Abstract
The anti-Toxoplasma activities of nine antiretroviral drugs were examined in vitro. Nucleoside analogs had no effect on parasite growth, whereas ritonavir and nelfinavir were inhibitory for Toxoplasma, with 50% inhibitory concentrations of 5.4 and 4.0 μg/ml, respectively. None of the antiviral drugs affected the anti-Toxoplasma activity of pyrimethamine or sulfadiazine.
In human immunodeficiency virus (HIV)-infected patients, the recommended treatment or prophylaxis for toxoplasmic encephalitis is a combination of a sulfonamide and a dihydrofolate reductase inhibitor (8, 13). An additional therapeutic benefit can also be expected from antiretroviral therapy through the restoration of host antimicrobial immunity. However, one study showed that azidovudine (AZT) negatively interfered with the antiparasitic effect of pyrimethamine (7); in contrast, didanosine (ddI) was found to have in vitro and in vivo activity against Toxoplasma gondii (12). Thus, our objective was to extend these studies by testing the anti-Toxoplasma activities of five nucleoside analogs and four protease inhibitors and their possible in vitro interaction with pyrimethamine and sulfadiazine, as first-line anti-Toxoplasma drugs.
We used tachyzoites of the virulent RH strain of T. gondii. Tissue cultures and drug tests were carried out using MRC5 fibroblast tissue cultures as previously described, with quantification of Toxoplasma growth by enzyme-linked immunosorbent assay (ELISA) (4). The following drugs were tested: AZT, lamivudine, ddI, stavudine, zalcitabine, ritonavir, indinavir, nelfinavir, and saquinavir. Stock solutions (10 mg/ml) were prepared in distilled water, except for ritonavir, which was dissolved in methanol, and saquinavir, which was dissolved in 50% methanol–50% acetone. Pyrimethamine and sulfadiazine (Sigma) were dissolved at 1 mg/ml in 50% methanol–50% acetone. Serial dilutions were then prepared in culture medium. Each antiviral drug was tested at serial concentrations ranging from 5 × 10−5 to 100 μg/ml (final concentration). Each concentration was tested in eight replicate wells and in two replicate culture plates. For assessing the interaction between drugs, combinations of three concentrations of each antiparasitic drug and four concentrations of each antiviral drug were tested in a two-dimensional test. From the results of previous experiments (4) and from individual tests of antiviral drugs, we selected concentrations that resulted in a range of inhibition from noninhibitory to completely inhibitory. For antiviral drugs that had no anti-Toxoplasma activity, we selected the highest concentration that was found to be nontoxic for the monolayer and three fivefold dilutions of this concentration. Each experiment used two or three replicate plates in which each drug or drug combination was tested in four replicate wells. All experiments were repeated twice. The effect of each drug at various concentrations was described by plotting the optical density (OD) values generated by the ELISA as a function of the logarithm of the concentration. A linear regression model was used to summarize the concentration-effect relationship and to determine the 50% inhibitory concentration (IC50). The effect of the drugs in combination was tested by using a two-way analysis of variance which included the estimation of an interaction factor (4).
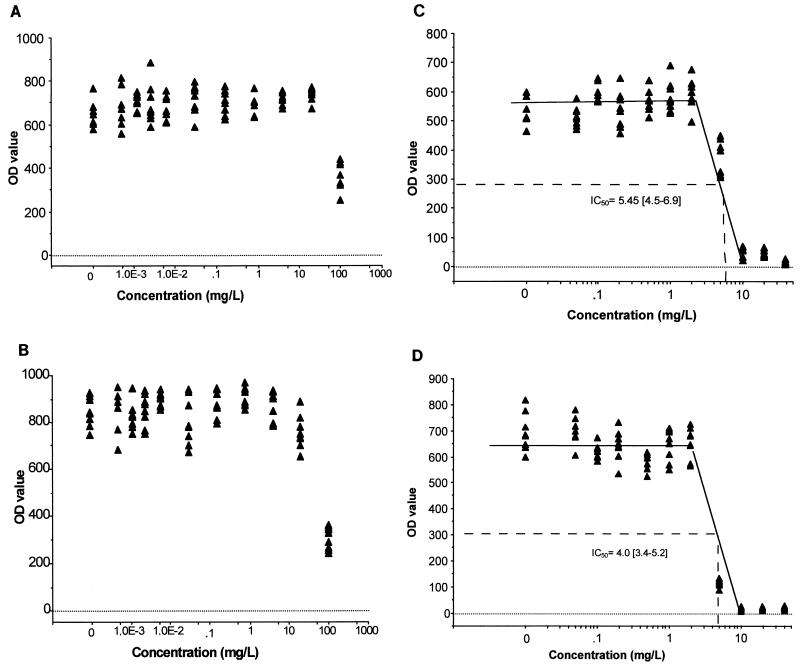
ddI, zalcitabine, stavudine, and lamivudine had no inhibitory effect on Toxoplasma growth, and no toxicity to the monolayers was observed by microscopic examination. With AZT and indinavir, an inhibitory effect was noted at 100 μg/ml without significant toxicity to the host cells (Fig. 1A and B). Saquinavir was inhibitory at concentrations of ≥20 μg/ml, which were also found to be toxic for the monolayers. Ritonavir and nelfinavir were inhibitory for Toxoplasma at concentrations of <10 μg/ml (Fig. 1C and D). Both ritonavir and nelfinavir were noninhibitory at concentrations of ≤2 μg/ml, and a marked increase of the inhibitory effect was observed with between 2 and 10 μg/ml, the concentration at which Toxoplasma growth was completely inhibited. A toxic effect on the monolayers was noted with nelfinavir at 20 μg/ml and with ritonavir at 40 μg/ml. The IC50s were estimated to be 5.4 μg/ml (95% confidence interval, 4.5 to 6.9 μg/ml) for ritonavir and 4.0 μg/ml (95% confidence interval, 3.4 to 5.2 μg/ml) for nelfinavir.
FIG. 1.
In vitro effect of antiviral drugs on T. gondii growth in MRC5 tissue culture. Each graph shows the OD value (×1,000) from ELISA with infected monolayers (y axis) versus drug concentration (x axis) for AZT (A), indinavir (B), ritonavir (C), and nelfinavir (D).
With the two-way analysis of variance that was used for assessing the drug combinations, the effect of each drug and the interaction effects of drugs in combination were estimated, taking into account the individual effect of each drug as well as the intra- and interplate variability. The synergistic effect of the combination of pyrimethamine with sulfadiazine was confirmed (Table 1), and combinations of one of the antiretroviral drugs with pyrimethamine or sulfadiazine were analyzed. For all combinations tested, there was no significant interaction effect. Drugs that individually had no inhibitory effect against T. gondii did not alter or enhance the anti-Toxoplasma activity of sulfadiazine or pyrimethamine. When antiretroviral drugs that were found to have an inhibitory effect on T. gondii were combined with pyrimethamine or sulfadiazine, an additional but not synergistic effect resulted (Tables 2 and 3). We conclude that these antiviral drugs had no interaction effect in vitro on the anti-Toxoplasma activity of pyrimethamine or sulfadiazine. In particular, we found no antagonistic effect between AZT and pyrimethamine, as had been previously reported by Israelski et al. (7), and this was confirmed with four other nucleoside analogs.
TABLE 1.
Inhibitory effect of pyrimethamine combined with sulfadiazine
| Concn of sulfadiazine (μg/ml) | OD value (103) at the indicated pyrimethamine concn (μg/ml)a
|
|||
|---|---|---|---|---|
| 0 | 0.01 | 0.05 | 0.5 | |
| 0 | 838 ± 60 | 855 ± 65 | 679 ± 70 | 73 ± 23 |
| 0.02 | 756 ± 74 | 819 ± 32 | 233 ± 51* | 42 ± 27 |
| 0.2 | 393 ± 54 | 140 ± 39* | 26 ± 14* | 15 ± 14* |
| 2 | 37 ± 9 | 18 ± 11 | 14 ± 13 | 14 ± 6 |
Mean ± standard deviation; each value represents the results from 8 to 12 replicate wells from 2 or 3 replicate plates. P values were calculated for the interaction effect. *, P < 10−4.
TABLE 2.
Inhibitory effect of AZT and indinavir, combined with sulfadiazine or pyrimethamine
| Drug and concn (μg/ml) | OD value (103) at indicated drug concn (μg/ml)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AZT
|
Indinavir
|
|||||||||
| 0 | 1 | 5 | 25 | 100 | 0 | 1 | 5 | 25 | 100 | |
| Sulfadiazine | ||||||||||
| 0 | 630 ± 106 | 642 ± 131 | 626 ± 97 | 648 ± 78 | 376 ± 103 | 734 ± 86 | 762 ± 97 | 692 ± 71 | 654 ± 107 | 287 ± 85 |
| 0.02 | 512 ± 91 | 523 ± 103 | 554 ± 97 | 537 ± 81 | 309 ± 77 | 605 ± 111 | 666 ± 55 | 637 ± 122 | 624 ± 114 | 254 ± 91 |
| 0.2 | 209 ± 51 | 172 ± 40 | 201 ± 45 | 169 ± 45 | 135 ± 27§ | 188 ± 92 | 284 ± 122 | 400 ± 116 | 249 ± 107 | 62 ± 31§ |
| 2 | 31 ± 26 | 27 ± 15 | 30 ± 20 | 27 ± 20 | 27 ± 19 | 28 ± 18 | 42 ± 30 | 36 ± 16 | 26 ± 20 | 31 ± 21 |
| Pyrimethamine | ||||||||||
| 0 | 932 ± 104 | 1,013 ± 75 | 989 ± 109 | 898 ± 145 | 743 ± 83 | 697 ± 143 | 813 ± 120 | 837 ± 84 | 837 ± 90 | 330 ± 32 |
| 0.01 | 959 ± 104 | 1,062 ± 105 | 927 ± 106 | 908 ± 112 | 703 ± 82 | 643 ± 136 | 755 ± 110 | 800 ± 164 | 791 ± 122 | 339 ± 56 |
| 0.05 | 706 ± 84 | 702 ± 52 | 666 ± 97 | 679 ± 57 | 587 ± 65§ | 552 ± 38 | 603 ± 86 | 745 ± 112 | 795 ± 103 | 250 ± 51§ |
| 0.25 | 100 ± 36 | 107 ± 65 | 92 ± 50 | 92 ± 19 | 81 ± 22 | 80 ± 40 | 79 ± 16 | 76 ± 11 | 78 ± 29 | 84 ± 29 |
Each value represents the results from 8 to 12 replicate wells from 2 or 3 replicate plates. P values were calculated for the interaction effect. §, additive but not synergistic effect (P > 0.05).
TABLE 3.
Inhibitory effect of ritonavir and nelfinavir, combined with sulfadiazine or pyrimethamine
| Drug and concn (μg/ml) | OD (103) at indicated drug concn (μg/ml)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ritonavir
|
Nelfinavir
|
|||||||||
| 0 | 1 | 2 | 5 | 25 | 0 | 0.5 | 2 | 5 | 10 | |
| Sulfadiazine | ||||||||||
| 0 | 633 ± 43 | 611 ± 50 | 596 ± 49 | 472 ± 38 | 33 ± 16 | 762 ± 128 | 810 ± 87 | 767 ± 73 | 423 ± 133 | 80 ± 113 |
| 0.02 | 632 ± 34 | 631 ± 88 | 632 ± 63 | 463 ± 43 | 29 ± 12 | 695 ± 62 | 754 ± 109 | 690 ± 64 | 454 ± 106 | 15 ± 11 |
| 0.2 | 421 ± 77 | 455 ± 99 | 555 ± 39 | 383 ± 63 | 14 ± 13§ | 264 ± 69 | 370 ± 84 | 526 ± 109 | 556 ± 95 | 14 ± 12§ |
| 2 | 14 ± 11 | 52 ± 25 | 31 ± 17 | 64 ± 75 | 6 ± 6§ | 14 ± 12 | 31 ± 23 | 38 ± 29 | 57 ± 30 | 24 ± 16 |
| Pyrimethamine | ||||||||||
| 0 | 705 ± 58 | 766 ± 107 | 756 ± 105 | 628 ± 47 | 32 ± 29 | 821 ± 77 | 855 ± 65 | 842 ± 67 | 578 ± 190 | 15 ± 17 |
| 0.01 | 672 ± 128 | 830 ± 115 | 854 ± 128 | 614 ± 43 | 24 ± 17 | 764 ± 69 | 819 ± 32 | 808 ± 65 | 625 ± 106 | 9 ± 7 |
| 0.05 | 595 ± 69 | 543 ± 120 | 607 ± 55 | 500 ± 45 | 15 ± 17§ | 603 ± 48 | 565 ± 80 | 560 ± 50 | 583 ± 97 | 10 ± 8 |
| 0.25 | 120 ± 35 | 83 ± 50 | 105 ± 32 | 75 ± 44 | 17 ± 16§ | 11 ± 9 | 25 ± 14 | 16 ± 15 | 29 ± 23 | 11 ± 8 |
Each value represents the results from 8 to 12 replicate wells from 2 or 3 replicate plates. P values were calculated for the interaction effect. §, additive but not synergistic effect (P > 0.05).
Our study revealed that several antiretroviral drugs were inhibitory for T. gondii. In contrast to Sarciron et al. (12), we found no inhibitory effect with ddI at concentrations up to 100 μg/ml. There is no clear explanation for this discrepancy, except that we used a more virulent strain of T. gondii and that cultures were prepared with fibroblasts instead of phagocytic THP1 cells. We found that ritonavir and nelfinavir were highly inhibitory for Toxoplasma growth, with IC50s of 5.4 and 4.0 μg/ml, respectively (i.e., concentrations that can be achieved in humans [5, 6, 9, 10]). The mode of action of HIV protease inhibitors on T. gondii remains to be elucidated. Indeed, several proteases already have been found in protozoa (3, 11), but evidence of the target enzyme of HIV protease inhibitors, i.e., aspartyl protease, has not yet been found in T. gondii. Our data suggest that this enzyme is present in T. gondii and that it plays a role in parasitic replication. Recent data showing that aspartyl protease is also present in fungi and can be inhibited by anti-HIV protease inhibitors (1, 2) also indicate that this enzyme could be a target for various microorganisms. In these other two studies, ritonavir was a potent inhibitor of Candida albicans and Pneumocystis carinii, as we found in this study for T. gondii. In addition to this direct pharmacological effect on parasitic growth, ritonavir and nelfinavir also may have an indirect effect on the host cell through enhancement of the respiratory burst of neutrophils (D. Ghanimi, A. Perianin, J. Morini, M. Levacher, I. Florentin, J. Giroud, and B. Rouveix, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1831, 1999). This mechanism could be of particular importance for an intracellular parasite such as T. gondii.
REFERENCES
- 1.Atzori C, Angeli E, Mainini A, Agostoni F, Micheli V, Cargnel A. In vitro activity of human immunodeficiency virus protease inhibitors against Pneumocystis carinii. J Infect Dis. 2000;181:1629–1634. doi: 10.1086/315437. [DOI] [PubMed] [Google Scholar]
- 2.Cassone A, De Bernadis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:448–453. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 3.Conseil V, Soête M, Dubremetz J F. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:1358–1361. doi: 10.1128/aac.43.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derouin F, Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob Agents Chemother. 1989;33:1753–1759. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flexner C. HIV protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 6.Gatti G, Di Biagio A, Casazza R, De Pascalis C, Bassetti M, Cruciani M, Vella S, Bassetti D. The relationship between ritonavir plasma levels and side-effects: implication for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- 7.Israelski D M, Tom C, Remington J S. Zidovudine antagonizes the action of pyrimethamine in experimental infection with Toxoplasma gondii. Antimicrob Agents Chemother. 1989;33:30–34. doi: 10.1128/aac.33.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luft B J, Remington J S. Toxoplasmic encephalitis. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 9.Merry C, Barry M G, Mulcahy F, Ryan M, Heavey J, Tjia J F, Gibbons S E, Breckenridge A M, Back D J. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11:F29–F33. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Pai V B, Nahata M C. Nelfinavir mesylate: a protease inhibitor. Ann Pharmacother. 1999;33:325–339. doi: 10.1345/aph.18089. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal P J. Proteases of protozoan parasites. Adv Parasitol. 1999;43:105–159. doi: 10.1016/s0065-308x(08)60242-0. [DOI] [PubMed] [Google Scholar]
- 12.Sarciron M-E, Lawton P, Saccharin C, Petavy A-F, Peyron F. Effects of 2′,3′-dideoxyinosine on Toxoplasma gondii cysts in mice. Antimicrob Agents Chemother. 1997;41:1531–1536. doi: 10.1128/aac.41.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.USPHS/IDSA Prevention of Opportunistic Infections Working Group; Infectious Diseases Society of America. 1999. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Ann Intern Med. 1999;131:873–908. doi: 10.7326/0003-4819-131-11-199912070-00022. [DOI] [PubMed] [Google Scholar]