Abstract
Background
Anemia is one of the risk factors for tuberculosis (TB), and more than 90% of TB patients suffer from anemia. The majority tuberculosis patients who had poor prognosis experienced anemia during the course of treatment. The objective of our study is to analyse the influences of anemia on the prognosis of tuberculosis patients in terms of pulmonary M. tuberculosis loads, lung pathology, and clinical factors.
Methods
In this retrospective cohort study, 155 TB patients in Shanghai were divided into the anemia-tuberculosis (A-TB) group and non-anemia-tuberculosis (NA-TB) group. We analysed bacteria counts in sputum smear and sputum smear conversion time between two groups. We evaluated the pulmonary pathology of cavity and effusion in A-TB patients. Logistic regression analysis was performed to explore the potential correlations of anemia with sputum bacterial load and pulmonary pathology. We compared clinical factors including the immune factors and inflammatory cells.
Results
Compared with the NA-TB (n=89) group, the A-TB group (n=66) had poorer improvement of lung injury in terms of cavity closure (4.7±3.59 vs. 10.56±7.42; P=0.036) and fluid improvement [4 (30.77%) vs. 12 (92.31%); P=0.001] during conventional treatment. At the start of treatment, the immune factors complement 4 (C4) [0.25 (0.19, 0.295) vs. 0.3086±0.076; P=0.006] and C-reactive protein (CRP) [3.2 (3.2, 21.5) vs. 19.5 (6.25, 78.35); P=0.016] were significantly higher in A-TB with NA-TB. During the course of treatment, the gradual decrease in the absolute number of lymphocytes (LYM#) (P=0.0012, r=−0.3400) and the gradual increase in the absolute number of monocytes (MONO#) (P=0.0050, r=0.2968), the absolute number of basophils (BASO#) (P=0.0213, r=0.2451), the red blood cell distribution width-coefficient (RDW-CV) (P=0.0136, r=0.2651), suggesting poor prognosis in anemic TB patients.
Conclusions
Anemia is a risk factor for lung injury in TB patients. Inflammatory factors and inflammatory cells are increased during treatment in A-TB patients.
Keywords: Tuberculosis, anemia, lung injury, inflammatory factors
Introduction
According to estimates of cause of death published by the World Health Organization (WHO) in 2019, tuberculosis was the top cause of death from a single infectious agent and the 13th leading cause of death worldwide (1). In 2020, there were 9.87 million new tuberculosis (TB) cases and an estimated 1.28 million deaths due to TB globally. Anemia is one of the risk factors for TB and is also a common complication of TB (2). The prevalence of anemia in TB patients ranges from 20% to 94% (3-6). Anemic patients were 3.56 times (95% CI: 2.53–5.01) more likely to have TB than non-anemic patients, and the risk of TB in anemic patients was 2.01 times (95% CI: 1.70–2.37) higher than in non-anemic patients. In addition, the risk of TB increases with the severity of anemia (7).
In most cases, tuberculosis-associated anemia is caused by anemia of inflammation (AI) (7-9) and can be partially resolved by anti-TB treatment. In some Asian studies, the prevalence of anemia at TB diagnosis decreased by 67% and 88%, respectively, after completion of TB treatment without additional iron-based intervention for anemia (10,11), suggesting a good recovery from anemia in cured TB patients. However, data for uncured TB patients have not been available. The incidence of malnutrition is high in TB patients. In Ethiopia, anemic TB patients had a 3.23 times higher risk of malnutrition than non-anemic TB patients (9). Iron deficiency is another major cause of TB anemia (12,13).
Anemia has been shown to be an independent predictor of disease progression and death in TB patients, and studies have shown that anemia is associated with poor prognosis and increased risk of death in TB patients (14-17). A Brazilian study (10) showed that among 191 TB patients living with human immunodeficiency virus (HIV), persistent anemia was the major determinant of unfavorable outcomes (14 of 18 participants, 77.8%). A retrospective study analyzed hematological markers in 118 TB patients during the first 60 days of treatment and found that the inflammation indicators in anemic TB patients were partially restored on the 60th day of treatment (9). However, only changes within 60 days were observed, and the authors did not analyze changes in hematological indicators during the complete anti-TB treatment course.
In the current study, biomarkers such as prealbumin (PA), transferrin (TRF) and uric acid have been found to be related to anemia (18,19). C-reactive protein (CRP) has been found to be a useful biomarker in screening TB-HIV (20). Platelet distribution width, low density lipoprotein cholesterol, HDL-C, and apolipoprotein A have been found to be risk factors for severe lung injury in tuberculosis patients with diabetes (21). However, there is a lack of biomarker studies on tuberculosis associated anemia.
Only a small number of studies have explored the poor treatment outcomes in anemia-tuberculosis (A-TB) patients. Here, we performed a detailed analysis of sputum bacterial load, imaging findings, and peripheral biomarkers through a 3-year retrospective study in cured and uncured A-TB patients to determine the impact of anemia on the prognosis of TB patients. Meanwhile, we aimed to identify new clinical factors that are related to severe lung injury in A-TB patients, which may be helpful for early prevention and intervention in A-TB patients with poor outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-679/rc).
Methods
Subjects
The study we used was a retrospective cohort study that selected 155 patients aged 18–70 years who tested positive for Mycobacterium tuberculosis (culture-positive, smear-positive, and Xpert MTB-RIF-positive) and were admitted to Shanghai Pulmonary Hospital from January 1, 2018 to December 31, 2018.Patient information including current medical history, discharge diagnosis, symptoms, signs, and comorbidities, as well as biochemical, hematological, and immunological parameters and imaging findings were collected from the Shanghai Pulmonary Hospital information processing database and electronic medical recording system. Patients with a variety of comorbidities (e.g., liver and kidney diseases and diabetes) were excluded, since these comorbidities may affect prognosis. Patients with incomplete information were also excluded.
Criteria for assessing TB response to treatment
According to the definitions and reporting framework for tuberculosis (2013 revision), “cured” was defined as a patient who had completed treatment according to the program protocol and who has been consistently bacteriologically negative (with at least 2 results), while patients who tested positive for Mycobacterium tuberculosis or whose clinical symptoms were not improved were considered treatment failures (22).
Computed tomography (CT) findings
CT data were collected from 155 TB patients over the period from their first admission in 2018 to their last visit to detect the degree of lung infection, cavitation, and fluid accumulation. CT images were read and interpreted by 2 experienced radiologists. The lungs were divided into 6 regions, with upper, middle, and lower zones on the left and right sides, to observe the infection zones in the lungs (11). The rate of cavity closure was calculated based on cavity reduction and closure status (12). The presence of pleural effusion, if any, and the effusion improvement were learned from previous CT reports.
Clinical factors measurements
The biochemical data were retrieved from the electronic case management system, including the results of pulmonary function testing, blood gas analysis, routine blood tests, automatic biochemical analysis, and protein electrophoreses. The records of routine blood tests were complete for all subjects, and changes in different clinical factors in the cured and uncured groups were collected.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software package or GraphPad Prism 8 software. The mean ± standard deviation () was used for normally distributed measurement data, and medians (M) and quartiles (Q) were used for non-normally distributed data. Count data are expressed by frequencies. The normally distributed continuous data were analyzed by the t-test and categorical data by the χ2 test. Fisher’s exact test was performed when the theoretical frequency was <5 or when the total sample size was <40. The non-normally distributed continuous variables were compared using a non-parametric test (i.e., Wilcoxon signed-ranks test). Logistic regression analysis was performed to explore the potential correlations of anemia with sputum bacterial load and pulmonary injury. P<0.05 shows that there is statistical significance.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (Shanghai, China) (K19-060Y). Verbal informed consent was given by all the subjects to use their clinical information for this study.
Results
Patient characteristics
A total of 155 patients entered the final analysis and were divided into the A-TB group (n=66, 42.58%) and non-anemia-tuberculosis (NA-TB) group (n=89, 57.42%) for anemia at the initiation of tuberculosis therapy. There were 33 males (50.00%) in the A-TB group and 52 males (58.43%) in the NA-TB group. The median age was not significantly different between the A-TB group (35 years) and NA-TB group (36 years) (P=0.348). Symptoms including cough, fever, and hemoptysis did not significantly differ between these 2 groups (Table 1).
Table 1. Demographic and clinical profiles of anemic and non-anemic tuberculosis patients.
| Characteristics | Non-anemia (n=89) | Anemia (n=66) | P value |
|---|---|---|---|
| Sexb | |||
| Male | 52 (58.43) | 33 (50.00) | 0.297 |
| Female | 37 (41.57) | 33 (50.00) | |
| Age (y)c | 36 (25.50–52.00) | 35 (29.75–54.25) | 0.348 |
| 18–30 | 35 (39.32) | 18 (27.27) | 0.227 |
| 31–50 | 30 (33.71) | 30 (45.46) | |
| 51–70 | 24 (26.97) | 18 (27.27) | |
| Hemoglobin (g/dL)c | 9.85 (8.5–11.3) | 14.75 (13.4–16.2) | <0.0001a |
| Coughb | 56 (62.92) | 46 (69.70) | 0.379 |
| Feverb | 16 (17.98) | 18 (27.27) | 0.167 |
| Hemoptysisb | 16 (17.98) | 8 (12.12) | 0.319 |
a, P <0.0001 is considered statistically significant; b, χ2 test, data are presented as percentage (%); c, Wilcoxon signed-rank test, data are presented as medians (M) with quartiles (Q).
Reduced cure rate in anemic TB patients
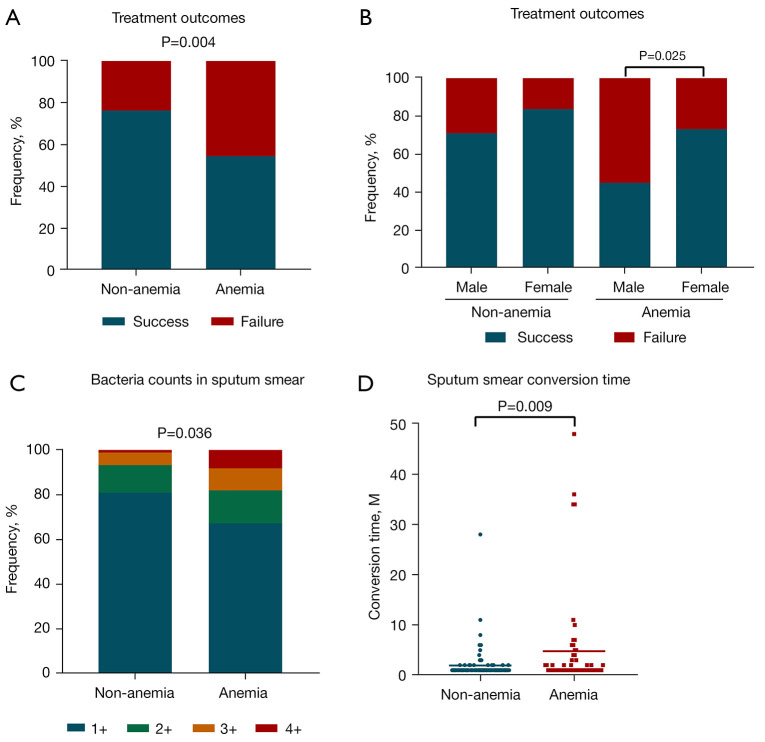
The retrospective analysis over a 3-year period revealed that 36 patients (54.55%) in the A-TB group and 68 patients (76.40%) in the NA-TB group were cured, showing a significant decrease in the cure rate for A-TB (by 21.85%; P=0.004) (Figure 1A). In the A-TB group, the cure rate was significantly higher in females than in males (P=0.025) (Figure 1B). The analysis of the sputum bacterial load showed that the A-TB group had a significantly higher bacterial load (Figure 1C), and the analysis of sputum smear turnaround time showed that the A-TB turnaround time was significantly longer (P=0.009) (Figure 1D).
Figure 1.
Effect of anemia on the cure rate and the results of sputum smear examination in tuberculosis patients (P<0.05 is considered statistically significant). (A) Comparison of the cure rate between the non-anemia-tuberculosis (NA-TB) and anemia-tuberculosis (A-TB) groups at the time of diagnosis. (B) Comparison of cure rates between male and female patients in the NA-TB and A-TB groups at the time of diagnosis. (C) Difference in sputum bacterial load between the NA-TB and A-TB groups. 1+, 1–9 acid-fast bacillus (AFB)/100 fields; 2+, 1–9 AFB/10 fields; 3+, 1–9 AFB/field; 4+, >9 AFB/field. (D) Comparison of time to sputum smear conversion between the NA-TB and A-TB groups.
Increased pathological damage in the lungs of patients in the A-TB group
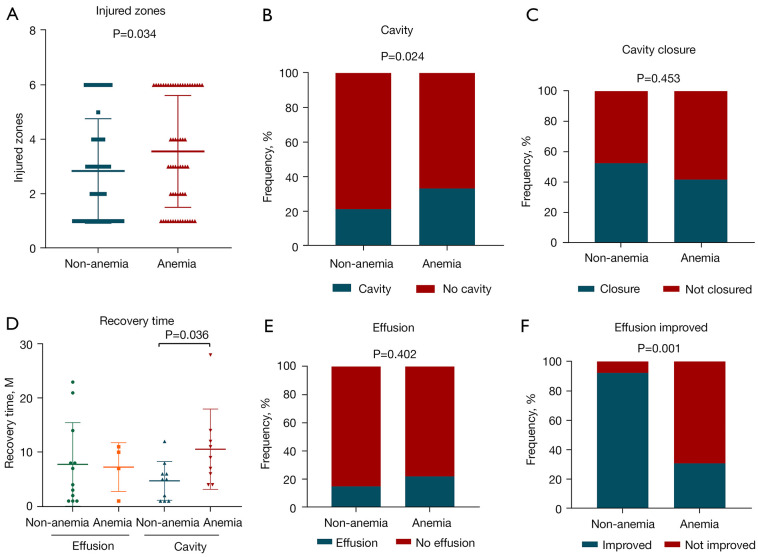
According to the CT reports of TB patients, there were significantly more infection zones in the lungs of patients in the A-TB group (M=3, Q: 1-6) than in the NA-TB group (M=2, Q: 1-4) (P=0.034) (Figure 2A). Analysis of the CT reports at the start of treatment revealed that 25 patients (37.88%) in the A-TB group had cavities in the lungs, which was significantly higher compared to that in the NA-TB group (P=0.024) (Figure 2B). During treatment, cavity reduction and closure were similar between the 2 groups (Figure 2C), but the time required for cavity closure was significantly longer in the A-TB group (P=0.036) (Figure 2D). Thirteen patients from either the A-TB group and NA-TB group developed pleural effusion (Figure 2E), and the rate of effusion improvement was significantly lower in the A-TB group (P=0.001) (30.77% vs. 92.31%) (Figure 2F).
Figure 2.
Correlation between anemia and lung injury in patients with tuberculosis. (A) Comparison of lung injured zones between the non-anemia-tuberculosis (NA-TB) group and anemia-tuberculosis (A-TB) group. (B,C) Comparisons of cavities and cavity closure between the NA-TB group and A-TB group. (D) Comparison of the time to effusion improvement and cavity closure between the NA-TB group and A-TB group. (E,F) Comparison of effusion and effusion improvement between the NA-TB group and A-TB group.
Anemia was associated with increased sputum bacterial load and severe pulmonary injury
Logistic regression analysis showed that anemia was a risk factor for poor prognosis in TB patients, mainly by causing increased sputum bacterial load and severe lung injury (Table 2). Compared with those in the NA-TB group, patients in the A-TB group had a reduced ability to clear the TB bacilli or control their proliferation, putting patients at a higher risk of having more TB bacilli in their lungs (OR, 2.217; 95% CI, 1.054–4.655; P=0.036). In terms of lung injury in TB patients, anemia was associated with increased risk factors including enlargement of the infection zones in lungs (OR, 1.919; 95% CI, 1.056–3.494; P=0.033) and formation of cavities (OR, 2.078; 95% CI, 1.003–4.305; P=0.049).
Table 2. Associations of anemia with sputum bacterial load and pulmonary pathology.
| Lung injury | OR (95% CI) | P value | OR1 (95% CI) | P value | OR2 (95% CI) | P value |
|---|---|---|---|---|---|---|
| Effusion | 1.583 (0.677–3.702) | 0.289 | 1.657 (0.703–3.907) | 0.248 | 1.585 (0.67–3.747) | 0.294 |
| Cavity | 2.078 (1.003–4.305) | 0.049 | 2.7 (1.211–6.021) | 0.015 | 2.145 (1.011–4.552) | 0.047 |
| Infection zone (1, 2, 3, 4, 5, and 6) | 1.919 (1.056–3.494) | 0.033 | 2.018 (1.105–3.688) | 0.022 | 2.065 (1.127–3.778) | 0.019 |
| Sputum bacterial load (1+, 2+, 3+, and 4+) | 2.217 (1.054–4.655) | 0.036 | 2.277 (1.08–4.802) | 0.031 | 2.273 (1.074–4.811) | 0.032 |
The OR value was obtained by logistic regression analysis. OR1, after gender adjustment; OR2, after age adjustment. P<0.05 is considered statistically significant.
Immune factors suggest poor prognosis in the A-TB group
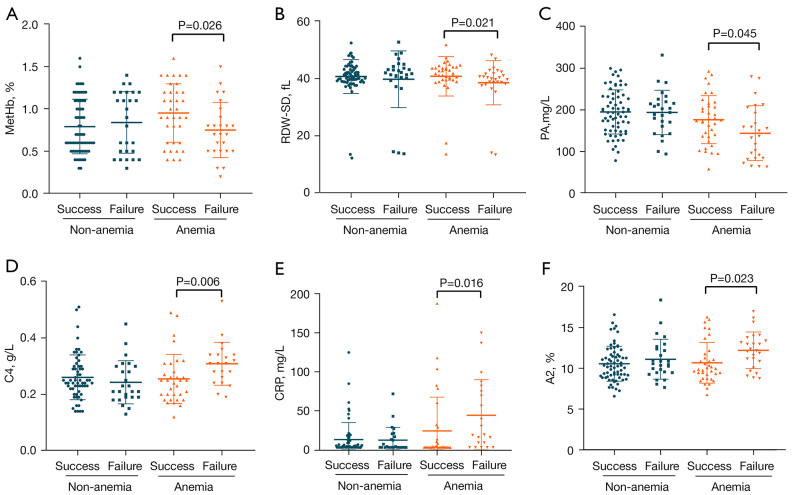
We analyzed some clinical factors in the A-TB and NA-TB groups (Table 3). In the A-TB group, methemoglobin (MetHb) (P=0.026) (Figure 3A), red blood cell distribution width-standard deviation (RDW-SD) (P=0.021) (Figure 3B), prealbumin (PA) (P=0.045) (Figure 3C), complement 4 (C4) (P=0.006) (Figure 3D), C-reactive protein (CRP) (P=0.016) (Figure 3E), and globulin α2 (A2) (P=0.023) (Figure 3F) were significantly different between cured patients and non-cured patients, while no such differences were found in the NA-TB group. It suggests that conventional anti-TB treatment maybe cause poor prognosis when the above clinical factors are abnormal in the A-TB group.
Table 3. Differences in immune factors in anemic and non-anemic tuberculosis patients.
| Parameter | Non-anemia | Anemia | |||||
|---|---|---|---|---|---|---|---|
| Success | Failure | P value | Success | Failure | P value | ||
| MetHb | 0.7 (0.5–1.1) | 0.9 (0.5–1.2) | 0.699 | 0.9528 | 0.6926 | 0.026 | |
| RDW-SD | 40.9 (39.1–43.3) | 42.1 (40.2–44.3) | 0.252 | 41.85 (39.63–44.03) | 40 (38.2–41.8) | 0.021 | |
| PA | 192.3706 | 194.0769 | 0.896 | 176.5556 | 143.88 | 0.045 | |
| C4 | 0.24 (0.22–0.30) | 0.21 (0.19–0.3) | 0.236 | 0.25 (0.19–0.29) | 0.31 (0.25–0.34) | 0.006 | |
| CRP | 4.4 (3.2–12.5) | 3.2 (3.2–18.75) | 0.825 | 3.2 (3.2–21.5) | 19.5 (6.25–78.35) | 0.016 | |
| A2 | 10.5926 | 7.25 (5.9–10.35) | 0.357 | 9.95 (8.92–11.67) | 11.8269 | 0.023 | |
| TRF | 2.0026 | 1.8196 | 0.039 | 2.0806 | 1.75 | 0.035 | |
| EO% | 1.9 (1–3.3) | 2.7 (1.7–3.8) | 0.031 | 1.55 (0.925–2.7) | 2 (0.9–2.6) | 0.607 | |
| AFU | 16 [14–20] | 19.346 | 0.049 | 14.889 | 15.5 [13–18] | 0.495 | |
| LDL-C | 2.4298 | 2.828 | 0.039 | 2.37 (1.93–2.65) | 2.579 | 0.394 | |
| IgM | 1.13 (0.84–1.56) | 0.9528 | 0.034 | 1.322 | 1.06 (0.825–1.2) | 0.054 | |
| LYM# | 1.69 (1.27–2.02) | 1.591 | 0.33 | 1.564 | 1.15 (0.98–1.79) | 0.219 | |
| MONO | 0.574 | 0.5 (0.41–0.69) | 0.444 | 0.525 (0.4–0.61) | 0.650 | 0.053 | |
| NEUT# | 3.88 (3.12–4.96) | 3.81 | 0.23 | 4.104 | 4.293 | 0.300 | |
P<0.05 is considered statistically significant. MetHb, methemoglobin; RDW-SD, red blood cell distribution width-standard deviation; PA, prealbumin; C4, complement 4; CRP, C-reactive protein; A2, globulin α2; TRF, transferrin; EO%, eosinophil %; AFU, a-L-fucosidase; LDL-C, low-density lipoprotein cholesterol; IgM, immunoglobulin M; LYM#, absolute lymphocyte count; MONO, absolute monocyte count; NEUT#, absolute neutrophil count.
Figure 3.
Differences in clinical factors in tuberculosis patients at the start of treatment (P value of <0.05 is considered statistically significant). (A) MetHb = methemoglobin; (B) RDW-SD = red blood cell distribution width-standard deviation; (C) PA = prealbumin; (D) C4 = complement 4; (E) CRP = C-reactive protein; (F) A2 = globulin α2.
The level of transferrin (TRF) significantly differed between cured and uncured patients in both the A-TB and NA-TB groups. In the NA-TB group, eosinophil % (EO%) (P=0.031), low-density lipoprotein (LDL) level (P=0.039), and immunoglobulin M (IgM) level (P=0.034) in uncured patients were significantly different from those in cured patients. However, no such differences were observed in the A-TB group (Table 3). Notably, most clinical factors including absolute LYM# and MONO showed no significant differences between cured and uncured patients at the start of treatment.
Inflammatory cells suggested a poor prognosis during treatment
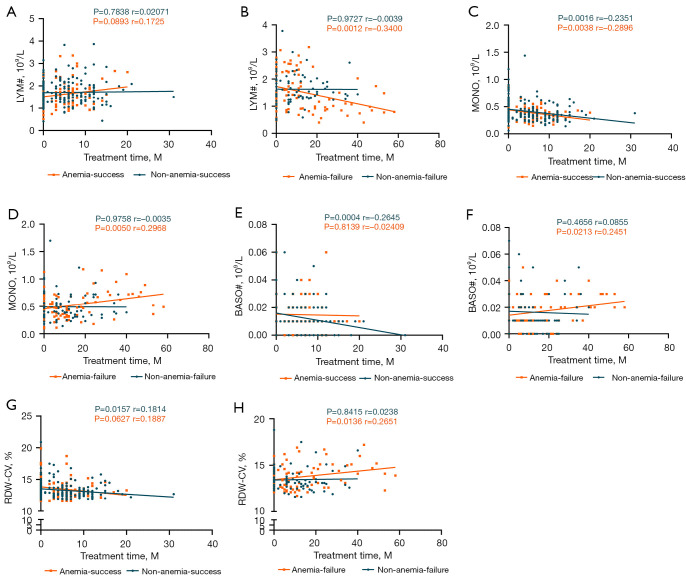
Serial analyses of the changes in peripheral blood clinical factors in cured and non-cured patients in both the A-TB group and NA-TB group using multiple sample sizes over a long period of time showed that LYM# (P=0.0012, r=−0.3400) (Figure 4A,4B), MONO (P=0.0050, r=0.2968) (Figure 4C,4D), absolute basophil count (BASO#) (P=0.0213, r=0.2451) (Figure 4E,4F), and red blood cell distribution width-coefficient (RDW-CV) (P=0.0136, r=0.2651) (Figure 4G,4H) were important clinical factors of poor outcomes during treatment in the A-TB group. The above indicators were consistently decreased or increased only in non-cured patients in the A-TB group. In the NA-TB group, however, the changes were opposite between cured and non-cured patients, suggesting a similar trend in only uncured anemic patients.
Figure 4.
Changes in clinical factors in tuberculosis patients during treatment (P value of <0.05 is considered statistically significant; r>0 means a positive correlation and r<0 indicates a negative correlation). (A,B) LYM# = absolute lymphocyte count; (C,D) MONO = absolute monocyte count; (E,F) BASO# = absolute basophil count; (G,H) RDW-SD = red blood cell distribution width-standard deviation.
Discussion
Anemia is a common complication of TB. In TB patients, the prevalence of anemia ranges from 20% to 94% (2-6). In our current study, the prevalence of anemia in 155 TB patients was 42.58%. Anemia is associated with the prognosis of TB patients (8,23,24), but this link has not been well elucidated. As a recently proven immune factor (25) and one of the manifestations of malnutrition (26), anemia typically co-exists with other immune-impairing diseases (e.g., diabetes and hepatitis) in TB patients, and therefore it is difficult to specify its clinical significance as a single factor in TB. In our current study, a detailed background classification of a large number of TB patients from a single region (Shanghai) was performed to screen patients with TB alone and TB patients with abnormal anemia indicators alone. This 3-year retrospective study revealed that anemia is a key risk factor for the poor prognosis of TB. Anemia resulted in lower TB cure rates, higher sputum bacterial load, and larger infection zones in the lungs, and as a result, more cavities were formed and cavity healing was slower.
The A-TB group had a higher sputum bacterial load. Sputum TB bacillary load and time to conversion are suggestive of good or bad prognosis (27,28). While immunodeficiency diseases such as diabetes mellitus and nephritis (21,29) have been reported to result in higher sputum bacillary load, few studies have described the similar role of anemia. In our current study, the bacillary load in the sputum smear was significantly higher in the A-TB group than in the NA-TB group. In addition, anemia was associated with the delay in sputum smear conversion (30). Time to sputum conversion in anemic TB patients was significantly longer than in non-anemic TB patients, suggesting a higher likelihood of poor prognosis in the anemic group (31).
Lung injury is more severe in TB patients. It can be used to predict the severity of TB (32-34). It was found that increased lung injury in HIV-TB patients was associated with higher CD4 counts (35) and that DM-TB patients had more severe lung damage and more cavities (21). Studies that include anemia as a single factor are lacking. In our current study, we found that anemia as a single influencing factor equally led to increased lung injury and delay in cavity closure and effusion improvement. Furthermore, logistic regression analysis showed that anemia was a risk factor for severe lung injury in terms of sputum bacterial load, infection zones, cavities, and effusion, again confirming the above findings.
Anemia has been found to cause significant changes in CRP, TRF, and uric acid levels (18,19), which were shown to correlate with anemia prevalence; however, no molecular markers associated with poor A-TB prognosis were noted in these studies. The present study focused on biomarkers at the start of treatment for non-cured A-TB patients and found significant differences in CRP, C4, PA, and A2 levels, which were associated with poor prognosis. PA is regarded as an inflammatory marker that is suggestive of anemia and malnutrition (36,37). CRP has been found to be a useful biomarker in screening TB-HIV (20). CRP, C4, PA, and A2 are molecular markers of poor prognosis in A-TB patients, as shown in our current study.
In an attempt to comprehensively the changes in clinical factors in A-TB patients during treatment, we analyzed all patients with untreated A-TB over a long period of time, with an interval of 3 months. Data by patient report or hospital records were statistically analyzed. The results showed that LYM# decreased with treatment time, BASO#, RDW-SD, and MONO# increased during treatment, which changes in these factors can be used to assess the prognosis in A-TB patients.
Generally, our data include immune factors, inflammatory cells and lung injury revealed poor prognosis in A-TB, this is useful a new treatment regimens that refer to these clinical factors may increase the cure rate and shorten the duration of treatment in A-TB patients.
Nevertheless, our research had some limitations. First, the relatively small sample size might have caused potential biases. For example, when we analyzed the resolution time of pleural effusion, the small number of patients showing improved effusion in the A-TB group resulted in a small sample size, and thus might have caused bias. Second, during the analysis of the treatment process, we only had complete data on routine blood tests in our database, and no complete information related to other clinical factors was available. As a result, dynamic analyses of the remaining clinical factors during treatment were not possible.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The work was supported by the Natural Science Foundation of China (grant No. 81970009).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (Shanghai, China) (K19-060Y). Verbal informed consent was given by all the subjects to use their clinical information for this study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-679/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-679/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-679/coif). The authors have no conflicts of interest to declare.
References
- 1.WHO. Global tuberculosis report 2021. Available online: https://www.who.int/publications/i/item/global-tuberculosis-report-2021
- 2.Mukherjee A, Kaeley N, Dhar M, et al. Prevalence, characteristics, and predictors of tuberculosis associated anemia. J Family Med Prim Care 2019;8:2445-9. 10.4103/jfmpc.jfmpc_311_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzegari S, Afshari M, Movahednia M, et al. Prevalence of anemia among patients with tuberculosis: A systematic review and meta-analysis. Indian J Tuberc 2019;66:299-307. 10.1016/j.ijtb.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Nagu TJ, Spiegelman D, Hertzmark E, et al. Anemia at the initiation of tuberculosis therapy is associated with delayed sputum conversion among pulmonary tuberculosis patients in Dar-es-Salaam, Tanzania. PLoS One 2014;9:e91229. 10.1371/journal.pone.0091229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hella J, Cercamondi CI, Mhimbira F, et al. Anemia in tuberculosis cases and household controls from Tanzania: Contribution of disease, coinfections, and the role of hepcidin. PLoS One 2018;13:e0195985. 10.1371/journal.pone.0195985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira MG, Delogo KN, Oliveira HM, et al. Anemia in hospitalized patients with pulmonary tuberculosis. J Bras Pneumol 2014;40:403-10. 10.1590/S1806-37132014000400008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelaw Y, Getaneh Z, Melku M. Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ Health Prev Med 2021;26:13. 10.1186/s12199-020-00931-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kourbatova EV, Borodulin BE, Borodulina EA, et al. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis 2006;10:1224-30. [PubMed] [Google Scholar]
- 9.Dallman PR. Iron deficiency and the immune response. Am J Clin Nutr 1987;46:329-34. 10.1093/ajcn/46.2.329 [DOI] [PubMed] [Google Scholar]
- 10.Demitto FO, Araújo-Pereira M, Schmaltz CA, et al. Impact of Persistent Anemia on Systemic Inflammation and Tuberculosis Outcomes in Persons Living With HIV. Front Immunol 2020;11:588405. 10.3389/fimmu.2020.588405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isanaka S, Mugusi F, Urassa W, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr 2012;142:350-7. 10.3945/jn.111.144287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Mendonça EB, Schmaltz CA, Sant'Anna FM, et al. Correction: Anemia in tuberculosis cases: A biomarker of severity? PLoS One 2021;16:e0249545. 10.1371/journal.pone.0249545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahiratmadja E, Wieringa FT, van Crevel R, et al. Iron deficiency and NRAMP1 polymorphisms (INT4, D543N and 3'UTR) do not contribute to severity of anaemia in tuberculosis in the Indonesian population. Br J Nutr 2007;98:684-90. 10.1017/S0007114507742691 [DOI] [PubMed] [Google Scholar]
- 14.Zhang SY, Fu JY, Guo XY, et al. Improvement cues of lesion absorption using the adjuvant therapy of traditional Chinese medicine Qinbudan tablet for retreatment pulmonary tuberculosis with standard anti-tuberculosis regimen. Infect Dis Poverty 2020;9:50. 10.1186/s40249-020-00660-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1998;19:29-33. 10.1097/00042560-199809010-00004 [DOI] [PubMed] [Google Scholar]
- 16.Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS Rev 2002;4:13-20. [PubMed] [Google Scholar]
- 17.O'Brien ME, Kupka R, Msamanga GI, et al. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr 2005;40:219-25. 10.1097/01.qai.0000166374.16222.a2 [DOI] [PubMed] [Google Scholar]
- 18.de Mendonça EB, Schmaltz CA, Sant'Anna FM, et al. Anemia in tuberculosis cases: A biomarker of severity? PLoS One 2021;16:e0245458. 10.1371/journal.pone.0245458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil-Santana L, Cruz LAB, Arriaga MB, et al. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of antitubercular therapy. Sci Rep 2019;9:1381. 10.1038/s41598-018-37860-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon C, Semitala FC, Atuhumuza E, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis 2017;17:1285-92. 10.1016/S1473-3099(17)30488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Z, Shi J, Dorhoi A, et al. Hemostasis and Lipoprotein Indices Signify Exacerbated Lung Injury in TB With Diabetes Comorbidity. Chest 2018;153:1187-200. 10.1016/j.chest.2017.11.029 [DOI] [PubMed] [Google Scholar]
- 22.WHO. Definitions and reporting framework for tuberculosis. Available online: https://www.who.int/publications-detail-redirect/9789241505345
- 23.van Lettow M, West CE, van der Meer JW, et al. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr 2005;59:526-32. 10.1038/sj.ejcn.1602116 [DOI] [PubMed] [Google Scholar]
- 24.Ramos LM, Sulmonett N, Ferreira CS, et al. Functional profile of patients with tuberculosis sequelae in a university hospital. J Bras Pneumol 2006;32:43-7. 10.1590/S1806-37132006000100010 [DOI] [PubMed] [Google Scholar]
- 25.Lam LKM, Murphy S, Kokkinaki D, et al. DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci Transl Med 2021;13:eabj1008. 10.1126/scitranslmed.abj1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med 2019;19:182. 10.1186/s12890-019-0953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabiiti W, Azam K, Farmer ECW, et al. Tuberculosis bacillary load, an early marker of disease severity: the utility of tuberculosis Molecular Bacterial Load Assay. Thorax 2020;75:606-8. 10.1136/thoraxjnl-2019-214238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Güler M, Unsal E, Dursun B, et al. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract 2007;61:231-5. 10.1111/j.1742-1241.2006.01131.x [DOI] [PubMed] [Google Scholar]
- 29.Igari H, Imasawa T, Noguchi N, et al. Advanced stage of chronic kidney disease is risk of poor treatment outcome for smear-positive pulmonary tuberculosis. J Infect Chemother 2015;21:559-63. 10.1016/j.jiac.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 30.Morris CD, Bird AR, Nell H. The haematological and biochemical changes in severe pulmonary tuberculosis. Q J Med 1989;73:1151-9. [PubMed] [Google Scholar]
- 31.Tiwari S, Kumar A, Kapoor SK. Relationship between sputum smear grading and smear conversion rate and treatment outcome in the patients of pulmonary tuberculosis undergoing dots--a prospective cohort study. Indian J Tuberc 2012;59:135-40. [PubMed] [Google Scholar]
- 32.Maduskar P, Hogeweg L, de Jong PA, et al. Cavity contour segmentation in chest radiographs using supervised learning and dynamic programming. Med Phys 2014;41:071912. 10.1118/1.4881096 [DOI] [PubMed] [Google Scholar]
- 33.Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, et al. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis 2020;20:e117-28. 10.1016/S1473-3099(20)30148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu Y, Soodeen-Lalloo AK, Huang J, et al. Sex Disparity in Severity of Lung Lesions in Newly Identified Tuberculosis Is Age-Associated. Front Med (Lausanne) 2019;6:163. 10.3389/fmed.2019.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011;24:351-76. 10.1128/CMR.00042-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham LL, Jr, Madsen MJ, Van Sickels JE. Using prealbumin as an inflammatory marker for patients with deep space infections of odontogenic origin. J Oral Maxillofac Surg 2006;64:375-8. 10.1016/j.joms.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Xue Y, Yuan X, et al. Combination of prealbumin and tuberculosis-specific antigen/phytohemagglutinin ratio for discriminating active tuberculosis from latent tuberculosis infection. Int J Clin Pract 2021;75:e13831. 10.1111/ijcp.13831 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as