findings of our study should be interpreted with caution.
Conclusions
Despite some limitations and uncertainties, our findings demonstrate the benefits of regular PA in terms of health-related outcomes for asthmatic patients and their long-term consequences. In the future, well-designed research addressing the precise association between PA and asthmatic patients is encouraged.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1170/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1170/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Asthma GIf. Global strategy for asthma management and prevention. 2019. Available online: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
- 2.Zazzali JL, Broder MS, Omachi TA, et al. Risk of corticosteroid-related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc 2015;36:268-74. 10.2500/aap.2015.36.3863 [DOI] [PubMed] [Google Scholar]
- 3.Dai L, Huang Y, Wang Y, et al. Serious systemic adverse events associated with allergen-specific immunotherapy in children with asthma. Zhongguo Dang Dai Er Ke Za Zhi 2014;16:58-61. [PubMed] [Google Scholar]
- 4.Pinto CR, Lemos AC, de Alcantara AT, et al. Systemic adverse events from inhaled corticosteroids self-reported by asthma patients: A "real-life" cross sectional study. Rev Port Pneumol (2006) 2016;22:243-5. [DOI] [PubMed] [Google Scholar]
- 5.Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res 2013;11:54-65. 10.3121/cmr.2013.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Z, Wang J, Xie Y, et al. Effects of exercise-based pulmonary rehabilitation on adults with asthma: a systematic review and meta-analysis. Respir Res 2021;22:33. 10.1186/s12931-021-01627-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126-31. [PMC free article] [PubMed] [Google Scholar]
- 8.Asthma Global Initiative for: Global Strategy for Asthma Management and Prevention. 2021.
- 9.Hansen ESH, Pitzner-Fabricius A, Toennesen LL, et al. Effect of aerobic exercise training on asthma in adults: a systematic review and meta-analysis. Eur Respir J 2020;56:2000146. 10.1183/13993003.00146-2020 [DOI] [PubMed] [Google Scholar]
- 10.Beggs S, Foong YC, Le HC, et al. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev 2013;(4):CD009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco DR, Silva MJ, Alexandrino AM, et al. Exercise-related quality of life in subjects with asthma: a systematic review. J Asthma 2012;49:487-95. 10.3109/02770903.2012.680636 [DOI] [PubMed] [Google Scholar]
- 12.Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev 2013;(9):CD001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ram FS, Robinson SM, Black PN, et al. Physical training for asthma. Cochrane Database Syst Rev 2005;(4):CD001116. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002;13:561-8. 10.1097/00001648-200209000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol 2005;115:928-34. 10.1016/j.jaci.2005.01.033 [DOI] [PubMed] [Google Scholar]
- 16.Neder JA, Nery LE, Silva AC, et al. Short-term effects of aerobic training in the clinical management of moderate to severe asthma in children. Thorax 1999;54:202-6. 10.1136/thx.54.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassim R, Koplin JJ, Dharmage SC, et al. The difference in amount of physical activity performed by children with and without asthma: A systematic review and meta-analysis. J Asthma 2016;53:882-92. 10.1080/02770903.2016.1175474 [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Powell A, Hoskins G, et al. Exploring and explaining low participation in physical activity among children and young people with asthma: a review. BMC Fam Pract 2008;9:40. 10.1186/1471-2296-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh L, Roberts RG, Kemp JG. Fitness and physical activity in children with asthma. Sports Med 2004;34:861-70. 10.2165/00007256-200434130-00001 [DOI] [PubMed] [Google Scholar]
- 20.Jago R, Salway RE, Ness AR, et al. Associations between physical activity and asthma, eczema and obesity in children aged 12-16: an observational cohort study. BMJ Open 2019;9:e024858. 10.1136/bmjopen-2018-024858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike KC, Griffiths LJ, Dezateux C, et al. Physical activity among children with asthma: Cross-sectional analysis in the UK millennium cohort. Pediatr Pulmonol 2019;54:962-9. 10.1002/ppul.24314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehead A, Omar RZ, Higgins JP, et al. Meta-analysis of ordinal outcomes using individual patient data. Stat Med 2001;20:2243-60. 10.1002/sim.919 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178-89. 10.1016/j.jclinepi.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 24.Tarsilla M. Cochrane Handbook for Systematic Reviews of Interventions. 2008.
- 25.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 27.Abou-Setta AM, Mousavi SS, Spooner C, et al. Newcastle-Ottawa Scale Assessment of Cohort Studies: Newcastle-Ottawa Scale Assessment of Cohort Studies; 2012.
- 28.Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (Eds). Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 2011;5:S38. [Google Scholar]
- 29.Hedges L. Advances in Statistical Methods for Meta-analysis. Evaluation and Statistical Review Annual; 1986. [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 31.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277-303. 10.1177/096228020101000404 [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Juni P, Bartlett C, et al. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7:1-76. 10.3310/hta7010 [DOI] [PubMed] [Google Scholar]
- 33.França-Pinto A, Mendes FA, de Carvalho-Pinto RM, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax 2015;70:732-9. 10.1136/thoraxjnl-2014-206070 [DOI] [PubMed] [Google Scholar]
- 34.Cochrane LM, Clark CJ. Benefits and problems of a physical training programme for asthmatic patients. Thorax 1990;45:345-51. 10.1136/thx.45.5.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw I, Shaw BS, Brown GA. Role of diaphragmatic breathing and aerobic exercise in improving pulmonary function and maximal oxygen consumption in asthmatics. Sci Sports 2010;25:139-45. 10.1016/j.scispo.2009.10.003 [DOI] [Google Scholar]
- 36.Turner S, Eastwood P, Cook A, et al. Improvements in symptoms and quality of life following exercise training in older adults with moderate/severe persistent asthma. Respiration 2011;81:302-10. 10.1159/000315142 [DOI] [PubMed] [Google Scholar]
- 37.Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc 2011;43:197-203. 10.1249/MSS.0b013e3181ed0ea3 [DOI] [PubMed] [Google Scholar]
- 38.Freitas PD, Silva AG, Ferreira PG, et al. Exercise Improves Physical Activity and Comorbidities in Obese Adults with Asthma. Med Sci Sports Exerc 2018;50:1367-76. 10.1249/MSS.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 39.Shaw BS, Shaw I. Pulmonary function and abdominal and thoracic kinematic changes following aerobic and inspiratory resistive diaphragmatic breathing training in asthmatics. Lung 2011;189:131-9. 10.1007/s00408-011-9281-8 [DOI] [PubMed] [Google Scholar]
- 40.Coelho CM, Reboredo MM, Valle FM, et al. Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: a randomized controlled trial. J Sports Sci 2018;36:1186-93. 10.1080/02640414.2017.1364402 [DOI] [PubMed] [Google Scholar]
- 41.Toennesen LL, Meteran H, Hostrup M, et al. Effects of Exercise and Diet in Nonobese Asthma Patients-A Randomized Controlled Trial. J Allergy Clin Immunol Pract 2018;6:803-11. 10.1016/j.jaip.2017.09.028 [DOI] [PubMed] [Google Scholar]
- 42.Farid R, Azad FJ, Atri AE, et al. Effect of aerobic exercise training on pulmonary function and tolerance of activity in asthmatic patients. Iran J Allergy Asthma Immunol 2005;4:133-8. [PubMed] [Google Scholar]
- 43.Refaat A, Gawish M. Effect of physical training on health-related quality of life in patients with moderate and severe asthma. Egyptian Journal of Chest Diseases and Tuberculosis 2015;64:761-6. 10.1016/j.ejcdt.2015.07.004 [DOI] [Google Scholar]
- 44.Scott HA, Gibson PG, Garg ML, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013;43:36-49. 10.1111/cea.12004 [DOI] [PubMed] [Google Scholar]
- 45.Mendes FA, Gonçalves RC, Nunes MP, et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest 2010;138:331-7. 10.1378/chest.09-2389 [DOI] [PubMed] [Google Scholar]
- 46.Gonçalves RC, Nunes MPT, Cukier A, et al. Effects of an aerobic physical training program on psychosocial characteristics, quality-of-life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Rev Bras Fisioter, São Carlos 2008;12:127-35. [Google Scholar]
- 47.Meyer A, Günther S, Volmer T, et al. A 12-month, moderate-intensity exercise training program improves fitness and quality of life in adults with asthma: a controlled trial. BMC Pulm Med 2015;15:56. 10.1186/s12890-015-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Strub P, Xiao L, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc 2015;12:1-11. 10.1513/AnnalsATS.201406-271OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freitas PD, Ferreira PG, Silva AG, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am J Respir Crit Care Med 2017;195:32-42. 10.1164/rccm.201603-0446OC [DOI] [PubMed] [Google Scholar]
- 50.Boyd A, Yang CT, Estell K, et al. Feasibility of exercising adults with asthma: a randomized pilot study. Allergy Asthma Clin Immunol 2012;8:13. 10.1186/1710-1492-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckett WS, Jacobs DR, Jr, Yu X, et al. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 2001;164:2045-50. 10.1164/ajrccm.164.11.2004235 [DOI] [PubMed] [Google Scholar]
- 52.Benet M, Varraso R, Kauffmann F, et al. The effects of regular physical activity on adult-onset asthma incidence in women. Respir Med 2011;105:1104-7. 10.1016/j.rmed.2011.03.016 [DOI] [PubMed] [Google Scholar]
- 53.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med 2003;97:273-80. 10.1053/rmed.2003.1419 [DOI] [PubMed] [Google Scholar]
- 54.Lucke J, Waters B, Hockey R, et al. Trends in women's risk factors and chronic conditions: findings from the Australian Longitudinal Study on Women's Health. Womens Health (Lond) 2007;3:423-32. 10.2217/17455057.3.4.423 [DOI] [PubMed] [Google Scholar]
- 55.Henriksen JM, Nielsen TT, Dahl R. Effects of physical training on plasma citrate and exercise-induced asthma. Scand J Clin Lab Invest 1981;41:225-9. 10.3109/00365518109092038 [DOI] [PubMed] [Google Scholar]
- 56.Ram FS, Robinson SM, Black PN. Effects of physical training in asthma: a systematic review. Br J Sports Med 2000;34:162-7. 10.1136/bjsm.34.3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crosbie A. The effect of physical training in children with asthma on pulmonary function, aerobic capacity and health-related quality of life: a systematic review of randomized control trials. Pediatr Exerc Sci 2012;24:472-89. 10.1123/pes.24.3.472 [DOI] [PubMed] [Google Scholar]
- 58.Bonini M, Palange P. Exercise-induced bronchoconstriction: new evidence in pathogenesis, diagnosis and treatment. Asthma Res Pract 2015;1:2. 10.1186/s40733-015-0004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiler JM, Anderson SD, Randolph C, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol 2010;105:S1-47. 10.1016/j.anai.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 60.Ali Z. How to diagnose exercise induced asthma? Asian J Sports Med 2011;2:63-7. 10.5812/asjsm.34776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Aymerich J, Varraso R, Antó JM, et al. Prospective study of physical activity and risk of asthma exacerbations in older women. Am J Respir Crit Care Med 2009;179:999-1003. 10.1164/rccm.200812-1929OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013;187:1016-27. 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- 63.Lazarinis N, Jørgensen L, Ekström T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax 2014;69:130-6. 10.1136/thoraxjnl-2013-203557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scichilone N, Contoli M, Paleari D, et al. Assessing and accessing the small airways; implications for asthma management. Pulm Pharmacol Ther 2013;26:172-9. 10.1016/j.pupt.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 65.Bahmer T, Waschki B, Schatz F, et al. Physical activity, airway resistance and small airway dysfunction in severe asthma. Eur Respir J 2017;49:1601827. 10.1183/13993003.01827-2016 [DOI] [PubMed] [Google Scholar]
- 66.Eijkemans M, Mommers M, Draaisma JM, et al. Physical activity and asthma: a systematic review and meta-analysis. PLoS One 2012;7:e50775. 10.1371/journal.pone.0050775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anokye NK, Trueman P, Green C, et al. Physical activity and health related quality of life. BMC Public Health 2012;12:624. 10.1186/1471-2458-12-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halaweh H, Willen C, Grimby-Ekman A, et al. Physical Activity and Health-Related Quality of Life Among Community Dwelling Elderly. J Clin Med Res 2015;7:845-52. 10.14740/jocmr2307w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO. Information Sheet: Global Recommendations on Physical Activity for Health 65 Years and Above. 2020. Available online: https://www.who.int/dietphysicalactivity/physical-activity-recommendations-65years.pdf
- 70.WHO. Information Sheet: Global Recommendations on Physical Activity for Health 18–64 Years Old. 2020. Available online: https://www.who.int/dietphysicalactivity/physical-activity-recommendations-18-64years.pdf
- 71.Panagiotou M, Koulouris NG, Rovina N. Physical Activity: A Missing Link in Asthma Care. J Clin Med 2020;9:706. 10.3390/jcm9030706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddel HK, Barnes DJ, Exacerbation Advisory Panel . Pharmacological strategies for self-management of asthma exacerbations. Eur Respir J 2006;28:182-99. 10.1183/09031936.06.00105305 [DOI] [PubMed] [Google Scholar]
- 73.Helmerhorst HJ, Brage S, Warren J, et al. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act 2012;9:103. 10.1186/1479-5868-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as