Abstract
Background
Sevoflurane can protect organs from ischemia-reperfusion (IR) injury, but the mechanism is still unclear. MicroRNA-122 (miR-122) is a liver-specific microRNA (miRNA) and regulates liver function. Therefore, this study aims to elucidate the relationship between the protective effect of sevoflurane and miR-122 in liver IR injury.
Methods
Wistar rats were divided into the following groups: sham, IR, IR + sevoflurane, IR + miR-122 antagomir, and IR + miR-122 antagomir + sevoflurane. Hematoxylin and eosin (H&E) staining and Suzuki score were used to evaluate the pathological damage of the liver. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10 in the serum and the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and nitric oxide (NO) in the liver homogenate supernatant were detected by using the corresponding kit. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) and flow cytometry was applied to evaluate the apoptosis of liver tissues. The expression of nuclear factor E2-related factor 2 (Nrf2), miR-122, p53, and HO-1 in liver tissue was evaluated by using immunohistochemistry, qRT-PCR, and western blot as needed.
Results
Compared to the IR group, the sevoflurane post-treatment or miR-122 antagomir groups showed improved liver injury, decreased Suzuki score, inhibited the levels of AST, ALT, LDH, MDA, NO, TNF-α, IL-1β, and IL-6, increased levels of SOD, IL-10, and inhibited hepatocyte apoptosis. Regarding the molecular mechanism, sevoflurane post-treatment fostered the expression of HO-1, promoted the transport of Nrf2 from cytoplasm to the nucleus, and decreased the expression of miR-122 and p53. The combined use of miR-122 antagomir and sevoflurane enhanced the protective effect of miR-122 antagomir in liver injury in IR rats.
Conclusions
Sevoflurane protected the liver from IR damage by regulating the miR-122/Nrf2/HO-1 pathway.
Keywords: Sevoflurane, miR-122, liver ischemia/reperfusion, Nrf2/HO-1
Introduction
Ischemia-reperfusion (IR) injury is an inevitable process of liver resection and liver transplantation; it is one of the main injury risk factors of liver transplantation and affects the long-term and short-term functions of patients after liver transplantation (1). IR injury can be divided into two stages: ischemia stage, and reperfusion stage. The former is mainly characterized by glycogen depletion, a lack of oxygen supply, and adenosine triphosphate (ATP) depletion, which leads to cell metabolism disorder (2). In addition to cell metabolism disorder, the latter stage also shows apoptosis caused by toxic substances, such as free radicals and direct or indirect inflammatory cascade reactions (2).
Study has confirmed that anesthetic inhalation after tissue ischemia can effectively reduce tissue reperfusion injury (3). Sevoflurane is a safe and universal inhalation anesthetic, which can be used for both adults and children. In addition, it has low flammability, does not cause irritation to the airway, has few side effects, and is regarded as the first choice for prevention and treatment of organ IR injury. Sevoflurane has been shown to play a protective role against IR damage in a variety of organs and tissues, such as the brain (4), heart (5), intestines (6), and lungs (7). Administering sevoflurane post-treatment can also reduce IR-induced liver damage (8). In Liu et al.’s report, by investigating the role of sevoflurane pre-conditioning in hepatic ischemia-reperfusion, they mainly found that sevoflurane relieves hepatic ischemia-reperfusion injury by inhibiting the expression of Grp78 (9), but there is no evidence to fully elucidate the molecular mechanism of sevoflurane’s protective effect on liver IR injury.
A large number of studies have confirmed that microRNAs (miRNAs) are involved in IR damage of various organs by regulating intracellular signaling pathways (10,11). MiRNA is a kind of non-coding RNA that regulates the expression of its bound mRNAs through mechanisms such as shearing and inhibiting transcription to perform biological functions (12). MiRNA-122 (miR-122) is a liver-specific miRNA, which accounts for 70% of the total amount of miRNA in liver tissue and regulates liver development, liver function and liver cell growth (13). Because of this specificity, miR-122 has become a star molecule in the diagnosis, prognosis, and treatment of liver diseases (14). The study of Van Caster et al. showed that, after liver IR injury, the level of circulating miR-122 was significantly increased and correlated with the activity of liver function indicators aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) (15). This means that the abnormal expression of miR-122 may have a causal relationship with liver IR injury. In addition, Nrf2 (Nrf2), a stress-activated transcription factor, plays a pivotal role in defense mechanism against oxidative stress damage, and growing evidence suggests this transcription factor as a key pharmacological target for the treatment of liver diseases, including liver IR injury (16). However, there is no research report on the relationship between the protective effect of sevoflurane and miR-122 in liver IR injury.
Therefore, this study aims to elucidate the relationship between the protective effect of sevoflurane and miR-122 in liver IR injury. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-115/rc).
Methods
Animals
Male Wistar rats aged six weeks (weighting 200–250 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The rats were raised under standard conditions of suitable humidity (30–70%) and temperature (21–22 ℃) and a 12-hour light-dark cycle, and they were allowed to eat and drink freely.
A protocol was prepared before the study without registration. All animal experiments were performed in Hangzhou Eyong Biotechnological Co., Ltd. Animal Experiment Center [Certificate No. SYXK (Zhe)2020-0024] under a project license (EYONG-20201201-02), in compliance with the Institutional Animal Care and Use Committee guidelines.
The animal experiments were divided into two parts. Mouse cages were randomly assigned to groups according to random numbers created using an online application (https://www.random.org). In the first part, rats were divided into the following three groups (ten per group): the sham group, the IR group, and the IR + sevoflurane (Sevo; 1612540, Merck, German) group. In the second part, rats were divided into the following three groups (ten per group): the IR group, the IR + miR-122 antagomir group, and the IR + miR-122 antagomir + Sevo group.
Rats in the IR + miR-122 antagomir group and the IR + miR-122 antagomir + Sevo group were injected with miR-122 antagonist (20 mg/kg/day, 0.2 mL/day; GenePharma, China) by the tail vein 3 days before modeling, and rats in the IR group were injected with the same amount of normal saline in the same way. The IR model was constructed 24 hours after the last injection.
Ischemia/reperfusion injury model
The IR model was constructed as previously described (17). Briefly, before the operation, the rats were anesthetized using an intraperitoneal injection of 1% sodium pentobarbital (40 mg/kg; P010, Merck, USA). After anesthesia, the livers of the rats were exposed by midline laparotomy, and the portal vein and hepatic lobe were clamped with atraumatic vascular clamps to cause ischemia. After 30 minutes, the atraumatic vascular clamp was removed, and reperfusion was performed for 1 hour. Rats in the sham group did not have ischemia caused by a vascular clamp, but the other steps were the same as those in the IR group. Rats in the IR + Sevo and IR + miR-122 antagomir + Sevo groups were immediately put into an anesthesia box to inhale 2% sevoflurane for 30 minutes after reperfusion according to our preliminary experiments. The sevoflurane concentration was stabilized at 2% using an anesthetic drug concentration monitor. After the experiment, the rats were euthanized with an overdose of sodium pentobarbital, and the liver tissue and blood were collected. The blood was centrifuged at 4,000 rpm for 12 minutes to obtain serum.
Hematoxylin and eosin (H&E) staining
The liver tissues of specimens were immersed into formalin (SL1560, Coolaber, China) for fixation, embedded in paraffin, and sectioned. The sections were immersed in xylene (A530011, Sangon, China), gradient concentrations of alcohol (100%, 95%, 80%, 70%, and 50%), and distilled water, respectively. Afterwards, the sections were stained with hematoxylin (SL7050, Coolaber, China), then stained with eosin (SL7060, Coolaber, China). After rinsing the excess dye solution, the sections were dehydrated and transparent, and were then mounted using a neutral balsam mounting medium (E675007, Sangon Biotech, China). The pathological changes of the liver tissue were observed under a microscope.
The Suzuki score was used to quantify the severity of inflammation and tissue injury (18). The highest score was 4 and the lowest score was 0. The higher the score, the more serious the injury.
Detection of liver function markers, inflammatory factors, and antioxidants
Enzyme-linked immunosorbent assay (ELISA) kits of AST (ab263883, abcam, USA), ALT (ab234579, abcam, USA), tumor necrosis factor (TNF)-α (ab236712, abcam, USA), interleukin (IL)-1β (ab255730, abcam, USA), IL-6 (ab234570, abcam, USA) and IL-10 (ab214566, abcam, USA), and LDH kit (MAK066, Merck, German) were used to detect the levels of liver function markers and inflammatory factors in the serum according to the manufacturer’s instructions.
After washing the liver with a normal saline solution, 100 mg of liver tissue was cut and made into a 10% homogenate. The homogenate was centrifuged at 2,500 r/minute for 15 minutes to obtain the supernatant. The malondialdehyde (MDA) kit (MAK085, Merck, German), superoxide dismutase (SOD) kit (MM-0386R2, MEIMIAN, China), and nitric oxide (NO) kit (S0024, Beyotime, China) were used to detect the levels of MDA, SOD, and NO, respectively, in the supernatant.
Immunohistochemistry (IHC)
After deparaffinization, the paraffin sections were immersed in a box containing a citrate buffer solution (pH 6.0; 36319ES60, YEASEN, China) in a microwave oven for antigen retrieval. The sections were reacted with 3% hydrogen peroxide for 25 minutes, then were blocked with 3% bovine serum albumin (BSA, E661003, Sangon, China) for 30 minutes. After that, the sections were reacted with anti-nuclear factor E2-related factor 2 (Nrf2) (1:100; ab31163, Abcam, UK) followed by incubated with Goat Anti-Rabbit IgG H&L (HRP, 1:1,000; ab6721, Abcam, UK) at 37 ℃ for 1 hour. After developing with DAB Substrate Kit (36202ES01, YEASEN, China), the sections were counterstained by hematoxylin. The image was observed under a microscope.
Flow cytometry
The tissue homogenate was made into a single cell suspension by mechanical grinding. The apoptosis of liver cells was determined using the Annexin V Fluorescein Isothiocyanate (Annexin V-FITC) apoptosis detection kit (C1062S, Beyotime, China). Cells (6×105) re-suspended with binding solution were reacted with 5 µL of Annexin V-FITC and 10 µL of Propidium iodide for 15 minutes. The BD Accuri C6 flow cytometer was used to analyze the apoptosis rate.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining
TUNEL staining of liver tissues was performed with the help of a TUNEL kit (C1086, Beyotime, China). After conventional deparaffinization and hydration, the paraffin sections were reacted with proteinase K (ST533, Beyotime, China) for 30 minutes at room temperature. Afterwards, the sections were reacted with the pre-prepared TUNEL detection solution at room temperature and protected from light for 1 hour. 4',6-diamidino-2-phenylindole (DAPI) staining solution (C1006, Beyotime, China) was used to counterstain the nucleus. After sealing sections with anti-fluorescence quenching sealing solution (P0126, Beyotime, China), the results were observed under a fluorescence microscope.
Quantitative reverse transcription polymerase chain reaction
The total amounts of RNA of the serum were extracted using the Total RNA Extraction Kit (R1200, Solarbio, China). The expression of miR-122 was detected using the One-Step miRNAs qRT-PCR kit (AOMD-Q020, GeneCopoeia, USA) and using Real-Time PCR Detection system (LightCycler®96, Roche Diagnostics, Indianapolis, IN, USA) and normalized to RNU6 with the 2−ΔΔCt method. The sequences were as follows: miR-122, forward: 5'-AGCTCTGGAGTGTGACAATGG-3', and reverse: 5'-GCCTAGCAGTAGCTGTTTAGTG-3'.
Western blot
The protein sample was collected from liver tissues using a protein extraction kit (BC3710, Solarbio, China) or a Nuclear and Cytoplasmic Extraction Reagent (78833, Thermo Fisher, USA). The protein was quantified using a BCA kit (PC0020, Solarbio, China). After separating the protein by SDS-PAGE, the protein was transferred into a polyvinylidene fluoride membrane (10600023, GE Healthcare Life, USA). The membrane was blocked by 5% BSA for 1 hour, then the membrane was reacted with primary antibodies and secondary antibodies. The relative intensity of the protein bands was quantified using ana ECL kit (36208ES60, YEASEN, China) with the iBright FL1500 Imaging System (A44115, Invitrogen, USA). The results were counted by ImageJ2x (Rawak Software, Germany). The primary antibodies and secondary antibodies were as follows: anti-p53 (AF0879, Affinity, USA), anti-β-actin (AF7018, Affinity, USA), Nrf2 (AF0639, Affinity, USA), Lamin B (DF6687, Affinity, USA), Heme oxygenase-1 (HO-1, AF5393, Affinity, USA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AF7021, Affinity, USA), and goat anti-rabbit (S0001, Affinity, USA).
Statistical analysis
The results were represented as mean ± standard deviation and analyzed using Graph Prism v8.0 (Graphpad software, California, USA) and SPSS 20.0 (SPSS, Chicago, USA). Differences among multiple groups were analyzed by one-way analysis of variance, and Student-Newman-Keuls (SNK) was applied for pairwise comparisons between groups. The Kruskal-Wallis H test was used for those with uneven variance. P<0.05 was considered as statistically significant.
Results
Sevoflurane relieved liver damage caused by IR
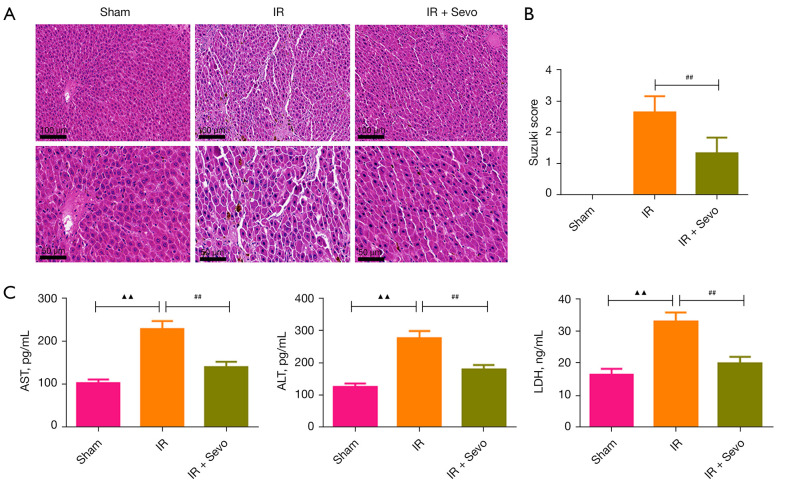
We evaluated the liver damage of rats in each group using H&E staining (Figure 1A). In the sham group, the morphology and structure of liver cells were normal, and the liver sinuses were clear, while, in the IR group, the liver cells were seriously damaged and swollen. However, compared with the IR group, the damage of liver cells in the IR + Sevo group was reduced, and the cell morphology was clearer. In addition, the Suzuki score of the IR + Sevo group was significantly lower than that of the IR group, which indicated that sevoflurane could alleviate the liver injury caused by IR (Figure 1B; P<0.01).
Figure 1.
Sevoflurane ameliorated IR-induced liver damage. The rats were randomly divided into the sham group, the IR group, and the IR + sevoflurane group. Liver injury for the rats in the IR group was induced through ischemia/reperfusion, and liver injury was induced in the IR + sevoflurane group through ischemia/reperfusion and then inhalation of 2% sevoflurane. (A) H&E staining of liver tissue sections (magnification: ×200, scale bar =100 mm; ×400, scale bar =50 mm). (B) The Suzuki score of livers. (C) The levels of AST, ALT, and LDH in the serum of rats. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the sham group; ##, P<0.01 vs. the IR group. IR, ischemia-reperfusion; H&E, hematoxylin and eosin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase.
We measured the levels of AST, ALT, and LDH in the rat serum, which were markers of liver function. As shown in Figure 1C, the levels of these three markers in the IR group were significantly higher than those in the sham group (P<0.01), but treatment with sevoflurane reduced the levels of AST, ALT, and LDH in the serum of the IR rats (P<0.01).
Sevoflurane inhibited oxidative stress and inflammatory response induced by IR
MDA, SOD, and NO are common oxidative stress parameters, so we detected the levels of these parameters in the supernatant of the liver homogenate. The level of MDA and NO in the IR group was higher than that in the sham group, but the level of SOD in the IR group was lower than that in the sham group (Figure 2A; P<0.01). However, treatment with sevoflurane reversed the increase of MDA and NO and the decrease of SOD in the rat livers caused by IR (P<0.01).
Figure 2.
Effects of sevoflurane on the antioxidant activity and inflammation induced by IR in liver tissues. (A) Levels of MDA, SOD, and NO in liver tissue. (B) The levels of inflammatory factors TNF-α, IL-1β, IL-6, and IL-10 in the serum of rats. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the sham group; ##, P<0.01 vs. the IR group. IR, ischemia-reperfusion; MDA, malondialdehyde; SOD, superoxide dismutase; NO, nitric oxide; TNF-α, tumor necrosis factor-α; IL, interleukin.
In addition, we also detected the levels of pro-inflammatory factors and anti-inflammatory factors in the serum. Similarly, the IR-induced increase of TNF-α, IL-1β, and IL-6 levels, and the decrease of IL-10 level, were reversed by sevoflurane (Figure 2B; P<0.01).
Sevoflurane fostered the expression of Nrf2 in liver tissue and suppressed the apoptosis of liver tissue
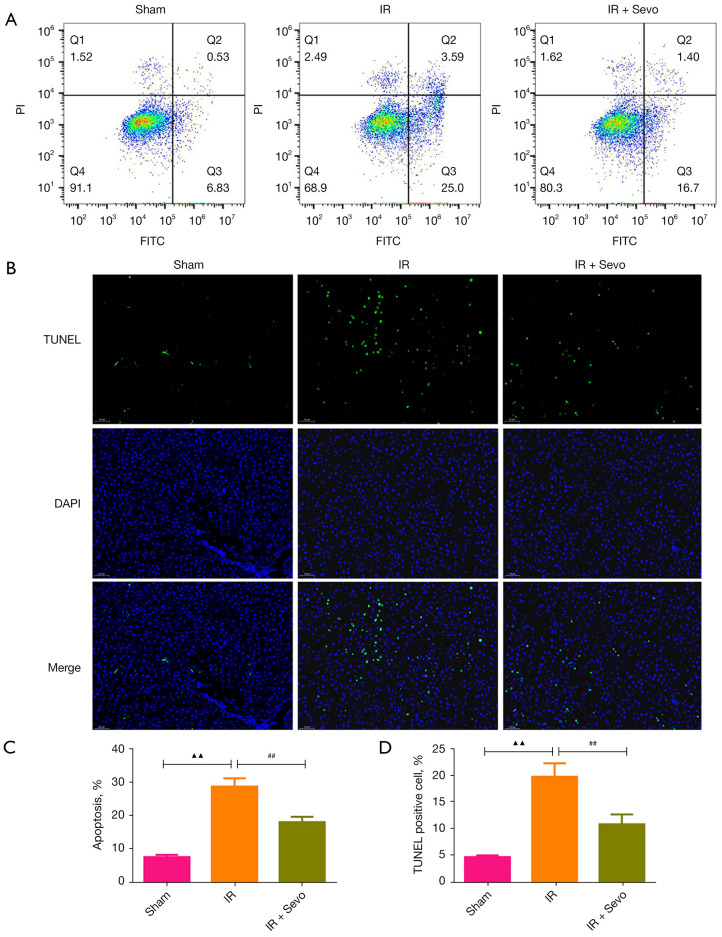
The activation of the Nrf2 pathway is very important for anti-oxidative stress. After IR, the expression of Nrf2 in liver tissue of rats was decreased significantly, but treatment with sevoflurane prevented the down-regulation of Nrf2 (Figure 3A,3B; P<0.05). Then, we evaluated the apoptosis in the livers by flow cytometry and TUNEL staining. The results of the two experiments were consistent and showed that sevoflurane reversed the increase of hepatocyte apoptosis caused by IR (Figure 4A-4D; P<0.01).
Figure 3.
Immunohistochemistry was used to detect the expression of Nrf2 in liver tissue. (A) Immunohistochemical staining of Nrf2 (magnification: ×200, scale bar =100 mm; ×400, scale bar =50 mm). (B) Relative positive expression analysis of Nrf2. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the sham group; #, P<0.05 vs. the IR group. Nrf2, nuclear factor E2-related factor 2; IR, ischemia-reperfusion.
Figure 4.
Sevoflurane improved IR-induced apoptosis of liver tissue. (A) Apoptosis of liver tissue in each group was detected by flow cytometry. (B) TUNEL staining was used to detect the apoptosis of liver tissue in each group (magnification, ×200). (C) Statistics of apoptosis rate were detected with flow cytometry. (D) Statistics of positive cell rate were detected with TUNEL staining. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the sham group; ##, P<0.01 vs. the IR group. IR, ischemia-reperfusion; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
Sevoflurane inhibited the expression of serum miR-122 and liver p53, and activated the Nrf2/HO-1 signaling pathway
We detected the content of the serum of miR-122 of rats in each group due to its specificity in liver injury. Surprisingly, the expression of miR-122 in the IR group increased sharply compared with the sham group (Figure 5; P<0.01). However, sevoflurane narrowed the increase of miR-122 caused by IR (P<0.01).
Figure 5.
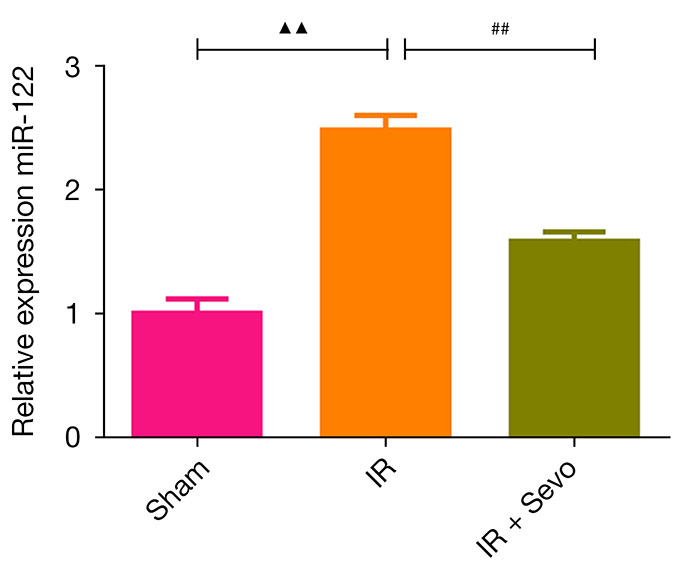
qRT-PCR detection of the effects of sevoflurane post-treatment on miR-122 levels after liver IR injury in rats. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the sham group; ##, P<0.01 vs. the IR group. IR, ischemia-reperfusion.
In addition, IR promoted the expression of p53 and Nrf2 in the cytoplasm (Figure 6A-6C; P<0.05) but had no significant effect on the expression of nuclear Nrf2 and HO-1. Nevertheless, sevoflurane reduced the expression of p53 and fostered the transport of Nrf2 from the cytoplasm to the nucleus, thereby increasing the expression of HO-1 (P<0.01).
Figure 6.
The effect of sevoflurane on the protein expression of p53 and Nrf2 in liver tissue induced by IR. (A) Western blot showed the expressions of p53. (B) Western blot showed the expressions of Nrf2 in the nucleus. (C) Western blot showed the expressions of Nrf2 in the cytoplasm. Quantified values were mean ± standard deviation of at least three independent experiments. ▲, P<0.05 vs. the sham group; ##, P<0.01 vs. the IR group. IR, ischemia-reperfusion; Nrf2, nuclear factor E2-related factor 2.
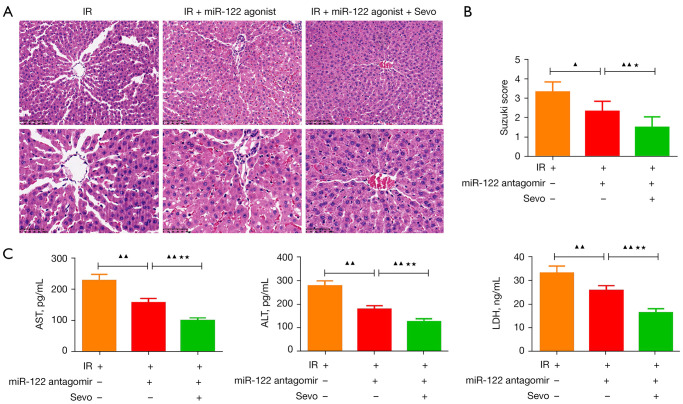
MiR-122 antagomir combined with sevoflurane further relieved the IR-induced liver damage
As shown in Figure 7, compared with the IR group, the use of miR-122 antagomirs reduced the liver damage of IR rats (Figure 7A), lowered the Suzuki score (Figure 7B; P<0.05), and reduced the levels of AST, ALT, and LDH in the serum of IR rats (Figure 7C; P<0.01). The combination of sevoflurane and miR-122 antagomir strengthened the miR-122 antagomir’s mitigation effect on liver injury in the IR rats (P<0.05).
Figure 7.
MiR-122 antagomir combined with sevoflurane further reduced IR-induced liver damage. The rats were randomly divided into the IR group, the IR + miR-122 antagomir group, and the IR + miR-122 antagomir + Sevo group. Liver injury was induced in the rats in the IR group through ischemia/reperfusion. The mice in the IR + miR-122 antagomir group were pretreated with miR-122 antagomir and then liver injury was induced through ischemia/reperfusion. The mice in the IR + miR-122 antagomir + Sevo group were pretreated with miR-122 antagomir, then liver injury was induced through ischemia/reperfusion, and then the rats inhaled 2% sevoflurane. (A) H&E staining of liver tissue sections (magnification: ×200, scale bar =100 mm; ×400, scale bar =50 mm). (B) The Suzuki score of livers. (C) The levels of AST, ALT, and LDH in the serum of rats. Quantified values were mean ± standard deviation of at least three independent experiments. ▲, P<0.05, ▲▲, P<0.01 vs. the IR group;★, P<0.05, ★★, P<0.01 vs. the IR + miR-122 antagomir group. +, this group rats were treated with indicated agent; −, this groups did not receive this indicated treatment. IR, ischemia-reperfusion; H&E, hematoxylin and eosin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase.
MiR-122 antagomir combined with sevoflurane further inhibited oxidative stress and inflammatory response induced by IR
The levels of MDA and NO in the miR-122 antagomir + IR group were lower than those in the IR group, but the SOD level was higher than that in the IR group (Figure 8A; P<0.01). At the same time, the miR-122 antagomir promoted the down-regulation of inflammatory factors (TNF-α, IL-1β, and IL-6) and the up-regulation of anti-inflammatory factors (IL-10) (Figure 8B; P<0.01). Moreover, the combination of miR-122 antagomirs and sevoflurane further strengthened the regulation of miR-122 antagomirs on the above-mentioned oxidative stress parameters and inflammation-related factors (P<0.05).
Figure 8.
MiR-122 antagomir combined with sevoflurane further inhibited oxidative stress and inflammatory response induced by IR. (A) Levels of MDA, SOD, and NO in liver tissue. (B) The levels of inflammatory factors TNF-α, IL-1β, IL-6, and IL-10 in the serum of rats. Quantified values were mean ± standard deviation of at least three independent experiments. ▲▲, P<0.01 vs. the IR group; ★, P<0.05, ★★, P<0.01 vs. the IR + miR-122 antagomir group. +, this group rats were treated with indicated agent; −, this groups did not receive this indicated treatment. IR, ischemia-reperfusion; MDA, malondialdehyde; SOD, superoxide dismutase; NO, nitric oxide; TNF-α, tumor necrosis factor-α; IL, interleukin.
MiR-122 antagomir combined with sevoflurane further inhibited the expression of miR-122 and activated the Nrf2/HO-1 signaling pathway
The miR-122 antagomir down-regulated the expression of miR-122 in the liver tissue of IR rats, but also increased the protein levels of Nrf2 (nucleus) and HO-1 and decreased the protein level of Nrf2 (cytoplasm) (Figure 9A,9B; P<0.05). Unexpectedly, the combination of miR-122 antagomir and sevoflurane further suppressed the expression of miR-122 and HO-1 and increased the transport of Nrf2 from the cytoplasm to the nucleus (P<0.05).
Figure 9.
MiR-122 antagomir combined with sevoflurane further promoted the expression of miR-122 and activated the Nrf2/HO-1 signaling pathway. (A) qRT-PCR showed the miR-122 levels in liver tissue. (B) Western blot showed the expressions of Nrf2 in the nucleus, and Nrf2 in the cytoplasm and HO-1. Quantified values were mean ± standard deviation of at least three independent experiments. ▲, P<0.05, ▲▲, P<0.01 vs. the IR group; ★, P<0.05, ★★, P<0.01 vs. the IR + miR-122 antagomir group. +, this group rats were treated with indicated agent; −, this groups did not receive this indicated treatment. IR, ischemia-reperfusion; Nrf2, nuclear factor E2-related factor 2.
Discussion
There are many causes contributing to the occurrence of liver IR injury, especially the surgical procedures including the hepatic resection and liver transplantation. The main influencing factors of liver IR are oxidative stress and inflammation, and their interaction causes liver cell damage and even liver dysfunction (2). Sevoflurane is widely used in clinical anesthesia and has a protective effect on IR damage to various organs. Liu et al. reported that sevoflurane pretreatment improves liver IR damage in rats (9). In this study, we found that sevoflurane post-treatment can also reduce liver damage caused by IR. AST, ALT, and LDH are highly expressed in liver diseases and are widely used to assess the degree of liver damage (19). Unsurprisingly, post-treatment with sevoflurane can significantly reduce the contents of AST, ALT, and LDH in the serum of IR rats, which is consistent with previous reports (20).
Oxidative stress is an imbalance between oxidation and antioxidant processes caused by an excess of reactive oxygen species. MDA is a product of a lipid peroxidation metabolism. The higher the level of MDA, the higher the degree of free radical attack on human cells. SOD can scavenge free radicals and protect cells from damage. NO is a kind of reactive nitrogen oxide, and its excess will cause the body’s oxidation and anti-oxidation imbalance. A previous study reported that the levels of MDA and NO produced in Sprague-Dawley rats of the liver IR model can be effectively inhibited by sevoflurane, while the level of SOD can be increased (9). Interestingly, we observed the same result in the IR model constructed by Wistar rats. In addition to inhibiting oxidative stress, sevoflurane can also exert liver protection by reducing inflammation. Yang et al. (21) reported that sevoflurane inhibited the increase of IR-induced inflammatory factors but promoted the expression of anti-inflammatory factor IL-10 in Wistar rats. Consistent with the research of Yang et al. (21), we found that sevoflurane post-treatment also inhibited the expression of inflammatory factors (TNF-α, IL-1β, and IL-6), but promoted the expression of IL-10 in the serum of IR rats.
The interaction of oxidative stress and inflammation can cause liver cell apoptosis, which is also a common phenomenon of liver IR injury (22). Notably, in the present study, sevoflurane was shown to inhibit cell apoptosis caused by IR by regulating the expression of miRNA and exerting a protective effect on the liver. Wu et al. reported that sevoflurane attenuates liver damage caused by liver IR through mediating miR-200c (23). Liao et al. pointed out that sevoflurane inhibits liver damage caused by liver IR through miR-9-5p (24). MiR-122 is a liver-specific miRNA which regulates liver development, liver function and liver cell growth (13). The increased expression of miR-122 is found to correlated with the liver function indicators after liver IR injury (15). Furthermore, Seyyed Ali Mard reported that IR induced an increase of miR-122, but the treatment group could significantly inhibit the levels of miR-122, indicating that the decrease of miR-122 was helpful to resist IR injury (25). Similar to the previous study (25), we found for the first time that sevoflurane can reverse the up-regulation of the miR-122 level caused by IR, thus preventing IR damage of the liver.
Nrf2 is one of the targets of miR-122 (26). Evidence showed that the mitigation effect of sevoflurane on liver IR injury was implicated in the activation of the Nrf2/HO-1 pathway (27). The Nrf2/HO-1 pathway has both anti-inflammatory and anti-oxidant capabilities and is an important signaling pathway for the body to resist oxidative stress damage. Under physiological conditions, Nrf2 and its negative regulator keap1 are located in the cytoplasm. Under stress conditions, the two are decoupled, allowing Nrf2 to be released into the nucleus and then combine with HO-1 to exert anti-oxidative stress effects (28). Previous research reported that the livers of mice that lacked Nrf2 were more susceptible to oxidative stress-induced damage (29). Therefore, activating the Nrf2/HO-1 pathway is particularly important for protecting the liver from damage. In this study, sevoflurane was shown to promote the transfer of Nrf2 from the cytoplasm to the nucleus and then promote the expression of HO-1, indicating that sevoflurane can regulate the activation of the Nrf2/HO-1 pathway by regulating miR-122.
p53 is a tumor suppressor that can promote tumor cell apoptosis, but p53 can be activated under oxidative stress conditions, which is not conducive to resisting IR-induced liver damage (30). The study by Derdak et al. pointed out that inhibition of p53 can reduce liver damage in mice with non-alcoholic fatty liver disease (31). In our study, sevoflurane post-treatment reversed the upregulated p53 in the IR group, which indicated that sevoflurane alleviated IR-induced liver injury by down-regulating p53.
In order to verify that sevoflurane had a hepatoprotective effect by inhibiting the expression of miR-122, we used miR-122 antagomir for verification. Previous study reported that miR-122-5p antagomir could reduce acetaminophen-induced oxidative stress and liver damage (32). In addition, the study by Qiang et al. pointed out that silencing of miR-122 can relieve liver pressure related to cadmium stress by regulating oxidative stress and stimulating the activity of antioxidant enzymes (33). Consistent with these findings, we found that the combination of miR-122 antagonists and sevoflurane further inhibited IR-induced oxidative stress and inflammatory response but promoted the activation of the Nrf2/HO-1 pathway, indicating that sevoflurane can reduce IR-induced liver damage by inhibiting miR-122.
Conclusions
This study emphasized that sevoflurane protects the liver from IR damage by regulating the miR-122/Nrf2/HO-1 pathway. In doing so, we provided a theoretical basis for the clinical application of sevoflurane in IR injury.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Ningbo Natural Science Foundation (Nos. 2018A610375 and 2019A610219).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were performed in Hangzhou Eyong Biotechnological Co., Ltd. Animal Experiment Center [Certificate No. SYXK (Zhe)2020-0024] under a project license (EYONG-20201201-02), in compliance with the Institutional Animal Care and Use Committee guidelines.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-115/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-115/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-115/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Mullens)
References
- 1.Yang W, Chen J, Meng Y, et al. Novel Targets for Treating Ischemia-Reperfusion Injury in the Liver. Int J Mol Sci 2018;19:1302. 10.3390/ijms19051302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konishi T, Lentsch AB. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr 2017;17:277-87. 10.3727/105221617X15042750874156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Álvarez P, Tapia L, Mardones LA, et al. Cellular mechanisms against ischemia reperfusion injury induced by the use of anesthetic pharmacological agents. Chem Biol Interact 2014;218:89-98. 10.1016/j.cbi.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 4.Zhong H, Chen H, Gu C. Sevoflurane Post-treatment Upregulated miR-203 Expression to Attenuate Cerebral Ischemia-Reperfusion-Induced Neuroinflammation by Targeting MyD88. Inflammation 2020;43:651-63. 10.1007/s10753-019-01147-2 [DOI] [PubMed] [Google Scholar]
- 5.Xie D, Zhao J, Guo R, et al. Sevoflurane Pre-conditioning Ameliorates Diabetic Myocardial Ischemia/Reperfusion Injury Via Differential Regulation of p38 and ERK. Sci Rep 2020;10:23. 10.1038/s41598-019-56897-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Ding R, Huang W, et al. Sevoflurane Protects against Intestinal Ischemia-Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma/Nuclear Factor-κB Pathway in Rats. Pharmacology 2020;105:231-42. 10.1159/000503727 [DOI] [PubMed] [Google Scholar]
- 7.Ohsumi A, Marseu K, Slinger P, et al. Sevoflurane Attenuates Ischemia-Reperfusion Injury in a Rat Lung Transplantation Model. Ann Thorac Surg 2017;103:1578-86. 10.1016/j.athoracsur.2016.10.062 [DOI] [PubMed] [Google Scholar]
- 8.Cavalcante FP, Coelho AM, Machado MC, et al. Mechanisms of the beneficial effect of sevoflurane in liver ischemia/reperfusion injury. Acta Cir Bras 2015;30:749-55. 10.1590/S0102-865020150110000005 [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Jin X, Zhang C, et al. Sevoflurane relieves hepatic ischemia-reperfusion injury by inhibiting the expression of Grp78. Biosci Rep 2018;38:BSR20180549. 10.1042/BSR20180549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehaini H, Awada H, El-Yazbi A, et al. MicroRNAs as Potential Pharmaco-targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 2019;8:152. 10.3390/cells8020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghafouri-Fard S, Shoorei H, Taheri M. Non-coding RNAs participate in the ischemia-reperfusion injury. Biomed Pharmacother 2020;129:110419. 10.1016/j.biopha.2020.110419 [DOI] [PubMed] [Google Scholar]
- 12.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol 2018;141:1202-7. 10.1016/j.jaci.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H, Ewing LE, Koturbash I, et al. MicroRNAs as biomarkers for liver injury: Current knowledge, challenges and future prospects. Food Chem Toxicol 2017;110:229-39. 10.1016/j.fct.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, He Y, Mackowiak B, et al. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021;70:784-95. 10.1136/gutjnl-2020-322526 [DOI] [PubMed] [Google Scholar]
- 15.Van Caster P, Brandenburger T, Strahl T, et al. Circulating microRNA-122, -21 and -223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol Med Rep 2015;12:3146-50. 10.3892/mmr.2015.3742 [DOI] [PubMed] [Google Scholar]
- 16.Jayasuriya R, Dhamodharan U, Ali D, et al. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine 2021;92:153755. 10.1016/j.phymed.2021.153755 [DOI] [PubMed] [Google Scholar]
- 17.Wu HH, Huang CC, Chang CP, et al. Heat Shock Protein 70 (HSP70) Reduces Hepatic Inflammatory and Oxidative Damage in a Rat Model of Liver Ischemia/Reperfusion Injury with Hyperbaric Oxygen Preconditioning. Med Sci Monit 2018;24:8096-104. 10.12659/MSM.911641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993;55:1265-72. 10.1097/00007890-199306000-00011 [DOI] [PubMed] [Google Scholar]
- 19.Lala V, Goyal A, Bansal P, et al. Liver Function Tests. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 20, 2021. [Google Scholar]
- 20.Xu G, Wang X, Xiong Y, et al. Effect of sevoflurane pretreatment in relieving liver ischemia/reperfusion-induced pulmonary and hepatic injury. Acta Cir Bras 2019;34:e201900805. 10.1590/s0102-865020190080000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Du Y, Zeng H, et al. Comparison of Inflammatory Markers Between the Sevoflurane and Isoflurane Anesthesia in a Rat Model of Liver Ischemia/Reperfusion Injury. Transplant Proc 2019;51:2071-5. 10.1016/j.transproceed.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 22.Liu HQ, Li RJ, Sun X, et al. High-fat diet enhances hepatic ischemia-reperfusion injury-induced apoptosis: Role of glucocorticoid receptors. Life Sci 2017;191:227-35. 10.1016/j.lfs.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Gu C, Huang X. Sevoflurane protects against hepatic ischemia/reperfusion injury by modulating microRNA-200c regulation in mice. Biomed Pharmacother 2016;84:1126-36. 10.1016/j.biopha.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 24.Liao X, Zhou S, Zong J, et al. Sevoflurane exerts protective effects on liver ischemia/reperfusion injury by regulating NFKB3 expression via miR-9-5p. Exp Ther Med 2019;17:2632-40. 10.3892/etm.2019.7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mard SA, Akbari G, Dianat M, et al. The Effect of Zinc Sulfate on miR-122, miR-34a, Atioxidants, Biochemical and Histopathological Parameters Following Hepatic Ischemia/Reperfusion Injury in Rats. Biol Trace Elem Res 2019;188:434-40. 10.1007/s12011-018-1425-8 [DOI] [PubMed] [Google Scholar]
- 26.Meng M, Zhang R, Han R, et al. The polysaccharides from the Grifola frondosa fruiting body prevent lipopolysaccharide/D-galactosamine-induced acute liver injury via the miR-122-Nrf2/ARE pathways. Food Funct 2021;12:1973-82. 10.1039/D0FO03327H [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Yang B, Yu L, et al. Sevoflurane protects the liver from ischemia-reperfusion injury by regulating Nrf2/HO-1 pathway. Eur J Pharmacol 2021;898:173932. 10.1016/j.ejphar.2021.173932 [DOI] [PubMed] [Google Scholar]
- 28.Krajka-Kuźniak V, Paluszczak J, Baer-Dubowska W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol Rep 2017;69:393-402. 10.1016/j.pharep.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 2010;244:57-65. 10.1016/j.taap.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbari G, Mard SA, Dianat M, et al. The Hepatoprotective and MicroRNAs Downregulatory Effects of Crocin Following Hepatic Ischemia-Reperfusion Injury in Rats. Oxid Med Cell Longev 2017;2017:1702967. 10.1155/2017/1702967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derdak Z, Villegas KA, Harb R, et al. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol 2013;58:785-91. 10.1016/j.jhep.2012.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Wu W, Ou P, et al. MiR-122-5p knockdown protects against APAP-mediated liver injury through up-regulating NDRG3. Mol Cell Biochem 2021;476:1257-67. 10.1007/s11010-020-03988-0 [DOI] [PubMed] [Google Scholar]
- 33.Qiang J, Tao YF, He J, et al. miR-122 promotes hepatic antioxidant defense of genetically improved farmed tilapia (GIFT, Oreochromis niloticus) exposed to cadmium by directly targeting a metallothionein gene. Aquat Toxicol 2017;182:39-48. 10.1016/j.aquatox.2016.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as