Abstract
Background
Gemcitabine is among the most commonly utilized chemotherapeutic agents for treating pancreatic cancer (PC), yet patients ultimately develop chemoresistance and thus exhibit a poor prognosis. Long noncoding RNAs (lncRNAs) can function as key regulators of PC progression and may serve as prognostic biomarkers in individuals with gemcitabine-resistant PC. This study sought to explore the role of the lncRNA DBH-AS1 in this oncogenic setting.
Methods
Based on public databases and qRT-PCR analyses the expression of lncRNA DBH-AS1 in PC tissues and cell lines. The effects of lncRNA DBH-AS1 on proliferation and gemcitabine resistance were determined by in vitro and in vivo experiments. Luciferase reporter assay and RNA immunoprecipitation (RIP) were carried out to reveal the interaction between lncRNA DBH-AS1, miR-3163 and USP44.
Results
We found that PC tissues exhibited DBH-AS1 downregulation that was particularly pronounced in gemcitabine-resistant PC tissues and cells. This DBH-AS1 downregulation was negatively correlated with the malignancy of PC tumors and with patient survival outcomes. Additionally, decreased DBH-AS1 expression in PC was found to be linked to the METTL3-dependent m6A methylation of the lncRNA, with functional analyses revealing that DBH-AS1 was able to suppress the growth of PC cells. Mechanistically, DBH-AS1 was able to increase PC cell sensitivity to gemcitabine by sequestering miR-3163 and thus upregulating USP44 in these tumor cells. Clinically, patient-derived PC tumor xenografts exhibiting high levels of DBH-AS1 expression were found to be responsive to gemcitabine treatment.
Conclusions
Overall, these data underscore a key role for DBH-AS1 as a regulator of PC tumor growth and a promising therapeutic target capable of predicting PC patient responsiveness to gemcitabine treatment.
Keywords: m6A, lncRNADBH-AS1, gemcitabine, ceRNA, patient-derived xenograft (PDX)
Introduction
Pancreatic cancer (PC) is an increasingly common malignancy of the digestive system, the incidence of which continues to rise globally. PC is associated with high mortality rates, with an estimated 60% of patients exhibiting distant metastasis at initial diagnosis and only 10–15% being eligible for surgical tumor resection (1-3). Gemcitabine is the first-line chemotherapeutic agent used to treat most patients with advanced PC at present (4). However, patients almost universally acquire gemcitabine chemoresistance, and its clinical efficacy is thus profoundly limited. There is an urgent need to identify novel therapeutic approaches cable of overcoming such chemoresistance and improving PC patient prognosis.
m6A methylation was shown to play a crucial role in drug resistance development and intervention in cancer cells (5,6). Although previous study reported that METTL3 depletion enhanced chemo, radio, and chemoradio-sensitivity in pancreatic cancer cell (7), the role of m6A methylation in gemcitabine resistance remains largely unknown.
Long noncoding RNAs (lncRNAs), which are >200 nucleotide RNAs lacking significant coding potential (8), can exert diverse regulatory functions to enhance or suppress tumor growth through the modulation of processes including metabolism, migration, invasion, cell cycle progression, autophagy, proliferation, and apoptosis (9-13). The dysregulation of specific lncRNA expression patterns has been tied to tumor chemoresistance in a range of cancers, including breast cancer, colorectal cancer, and hepatocellular carcinoma (HCC), with some lncRNAs having been identified as promising prognostic biomarkers in gemcitabine-resistant PC (14-18). LncRNA DBH-AS1 plays an important role in the advancement of various malignant tumors including HCC (19), osteosarcoma (20), and diffuse large B-cell lymphoma (21). However, the role of this lncRNA in PC remained to be elucidated. This study explored the expression of DBH-AS1 in PC and examined the mechanistic relationship between its expression and the resistance of PC cells to gemcitabine treatment. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-556/rc).
Methods
Patients and tissue samples
In total, tumour and paracancerous tissue samples were collected from 156 individuals with PC were collected from individuals with PC undergoing surgical tumor resection in Changhai Hospital from 2006 to 2010. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Changhai Hospital (No. CHEX-Y2020-043) approved this collection procedure, and participants gave informed consent. Full patient clinicopathological details are compiled in Table 1. In addition, another 16 sets of fresh tumor and paracancerous tissues for m6A methylated RNA immunoprecipitation (MeRIP)-qPCR analysis collected from patients with PC undergoing surgical resection from 2019 to 2022 at Changhai Hospital were used to assess the correlation between DBH-AS1 expression and m6A modification-related molecules.
Table 1. Association of DBH-AS1 expression with the clinicopathological features of the 156 patients with PC.
| Features | n | lncRNA DBH-AS1 level | P value | |
|---|---|---|---|---|
| High | Low | |||
| All cases | 156 | 78 | 78 | |
| Age, years | 0.006 | |||
| <60 | 88 | 29 | 59 | |
| ≥60 | 68 | 49 | 19 | |
| Gender | <0.001 | |||
| Male | 98 | 38 | 60 | |
| Female | 58 | 40 | 18 | |
| Tumor size (cm) | <0.001 | |||
| <2 | 72 | 48 | 24 | |
| ≥2 | 84 | 30 | 54 | |
| Lymph node metastasis | 0.419 | |||
| Negative | 67 | 31 | 36 | |
| Positive | 89 | 47 | 42 | |
| TNM stage | 0.010 | |||
| I and II | 84 | 50 | 34 | |
| III and IV | 72 | 28 | 44 | |
| Distant metastasis | 0.005 | |||
| Negative | 97 | 57 | 40 | |
| Positive | 59 | 21 | 38 | |
| Perineural invasion | 0.170 | |||
| Negative | 50 | 29 | 21 | |
| Positive | 106 | 49 | 57 | |
PC, pancreatic cancer; TNM, tumor, nodes, and metastases.
Cell culture and transfection
Human pancreatic duct epithelial (HPDE) cells and PC cell lines were obtained from the American Type Culture Collection and cultured in Dulbecco’s minimal essential medium (DMEM) or Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen; Thermo Fisher Scientific, Inc.) and penicillin/streptomycin in a humidified 37 ℃, 5% CO2 incubator. Oligonucleotides used for this study were obtained from Ribobio (Guangzhou, China).
Quantitative polymerase chain reaction (qPCR)
RNA extraction kits (QIAGEN, Hilden, Germany) were used following the manufacturer’s instructions to extract RNA from samples of interest, after which qPCR analyses were conducted with the Bio-Rad CFX96 Real-Time PCR instrument and the SYBR qPCR Mix (Takara, Dalian, China). The expression levels of lncRNA and mRNA targets were normalized to those of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), while U6 levels were used for miRNA normalization.
m6A methylated RNA immunoprecipitation (MeRIP)-qPCR analysis
MeRIP assays were conducted using a Magna MeRIP m6A Kit (Epigentek, USA) following the manufacturer’s instructions. First, RNA was extracted from PC cells or tissues and fragmented via sonication at 0 ℃ for 10 seconds, after which magnetic beads (Sigma-Aldrich, Germany) were incubated at room temperature for 30 minutes with anti-m6A. Antibody-conjugated beads were then rinsed, combined with DNA-free RNA for 2 hours at 4 ℃ with constant rotation, then washed again. RNA was then eluted with 5'-monophosphate sodium salt (m6A). A miRNeasy mini-kit (QIAGEN) was used to isolate this RNA, after which qPCR analyses were conducted as appropriate.
In vitro cytotoxicity tests
Gemcitabine was obtained from Eli Lilly & Co., Indianapolis, IN, USA. PC cells were plated in triplicate in 96-well plates (8×103/well), and cytotoxicity tests were conducted as in prior studies (22).
Cell proliferation and colony formation assays
A cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used to assess cellular proliferation. Colony formation activity was assessed by plating 1.5×103 cells in triplicate in 6-well plates for 14 days. Plates were then rinsed with phosphate-buffered saline (PBS), fixed using methanol for 10 minutes, and stained for 10 minutes using 0.1% crystal violet prior to subsequent analyses.
Luciferase reporter assay
Luciferase reporter assays were conducted as in prior studies (22). Briefly, 48 hours post-transfection, cells were collected and assessed in triplicate with a dual-luciferase assay (Promega Corp., Madison, WI, USA) following the manufacturer’s instructions.
Nuclear and cytoplasmic protein extraction
PC cell nuclear and cytoplasmic fractions were collected using a nuclear and cytoplasmic protein extraction kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. GAPDH was used for the nuclear, and U6 for the cytoplasmic.
Patient-derived xenograft (PDX) modeling and in vivo assays
All female BALB/c nude mice (3–4 weeks old) used in our study were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. and then housed in specific pathogen-free units. PDX modeling in BALB/c nude female (male, 4–5 weeks old) and in vivo assays were performed as in prior studies (22).
Animal experiments were performed under a project license granted by ethics board of Second Military Medical University (No. CHE2020-144), in compliance with Second Military Medical University guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Statistical analysis
GraphPad Prism (GraphPad Software, Inc., CA, USA) was used for all statistical testing, with P<0.05 as the significance threshold. Intergroup comparison was carried out using a bilateral t-test, analysis of variance (ANOVA), or a chi-square test according to data type. Correlative analysis was performed using a Spearman test. Kaplan-Meier and log-rank analyses were used to evaluate the prognosis of patients with PC.
Results
Patients with PC exhibit DBH-AS1 downregulation that is correlated with poor survival outcomes
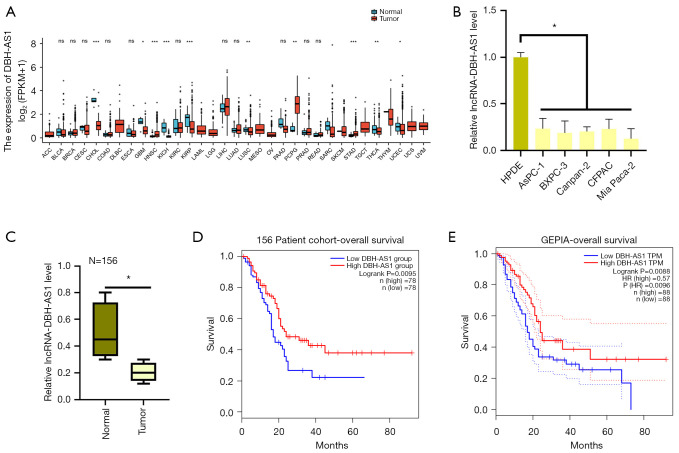
According to the gene expression profiling interactive analysis (GEPIA) database, PC tumors and several other cancer types exhibit downregulation of the lncRNA DBH-AS1 (Figure 1A). Consistent with this information, we observed lower DBH-AS1 levels in PC cell lines relative to HPDE control cells via qPCR analysis (Figure 1B) and found this lncRNA to be expressed at lower levels in 156 PC tumor samples than in precancerous normal tissue samples (Figure 1C). After using the median DBH-AS1 expression levels to separate patients with PC into 2 groups—1 with high levels and 1 with low levels of DBH-AS1 expression—we conducted correlation analyses to explore the link between this lncRNA and the clinicopathological parameters in these 156 patients with PC (Table 1). This approach revealed that low levels of DBH-AS1 expression were positively correlated with larger tumor size and a more advanced tumor, nodes, and metastases (TNM) stage. Patients with lower DBH-AS1 expression levels exhibited poorer overall survival (OS) outcomes in a Kaplan-Meier analysis than patients expressing high DBH-AS1 levels (Figure 1D). Analyses of the GEPIA and The Cancer Genome Atlas (TCGA) databases further confirmed that low levels of DBH-AS1 expression indicate poor survival in patients with PC (Figure 1E). Our results suggest that the downregulation of DBH-AS1 is associated with poor clinical outcomes in patients with PC.
Figure 1.
DBH-AS1 expression is reduced in PC and correlates with worse survival outcomes. (A) GEPIA data revealed DBH-AS1 downregulation in PC. (B) qPCR analysis of DBH-AS1 levels in PC cell lines. (C) qPCR analysis of DBH-AS1 levels in 156 individuals with PC were collected from individuals with PC. (D) Kaplan-Meier analysis of the OS of 156 patients with PC. (E) Kaplan-Meier analysis of the OS of 176 patients with PC, sourced from the GEPIA database. (B,C) t-test; (D,E) log-rank test. Data are means ± SEM from triplicate experiments. *, P<0.05; **, P<0.01; ***, P<0.001; ns, P>0.05. GEPIA, gene expression profiling interactive analysis database; PC, pancreatic cancer; qPCR, quantitative polymerase chain reaction; OS, overall survival.
m6A RNA methylation contributes to DBH-AS1 downregulation in patients with PC
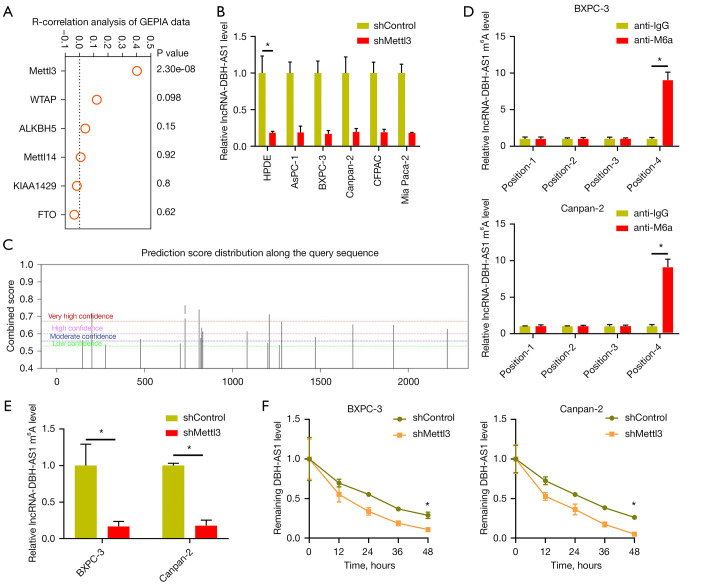
Given the above findings, we next sought to explore the mechanistic basis for DBH-AS1 downregulation in PC. To that end, we treated PC cells with inhibitors of DNA methylation or histone deacetylase activity, revealing DBH-AS1 expression to be independent of these forms of epigenetic modification (Figure S1A,S1B). Disrupting Dicer expression, which is critical to the processing of microRNAs (miRNAs), did not impact DNH-AS1 expression in these PC cells either (Figure S1C). We noted that in the GEPIA database, the expression of the m6A methyltransferase METTL3 was positively correlated with DBH-AS1 expression levels (Figure 2A), and METTL3 knockdown resulted in a significant drop in the expression of this lncRNA (Figure 2B and Figure S1D), highlighting a potential role for m6A modification in the observed changes to DBH-AS1 expression in PC cells. Subsequent MeRIP-qPCR analysis revealed DBH-AS1 m6A levels to be significantly lower in 16 tumor samples from patients with PC than in paracancerous tissue samples (Figure S1E). The sequence-based RNA adenosine methylation site predictor (SRAMP) database was then used to define 4 specific m6A methylation loci associated with DBH-AS1, consistent with the MeRIP-qPCR data (Figure 2C,2D, and Table 2). RIP assays additionally confirmed the m6A modification status of DBH-AS1in PC cells and clarified the link between METTL3 and the m6A modification of this lncRNA (Figure 2E). Rates of DBH-AS1 degradation were assessed over time following the treatment of cells with actinomycin D, revealing that METTL3 was able to modulate DBH-AS1 RNA stability (Figure 2F), thus suggesting that METTL3-induced m6A RNA methylation is capable of modulating levels of DBH-AS1 within PC cells.
Figure 2.
m6A RNA methylation drives the downregulation of DBH-AS1 in PC. (A) GEPIA data revealed a positive correlation between DBH-AS1 and METTL3 expression levels in PC. (B) qPCR analysis of DBH-AS1 expression in PC cells treated with shMETTL3 or shControl. (C) The SRAMP database was utilized to detect m6A methylation loci within DBH-AS1. (D) MeRIP-PCR analysis of m6A-modified DBH-AS1. (E) MeRIP-PCR analysis of m6A-modified DBH-AS1 levels in cells treated with shControl or shMETTL3. (F) The impact of METTL3 on the degradation of DBH-AS1. (B,D-F) t-test. Data are means ± SEM from triplicate experiments, *, P<0.05. GEPIA, gene expression profiling interactive analysis database; PC, pancreatic cancer; qPCR, quantitative polymerase chain reaction; SRAMP, sequence-based RNA adenosine methylation site predictor; MeRIP, methylated RNA immunoprecipitation; sh, short hairpin.
Table 2. m6A sites based on SRAMP database.
| # | Position | Sequence context | Score |
|---|---|---|---|
| 1 | 146 | GGCCCCCCAUGAGGCCUGCCUGACUGCAUGCCCCACCCGGGCCCA | (Moderate confidence) |
| 2 | 200 | ACCUGGAAGCGGACGGCUGAGGACUUGUUGCAGUGCAUGGAGAUG | (Very high confidence) |
| 3 | 277 | GCGGUUGAAGGAGUUCCAGGGAACAGAGGAUCCCGAAGCCCCCCU | (Low confidence) |
| 4 | 476 | AUUGCCACCAUUGGCAGGAGAGACAGGCAGUGAGUGAGUGAGUCA | (Moderate confidence) |
| 5 | 703 | UUGUCUGGAAGCUGUCCCCAUGACCUCCAGCGAGAGCCUGGAGCC | (Low confidence) |
| 6 | 730 | CAGCGAGAGCCUGGAGCCAAGGACUGCAAGGGAGGCCCUUUCCAC | (Very high confidence) |
| 7 | 810 | CGUGGCUCUGAGGGGAGCAGGGACUCUAUAAGACAGGACCGUGAC | (Very high confidence) |
| 8 | 820 | AGGGGAGCAGGGACUCUAUAAGACAGGACCGUGACUAGAGGCCGC | (Moderate confidence) |
| 9 | 825 | AGCAGGGACUCUAUAAGACAGGACCGUGACUAGAGGCCGCGACAA | (High confidence) |
| 10 | 831 | GACUCUAUAAGACAGGACCGUGACUAGAGGCCGCGACAACCUCCA | (High confidence) |
| 11 | 1083 | UCCCAGCAGGUCAGGGGCCGGGACAUGACGCCCUGGCCACCCUUG | (High confidence) |
| 12 | 1201 | GCAAUCUUUACUCUAUGGCAGAACAAAGGGACUGGGCAAAGCUUG | (Low confidence) |
| 13 | 1209 | UACUCUAUGGCAGAACAAAGGGACUGGGCAAAGCUUGGCGUGGCA | (Very high confidence) |
| 14 | 1266 | GCCGCCCCCUCCUCCCGUGCAGACAAAGAGCAUGGGACAGUUCUG | (Low confidence) |
| 15 | 1280 | CCGUGCAGACAAAGAGCAUGGGACAGUUCUGCCCAGAGACGAAAC | (High confidence) |
| 16 | 1471 | CCACCACAGACAUCAAAAGGGAACUGAGUCCGACCCUGUGUAGAA | (Moderate confidence) |
| 17 | 1685 | CAUGGGCCAGGGAAGCCACAGAACUGCCCACGGGGACGCUGGCAC | (High confidence) |
| 18 | 1917 | CAGAAUGGGGCUGAUGGUGGGGACUUGCCUGGGGUGCUCCACCUG | (High confidence) |
| 19 | 2222 | CCUCCUCUAGAGAGAAUAAAGGACUGACAAAGCUAAAAAAAAAAA | (High confidence) |
SRAMP, sequence-based RNA adenosine methylation site predictor.
DBH-AS1 inhibits the growth of PC cells
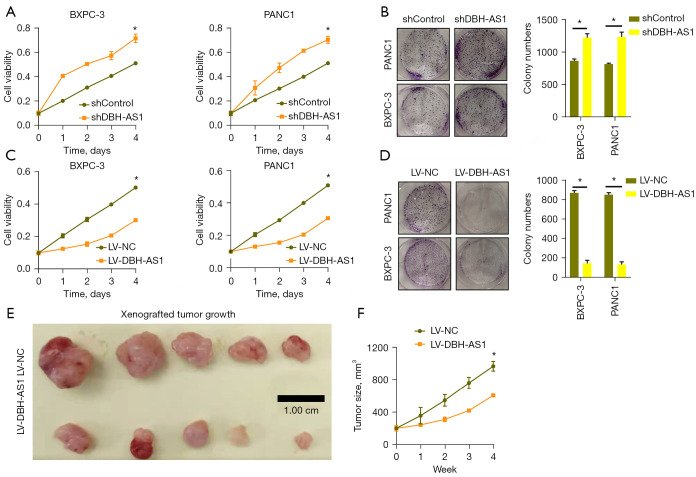
Given the observed relationship between DBH-AS1 and the size of tumors in patients with PC, we next explored the role of this lncRNA as a modulator of PC cell growth. DBH-AS1 was stably knocked down in Canpan-2 and BXPC-3 cells (Figure S2A), leading to the significant enhancement of Canpan-2 and BXPC-3 cells proliferation in both CCK-8 (Figure 3A) and colony formation assays (Figure 3B). In contrast, increasing the expression of DBH-AS1 resulted in the impaired growth of both of these cell lines (Figure 3C,3D, Figure S2B). To further explore the functional role of DBH-AS1 in oncogenic settings, nude BALB/C mice were implanted subcutaneously with Canpan-2 cells stably expressing LV-DBH-AS1, revealing the significant impairment of tumor growth in these BALB/C mice relative to those implanted with control tumors (Figure 3E,3F, Figure S2C). These results confirmed the ability of DBH-AS1 to suppress the in vivo growth of PC tumors.
Figure 3.
DBH-AS1 inhibits the growth of PC cells. (A,C) qPCR analysis of PC cell growth following LV-DBH-AS1 LV-NC, shDBH-AS1, or shControl treatment. (B,D) PC cell colony formation was assessed for cells treated as in (A). The colonies were dyed with crystal violet and photographed under the camera (×1). (E,F) Nude mice subcutaneously injected with Canpan-2 cells stably transfected with LV-DBH-AS1 (n=5) or LV-NC (n=5). Subsequent analyses of tumor growth revealed that LV-DBH-AS1 inhibited in vivo PC tumor growth. Scale bar: 1cm. (A-D,F) t-test. Data are means ± SEM from triplicate experiments. *, P<0.05. PC, pancreatic cancer; qPCR, quantitative polymerase chain reaction; sh, short hairpin.
DBH-AS1 increases the sensitivity of PC cells to gemcitabine
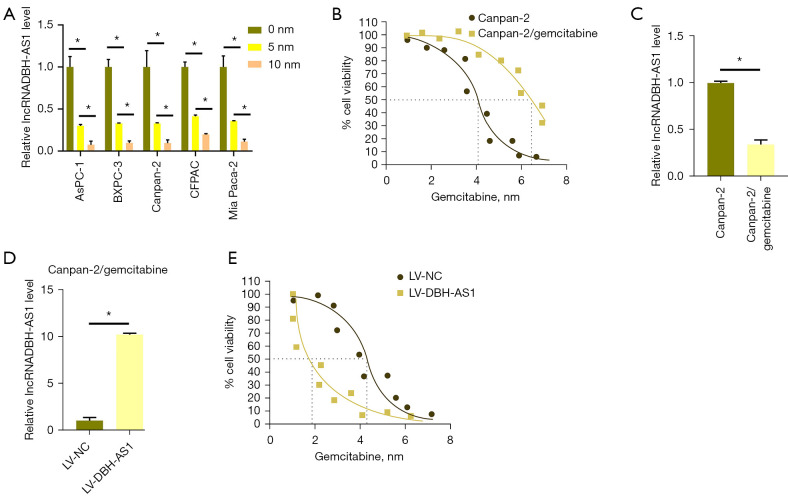
DBH-AS1 levels were reduced in PC cells treated with gemcitabine (Figure 4A). We then established a gemcitabine-resistant cell line by treating Canpan-2 cells for 1 month with this chemotherapeutic agent and selecting for drug-resistant Canpan-2/gemcitabine cells (Figure 4B). Notably, these Canpan-2/gemcitabine cells exhibited lower levels of DBH-AS1 expression than the parental cells (Figure 4C), and the lentivirus-mediated overexpression of the lncRNA increased. Canpan-2/gemcitabine cell sensitivity to gemcitabine treatment compared to that observed in cells transduced with a control lentiviral construct. Conversely, the short hairpin (sh) DBH-AS1 treatment of parental Canpan-2 cells decreased their sensitivity to gemcitabine treatment compared to shControl treatment (Figure 4D,4E), thus confirming the ability of DBH-AS1 to sensitize PC cells to treatment with gemcitabine.
Figure 4.
DBH-AS1 increases the sensitivity of PC cells to gemcitabine. (A) qPCR analysis of DBH-AS1 levels in PC cells via following gemcitabine (0, 5, and 10 nM) treatment. (B) Representative growth curves for Canpan-2/gemcitabine and Canpan-2 cells. (C) qPCR analysis of DBH-AS1 levels in Canpan-2/gemcitabine and Canpan-2 cells. (D) qPCR analysis of DBH-AS1 levels in Canpan-2/gemcitabine following LV-NC or LV-DBH-AS1 treatment. (E) Representative growth curves for gemcitabine-treated Canpan-2/gemcitabine cells treated with LV-NC and LV-DBH-AS1, or Canpan-2 cells treated with shControl or shDBH-AS1. IC50, half-maximal inhibitory concentration. (A,C,D) t-test; (B,E) variance test. Data are means ± SEM from triplicate experiments, *, P<0.05. PC, pancreatic cancer; qPCR, quantitative polymerase chain reaction; sh, short hairpin.
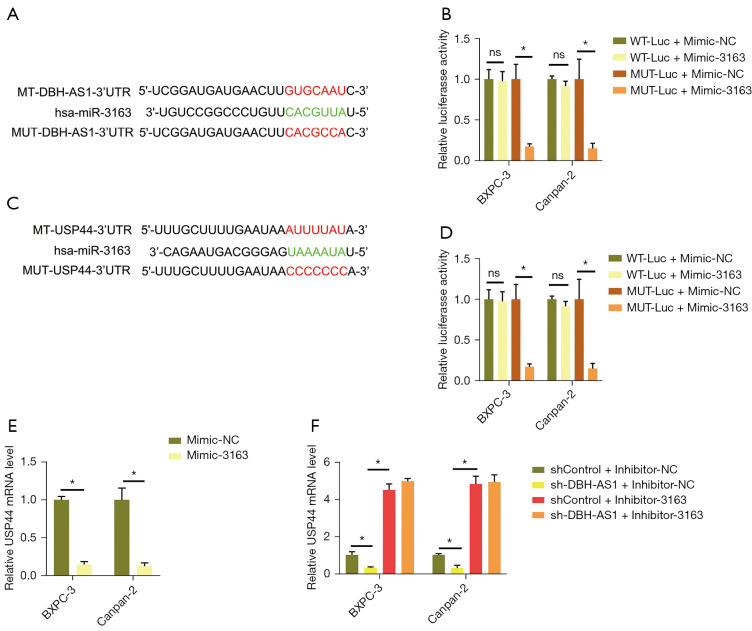
DBH-AS1 sequesters miR-3163 and thereby promotes USP44 upregulation
Several prior reports have highlighted the ability of a competing endogenous RNA (ceRNA) mechanism to explain many of the myriad regulatory effects of lncRNAs on cells. In a subcellular fractionation analysis, DBH-AS1 was found to be primarily expressed in the PC cell cytoplasm, consistent with potential ceRNA functionality (Figure S3A). Using bioinformatics tools, we selected potential miRNA targets with sequence complementarity for DBH-AS1, revealing 16 such targets that exhibited the maximum possible predictive score (>169) (Table 3). These interactions were then verified via RNA pull-down and dual-luciferase reporter assays. In an MS2bs RNA pull-down assay for all 16 targets, DBH-AS1 was found to be capable of binding miR-3163 (Figure S3B), while in a dual-luciferase reporter assay, it was found to bind miR-3163 (Figure 5A,5B). The primary mechanism whereby miRNAs regulate cellular activity is by inhibiting target gene translation through interactions with complementary 3'-untranslated region (UTR) sequences. Using the TargetScan database, the USP44 3'-UTR was found to harbor a miR-3163 binding site (Figure 5C). Dual-luciferase assays consistently indicated a binding interaction between miR-3163 and this USP44 3'-UTR region (Figure 5D). USP44 expression was reduced in PC cells following miR-3163 mimic transfection (Figure 5E and Figure S3C). USP44 mRNA levels were reduced when DBH-AS1 was knocked down, while inhibited miR-3163 expression partially reversed this effect (Figure 5F and Figure S3D,S3E). These results suggested that DBH-AS1 can competitively binding to miR-3163 to sequester it and thereby promote USP44 upregulation.
Table 3. Predicted target miRNAs with the lncRNA DBH-AS1 binding site based on the Miranda database.
| miRNA | Max score | miRNA length |
|---|---|---|
| hsa-miR-216a-3p | 180 | 22 |
| hsa-miR-6894-3p | 179 | 21 |
| hsa-miR-149-3p | 173 | 21 |
| hsa-miR-6821-5p | 172 | 23 |
| hsa-miR-6724-5p | 172 | 23 |
| hsa-miR-612 | 172 | 25 |
| hsa-miR-3163 | 171 | 22 |
| hsa-miR-766-3p | 171 | 22 |
| hsa-miR-4763-3p | 170 | 24 |
| hsa-miR-5008-5p | 170 | 22 |
| hsa-miR-4763-5p | 170 | 21 |
| hsa-miR-212-5p | 170 | 23 |
Figure 5.
DBH-AS1 sequesters miR-3163 and thereby promotes USP44 upregulation. (A) Schematic overview of complementary miR-3163 and DBH-AS1 sequences. (B) DBH-AS1 binding to miR-3163 assessed via dual-luciferase reporter assay. (C) Schematic overview of complementary miR-3163 binding sites within the USP44 3'-UTR. (D) miR-3163 binding to the USP44 3'-UTR assessed via dual-luciferase reporter assay. (E) USP44 mRNA levels measured in PC cells following miR-3163 mimic or mimic NC transfection. (F) USP44 mRNA levels were assessed in the indicated cells. (B,D,E) t-test; (F) variance test. Data are means ± SEM from triplicate experiments. *, P<0.05; ns, P>0.05. PC, pancreatic cancer; CCK-8, cell-counting kit 8; sh, short hairpin.
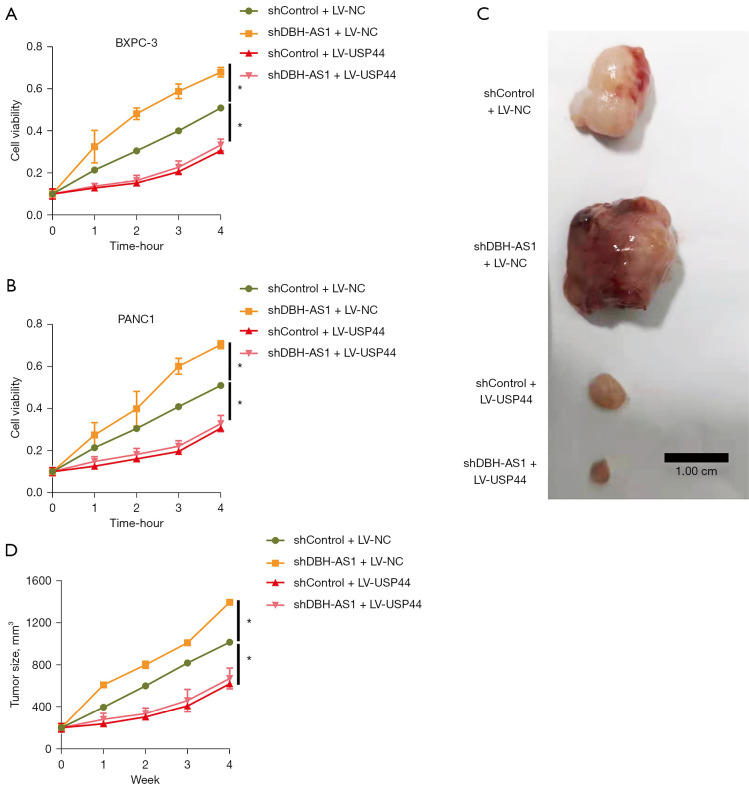
DBH-AS1 knockdown enhances PC cell growth via the downregulation of USP44
Next, we examined the ability of DBH-AS1 knockdown to enhance PC cell proliferation and gemcitabine chemoresistance via the reduction of USP44 expression. Knocking down DBH-AS1 significantly impaired PC cell growth in vitro and in vivo, while USP44 upregulation was sufficient to reverse these effects (Figure 6). These results confirmed the ability of a DBH-AS1 deficiency to enhance PC cell proliferation at least in part by promoting USP44 downregulation.
Figure 6.
DBH-AS1 knockdown enhances PC cell growth via the downregulation of USP44. (A,B) CCK-8 assay of PC cells treated with shControl or shDBH-AS1 and LV-USP44 or LV-NC. (C,D) Canpan-2 cells following stable shControl or shDBH-AS1 transfection and LV-USP44 or LV-NC treatment subcutaneously implanted in nude mice (n=5/group). LV-DBH-AS1 treatment was associated with slower PC tumor growth in these animals. Scale bar: 1cm. (A,B,D) t-test. Data are means ± SEM from triplicate experiments, *, P<0.05. PC, pancreatic cancer; CCK-8, cell-counting kit 8; sh, short hairpin.
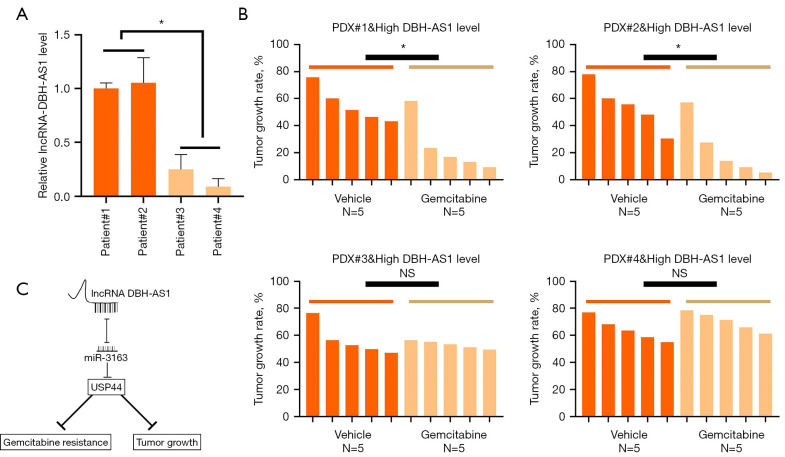
The expression of DBH-AS1 is correlated with beneficial outcomes in gemcitabine-treated patients with PC
In addition, PDXs exhibiting high levels of DBH-AS1 expression grew more slowly following gemcitabine treatment than PDXs expressing low levels of this lncRNA (Figure 7A,7B). These results suggest DBH-AS1 is correlated with gemcitabine responsiveness, making it a potentially valuable predictor of gemcitabine treatment outcomes in patients with PC.
Figure 7.
DBH-AS1 expression correlates with beneficial outcomes in gemcitabine-treated patients with PC. (A) qPCR analysis of DBH-AS1 expression in PDX samples. (B) PDXs expressing low or high levels of DBH-AS1 treated for 28 days with gemcitabine (30mg/kg body weight) or vehicle control, with tumor growth rates monitored over time. (C) Schematic demonstrating the mechanisms through which DBH-AS1 suppresses pancreatic cancer cell growth and gemcitabine resistance. (A,B) t-test. Data are means ± SEM from triplicate experiments, *, P<0.05; NS, P>0.05. PC, pancreatic cancer; qPCR, quantitative polymerase chain reaction; PDX, patient-derived xenograft; NS, no statistical.
Discussion
There is growing evidence demonstrating that lncRNA dysregulation is a key mediator of diverse physiological and pathological processes, including the onset and progression of various cancers (23,24). Moreover, several lncRNAs have been linked to tumor chemoresistance, as in the cases of MALAT1-related cisplatin resistance (25) and linc00173-mediated resistance to adriamycin and etoposide (26). Gemcitabine is a first-line chemotherapeutic drug used to treat patients with advanced PC, either alone or together with other chemotherapeutic compounds (27). Resistance to gemcitabine has been correlated with other lncRNAs, including lncRNA-low expression in tumor (LET) (28). While chemoresistant tumors have been shown to exhibit differential patterns of lncRNA expression, further research is essential to better clarify the functional roles of these lncRNAs in many oncogenic contexts. In this study, we found that DNH-AS1 was downregulated in gemcitabine-resistant human PC cells, with the downregulation of this lncRNA being sufficient to increase gemcitabine resistance in PC cells in vitro and in vivo (Figure 7C).
We found that DBH-AS1 was downregulated in patients with PC in a manner that correlated with survival outcomes, spurring us to explore its mechanistic role in this cancer type. Both DNA methylation and histone acetylation are key regulators of the expression of many genes (29,30) but were not found to affect DBH-AS1 expression in PC cells substantially. Moreover, Dicer interference failed to impact the expression of this lncRNA, suggesting that it is not downregulated by any miRNAs. Recent work suggests that the m6A modification of mRNAs can significantly alter their stability, transport, translation, splicing, and localization within cells (31,32), with such m6A modification being regulated in mammalian cells by a complex composed of METTL3, METTL14, and WTAP (33). Our results suggest that the expression of DBH-AS1 is at least partially controlled by RNA methylation, given that m6A methylase knockdown reduced DBH-AS levels in PC cells, while m6A demethylase knockdown had the opposite effect, increasing DBH-AS1 levels in PC cells. Moreover, we identified a potential role for the m6A methylase METTL3 in reducing DBH-AS1 in patients with PC. To our knowledge, this study is the first to have outlined a role for m6A modification in the dysregulation of DBH-AS1 expression in cancers.
Tumor differentiation status has been linked to responsiveness to targeted therapies, but the mechanisms underlying this relationship are not well understood (34). We found that DBH-AS1 overexpression was sufficient to increase PC cell sensitivity to gemcitabine-mediated growth inhibition. Mechanistically, DNH-AS1 was found to function as a ceRNA for miR-3163 to promote the upregulation of USP44, which is involved in the uptake of gemcitabine. Consistent with these findings, clinical samples revealed that patients with PC and high DBH-AS1 expression levels were more likely to benefit from gemcitabine treatment than patients with lower expression levels of this lncRNA. Given that histological and gene expressions in PDX models have been shown to be largely consistent with case-matched original tumors (35), we also assessed correlations between gemcitabine sensitivity and DBH-AS1 expression in PDX models, revealing that PDXs expressing higher DBH-AS1 levels exhibited more pronounced growth inhibition following treatment with gemcitabine. In contrast, those expressing low levels of this lncRNA were largely unresponsive to such treatment.
Overall, these results indicate that DBH-AS1 may serve as an individualized predictor of gemcitabine treatment sensitivity in patients with PC, although further research will be necessary to confirm this possibility.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Science Fund for Creative Research Groups, NSFC, China (No. 8190550), Shanghai Tongren Hospital (No. 2019shtrxx09), and National Natural Science Foundation of China Youth Fund (No. 81903059).
Ethical Statement: The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Changhai Hospital (No. CHEX-Y2020-043) approved this collection procedure and participants gave informed consent. Animal experiments were performed under a project license granted by ethics board of Second Military Medical University (No. CHE2020-144), in compliance with Second Military Medical University guidelines for the care and use of animals.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-556/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-556/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-556/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Roberts)
References
- 1.Binenbaum Y, Na'ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat 2015;23:55-68. 10.1016/j.drup.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. 10.1016/S0140-6736(16)00141-0 [DOI] [PubMed] [Google Scholar]
- 5.Yan F, Al-Kali A, Zhang Z, et al. A dynamic N6-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res 2018;28:1062-76. 10.1038/s41422-018-0097-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang M, Liu W, Tian W, et al. RNA N-6-methyladenosine enzymes and resistance of cancer cells to chemotherapy and radiotherapy. Epigenomics 2020;12:801-9. 10.2217/epi-2019-0358 [DOI] [PubMed] [Google Scholar]
- 7.Taketo K, Konno M, Asai A, et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol 2018;52:621-9. [DOI] [PubMed] [Google Scholar]
- 8.Hüttenhofer A, Schattner P, Polacek N. Non-coding RNAs: hope or hype. Trends Genet 2005;21:289-97. 10.1016/j.tig.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Taucher V, Mangge H, Haybaeck J. Non-coding RNAs in pancreatic cancer: challenges and opportunities for clinical application. Cell Oncol (Dordr) 2016;39:295-318. 10.1007/s13402-016-0275-7 [DOI] [PubMed] [Google Scholar]
- 10.Tang YT, Xu XH, Yang XD, et al. Role of non-coding RNAs in pancreatic cancer: the bane of the microworld. World J Gastroenterol 2014;20:9405-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Jiang H, Ping C, et al. Exploring the Wnt pathway-associated LncRNAs and genes involved in pancreatic carcinogenesis driven by Tp53 mutation. Pharm Res 2015;32:793-805. 10.1007/s11095-013-1269-z [DOI] [PubMed] [Google Scholar]
- 12.Tahira AC, Kubrusly MS, Faria MF, et al. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol Cancer 2011;10:141. 10.1186/1476-4598-10-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen R, Ghosal S, Das S, et al. Competing endogenous RNA: the key to posttranscriptional regulation. ScientificWorldJournal 2014;2014:896206. 10.1155/2014/896206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou C, Yi C, Yi Y, et al. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/β-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol Cancer 2020;19:118. 10.1186/s12943-020-01237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong G, Liu C, Yang G, et al. Long noncoding RNA GSTM3TV2 upregulates LAT2 and OLR1 by competitively sponging let-7 to promote gemcitabine resistance in pancreatic cancer. J Hematol Oncol 2019;12:97. 10.1186/s13045-019-0777-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Wang P, Yang W, et al. The role of lncRNA MSC-AS1/miR-29b-3p axis-mediated CDK14 modulation in pancreatic cancer proliferation and Gemcitabine-induced apoptosis. Cancer Biol Ther 2019;20:729-39. 10.1080/15384047.2018.1529121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Wu S, Ma J, et al. lncRNA GAS5 Reverses EMT and Tumor Stem Cell-Mediated Gemcitabine Resistance and Metastasis by Targeting miR-221/SOCS3 in Pancreatic Cancer. Mol Ther Nucleic Acids 2018;13:472-82. 10.1016/j.omtn.2018.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Li K, Huang X, et al. lncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCFβ-TRCP-Mediated Degradation of NRF2 in Pancreatic Cancer. Mol Ther Nucleic Acids 2020;19:974-85. 10.1016/j.omtn.2019.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang JL, Ren TY, Cao SW, et al. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget 2015;6:33791-804. 10.18632/oncotarget.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZB, Wang JA, Lv RQ. Downregulation of long non-coding RNA DBH-AS1 inhibits osteosarcoma progression by PI3K-AKT signaling pathways and indicates good prognosis. Eur Rev Med Pharmacol Sci 2019;23:1418-27. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Gao F, Peng Y, et al. Long non-coding RNA DBH-AS1 promotes cancer progression in diffuse large B-cell lymphoma by targeting FN1 via RNA-binding protein BUD13. Cell Biol Int 2020;44:1331-40. 10.1002/cbin.11327 [DOI] [PubMed] [Google Scholar]
- 22.Zheng H, Bi FR, Yang Y, et al. Downregulation of miR-196-5p Induced by Hypoxia Drives Tumorigenesis and Metastasis in Hepatocellular Carcinoma. Horm Cancer 2019;10:177-89. 10.1007/s12672-019-00370-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017;36:5661-7. 10.1038/onc.2017.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munschauer M, Nguyen CT, Sirokman K, et al. Publisher Correction: The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 2018;563:E32. 10.1038/s41586-018-0584-2 [DOI] [PubMed] [Google Scholar]
- 25.Zhang YF, Li CS, Zhou Y, et al. Propofol facilitates cisplatin sensitivity via lncRNA MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric cancer. Life Sci 2020;244:117280. 10.1016/j.lfs.2020.117280 [DOI] [PubMed] [Google Scholar]
- 26.Zeng F, Wang Q, Wang S, et al. Linc00173 promotes chemoresistance and progression of small cell lung cancer by sponging miR-218 to regulate Etk expression. Oncogene 2020;39:293-307. 10.1038/s41388-019-0984-2 [DOI] [PubMed] [Google Scholar]
- 27.Rothenberg ML. New developments in chemotherapy for patients with advanced pancreatic cancer. Oncology (Williston Park) 1996;10:18-22. [PubMed] [Google Scholar]
- 28.Zhuang J, Shen L, Yang L, et al. TGFβ1 Promotes Gemcitabine Resistance through Regulating the LncRNA-LET/NF90/miR-145 Signaling Axis in Bladder Cancer. Theranostics 2017;7:3053-67. 10.7150/thno.19542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XS, Wu H, Krzisch M, et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018;172:979-992.e6. 10.1016/j.cell.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goudarzi A, Zhang D, Huang H, et al. Dynamic Competing Histone H4 K5K8 Acetylation and Butyrylation Are Hallmarks of Highly Active Gene Promoters. Mol Cell 2016;62:169-80. 10.1016/j.molcel.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Eckert MA, Harada BT, et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol 2018;20:1074-83. 10.1038/s41556-018-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep 2014;8:284-96. 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S, Choe J, Du P, et al. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 2016;62:335-45. 10.1016/j.molcel.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Risom T, Langer EM, Chapman MP, et al. Differentiation-state plasticity is a targetable resistance mechanism in basal-like breast cancer. Nat Commun 2018;9:3815. 10.1038/s41467-018-05729-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Q, Zhang B, Sun H, et al. Genomic characterization of a large panel of patient-derived hepatocellular carcinoma xenograft tumor models for preclinical development. Oncotarget 2015;6:20160-76. 10.18632/oncotarget.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as