Abstract
Background
Patients with inflammatory bowel disease (IBD) often have low weight, malnutrition and sarcopenia. The criteria of sarcopenia used were European and American standards previously. The aim of the study was to evaluate the impact of sarcopenia on clinical outcomes in patients with IBD using the Asian Working Group for Sarcopenia 2019 (AWGS2019) criteria.
Methods
The inclusion of the subjects was IBD patients between 18 to 60 years. Sarcopenia, pre-sarcopenia and sarcopenic obesity were defined. Participants were followed up for 90 days. Information as to whether the symptoms improved, treatment plans changed, underwent surgery, were readmitted to the hospital, or died was recorded. Analyses of chi-square test, t-test, cumulative survival analysis and receiver operating characteristic (ROC) curves were done through SPSS25.0 software. Odds ratio (OR) and 95% confidence interval (CI) were calculated.
Results
A total of 110 patients with IBD were included. The prevalence of pre-sarcopenia was 44.6% and of sarcopenia 50.8%. Body mass index (BMI) (P=0.018; OR =0.449) and albumin (Alb) levels were lower (P=0.004; OR =0.608) in the sarcopenia group than the control and pre-sarcopenia groups, and they were risk factors for sarcopenia. Meanwhile, a history of more frequent alcohol consumption, parenteral manifestations, IBD-related complications, higher C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were significant statistic different for sarcopenia group compared with others. Rates of surgery (P<0.001; OR =6.651), re-hospitalization (P<0.001; OR =6.344) or death (P=0.003) were higher in the sarcopenia group than in the control group. The sarcopenia group had higher rates of surgery (P=0.022; OR =3.608) and re-hospitalization (P=0.048; OR =5.500) than the pre-sarcopenia group after adjustment analysis. Patients in the sarcopenic obesity group with body fat percentages ≥24.8% (P=0.039; 95% CI: 0.590–1.000) in men and ≥32.0% (P=0.006; 95% CI: 0.692–1.000) in women were more likely to receive surgery, female patients with that ≥24.5% (P=0.025; 95% CI: 0.556–1.000) were more likely to experience re-hospitalization.
Conclusions
Patients with IBD diagnosed with sarcopenia or sarcopenic obesity based on AWGS2019 criteria had poorer outcomes. The AWGS2019 criteria are comprehensive and more suitable for predicting outcomes in IBD patients, which helps doctors making precise treatment.
Keywords: Inflammatory bowel disease (IBD), ulcerative colitis (UC), Crohn’s disease (CD), sarcopenia, AWGS 2019 criteria
Introduction
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disease that includes ulcerative colitis (UC) and Crohn’s disease (CD) (1). Patients with IBD often suffer from malnutrition, sarcopenia and poor clinical outcomes, which are related to reduced energy intake, malabsorption, loss of nutrients, and inflammation (2-4). Previous studies showed that smoking (5,6), intaking certain diet constituents and drugs (7) are associated to the poor outcomes of IBD patients while whether sarcopenia is related to that was less studied. Due to the incidence of sarcopenia is not low in clinic, evaluating the relationship of sarcopenia and the outcomes of IBD patients is necessary and important.
Sarcopenia was firstly defined by Rosenberg in 1989 as the loss of lean muscle coupled with a loss in muscle strength (8,9). Loss of muscle mass and function is associated with considerable morbidity and mortality (2-4,10). Sarcopenia can contribute to negative outcomes in several diseases, such as liver cirrhosis, chronic obstructive pulmonary disease, and chronic kidney disease (11). In recent research, greater attention has been paid to sarcopenia in patients with IBD (12), as the prevalence of sarcopenia is relatively high in this group (1). Based on this recommendation, we designed this study to assess the impact of sarcopenia on clinical outcomes such as the need for surgery, re-hospitalization, or death in IBD patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1126/rc).
Methods
Study population and design
To answer the research question, this study calculated the sample size based on the formula for prospective cohort studies. Finally, this study included 110 consecutive patients (65 men and 45 women, with a median age of 45.5 years) who received a diagnosis of IBD in the Affiliated Hospital of Qingdao University from September 1, 2020 to September 1, 2021. The diagnosis of IBD was based on clinical, hematological, endoscopic, and pathological examination.
The inclusion of the subjects in the study was IBD patients whose age between 18 to 60 years to avoid age-related primary sarcopenia. This decision was based on the Chinese law on the protection of the rights and interests of the elderly, which defines 60 years as the onset of old age (13). Exclusion criteria were as follows: (I) the presence of comorbidities, such as cancer, liver diseases, or severe organ damage, such as nephrotic syndrome or proteinuria, which could reduce potential nutritional status; and (II) incomplete bioelectrical impendence analysis (BIA) results.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (No. QYFY WZLL 26290) and informed consent was taken from all the patients. The study protocol has been registered in the Chinese Clinical Trial Registry (identifier: ChiCTR2100045179).
Clinical, laboratory, and prognostic parameters
Participants’ socio-demographic, clinical, laboratory, and prognostic data (including surgery, re-hospitalization due to recurrence, and death within 90 days) were recorded. Laboratory data were recorded based on single measurements. Socio-demographic and clinical data included sex, age, body mass index (BMI), history of smoking and alcohol consumption, history of gastrointestinal surgery, parenteral manifestations, and IBD-related complications. Laboratory data included albumin (Alb), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), fasting blood glucose, total cholesterol (TC), total triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL), urea nitrogen (BUN), creatinine (Cr), hemoglobin (Hb), platelets (PLT), and white blood cells (WBC).
Evaluation of skeletal muscle function
Skeletal muscle function was assessed through handgrip strength at baseline. Handgrip strength was measured using a BIOM-H500+X5 standard measuring tool (Biometrics, London, UK). Participants were seated with their feet placed naturally on the ground, knees bent, and hips bent at 90°. They held the grip meter with their dominant hand and maintained a neutral shoulder adduction position. Elbow flexion was at 90° with the participant’s upper arm flat to their chest and forearm in a neutral position. Wrist extension was 0–30°, i.e., at a 0–15° angle to the ulna. Participants squeezed the grip with full force, and their grip strength was recorded. Besides, the low physical performance was defined as achieving a 6-meter walk at <1.0 m/s or Short Physical Performance Battery ≤9 (14).
Evaluation of skeletal muscle mass
BIA was used to analyze body composition, including fat mass, muscle mass, visceral fat area, and waistline circumference. Professionally trained testers input participants’ age, sex, height, and weight to an InBody S10 body component analyzer (Biospace Ltd., Seoul, Korea). Participants were barefoot with their feet and hands in close contact with the corresponding sensing area. They maintained the same posture during the entire measurement process.
Sarcopenia, pre-sarcopenia, and sarcopenic obesity assessment
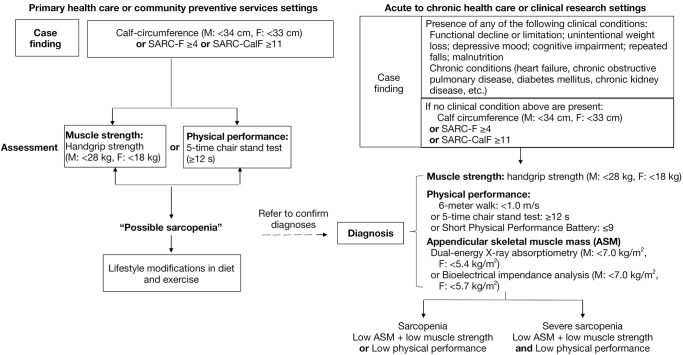
The Asian Working Group for Sarcopenia 2019 (AWGS2019) criteria were used to define sarcopenia (15) as low skeletal muscle mass (skeletal muscle index <7.0 kg/m2 for males, or <5.7 kg/m2 for females) together with low muscle function (handgrip strength <28 kg for males, and <18 kg for females) (Figure 1).
Figure 1.
Process of identifying sarcopenia using the AWGS criteria (15). SARC-F, strength, assistance in walking, rising from a chair, climbing stairs, and falls; SARC-CalF, adding calf circumference on the basis of SARC-F; AWGS, Asian Working Group for Sarcopenia.
Pre-sarcopenia was defined as low muscle mass (skeletal muscle index <7.0 kg/m2 for males, or <5.7 kg/m2 for females) without low muscle function (13). Severe sarcopenia was defined as sarcopenia with low physical performance. Sarcopenic obesity was defined as sarcopenia together with a body fat percentage >27% and >38% in men and women, respectively (14,16).
Follow-up
Participants were followed up for 90 days from the day they discharged from hospital through telephone or outpatient. During that time, information as to whether the symptoms improved (such as reduced diarrhea, abdominal pain and distension), treatment plans changed (adjustment to hormonal or biologic therapy), underwent surgery due to conservative treatment failed, were readmitted to the hospital due to recurrence, or died was recorded (8,17).
Statistical analysis
Quantitative variables are presented herein as mean ± standard deviation if normally distributed and as median and interquartile range when not normally distributed. Quantitative data were checked using IBM SPSS Statistics version 25.0 (IBM SPSS Inc., Chicago, IL, USA). When the P value of Kolmogorov Smirnov test was more than 0.05, the data were considered normally distributed. Qualitative variables are presented as numbers or percentages. A chi-square (χ2) test was used to compare categorical variables between the groups. A t-test was used for quantitative variables, and a Mann-Whitney U or Wilcoxon rank-sum test was used when normal distribution and homogeneity of variance did not meet the requirements of the t-test. Binary logistic regression was used for univariate analysis, factor with a P value <0.05 considered statistically significant. Statistically significant factors in the univariate analysis were then used as input data for multivariate analysis with P value <0.05 considered statistically significant. Cumulative survival analysis was done to evaluate the relationship between time to re-hospitalization and various subgroups. The odds ratios (ORs) and 95% confidence intervals (CIs) were also included in the analysis. The independent variables included socio-demographic, clinical, prognostic, and laboratory data, skeletal muscle mass, and function testing. The dependent variables included sarcopenia or poor outcomes during follow-up. All analyses were conducted using SPSS25.0 software (IBM).
Results
Demographic data of the participants with IBD diagnosed with sarcopenia according to the AWGS2019 criteria
A total of 110 consecutive patients with IBD, including 65 men (59%) and 45 women (41%) with a median age of 45.5 years, were included in this study. Of these, 85 had UC, and 25 had CD. There were 65 participants with suspected sarcopenia according to their low calf circumferences or low strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) scores, or SARC-CalF (adding the calf circumference) scores while 45 patients not. The participants were divided into a control group (normal skeletal muscle mass and function), a pre-sarcopenia group, and a sarcopenia group according to their muscle mass and function. Among the 65 suspected sarcopenia patients, 3 patients had normal skeletal muscle mass and function, 62 patients had low muscle mass and/or muscle function. Of those 62 patients, 29 patients had low muscle mass without low muscle function, 33 patients had low muscle mass and low muscle function. Ultimately, 48 participants were included in the control group; 29 in the pre-sarcopenia group, with a prevalence of 44.6% (29 of 65); and 33 (33 of 65, 50.8%) in the sarcopenia group, including 23 with UC and 10 with CD. In the sarcopenia group, there were 14 participants with sarcopenic obesity and 19 with non-sarcopenic obesity; for severity, 9 with severe sarcopenia and 24 non-severe sarcopenia (Figure 2).
Figure 2.
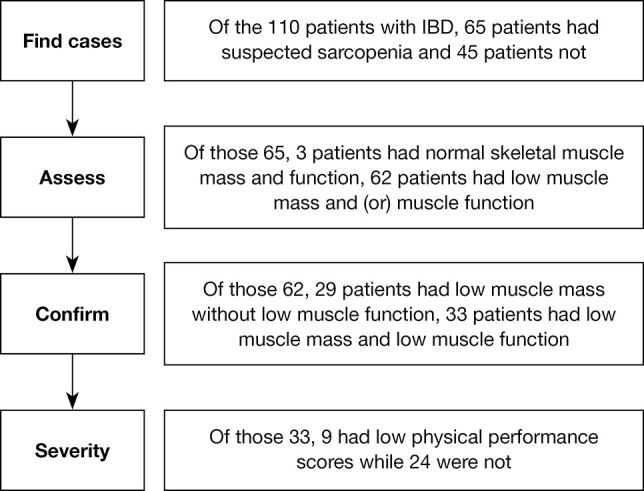
Process of diagnosing sarcopenia using the AWGS2019 criteria. IBD, inflammatory bowel disease; AWGS, Asian Working Group for Sarcopenia.
Clinicopathological characteristics among the pre-sarcopenia, sarcopenia, and control groups
Compared with the control group, the pre-sarcopenia group had lower BMIs (P=0.016), and a history of more parenteral manifestations (P=0.008).
In turn, participants in the sarcopenia group had lower BMIs (P<0.001, P<0.001), Alb levels (P<0.001, P<0.001), and a history of more frequent alcohol consumption (P<0.001, P<0.001), parenteral manifestations (P<0.001, P=0.002), and IBD-related complications (P<0.001, P<0.001) than the control and pre-sarcopenia groups. They also had higher CRP levels (P<0.001, P=0.002) and ESRs (P<0.001, P<0.001) (Table 1). Compared to the control group, patients in the sarcopenia group had a history of more frequent previous gastrointestinal surgery (P=0.020) and lower Hb (P=0.001). Patients in the control group had more medical improvement (P=0.023) such as reduced pus and blood defecation, lesser abdominal pain or abdominal distention than before.
Table 1. Comparison of the control, pre-sarcopenia, and sarcopenia groups.
| Factors | Control (n=48) | Pre-sarcopenia (n=29) | Sarcopenia (n=33) | P value (control vs. pre-sarcopenia) | P value (control vs. sarcopenia) | P value (pre-sarcopenia vs. sarcopenia) |
|---|---|---|---|---|---|---|
| Sex | 0.139 | 0.010** | 0.316 | |||
| Male | 35 | 16 | 14 | |||
| Female | 13 | 13 | 19 | |||
| Age (years) | 46.44±12.20 | 44.17±12.27 | 39.70±17.78 | 0.433 | 0.064 | 0.249 |
| BMI (kg/m2) | 23.56±2.17 | 21.86±3.2 | 19.11±2.73 | 0.016* | <0.001** | <0.001*** |
| Smoking history | 5 (10.42%) | 5 (17.24%) | 2 (6.06%) | 0.489 | 0.695 | 0.237 |
| Alcohol consumption history | 7 (14.58%) | 5 (17.24%) | 22 (66.67%) | 0.755 | <0.001** | <0.001*** |
| Gastrointestinal surgery history | 9 (18.75%) | 11 (37.93%) | 14 (42.42%) | 0.063 | 0.020** | 0.719 |
| Parenteral manifestations | 2 (4.17%) | 7 (24.13%) | 21 (63.64%) | 0.008* | <0.001** | 0.002*** |
| IBD-related complications | 1 (2.08%) | 2 (6.89%) | 22 (66.67%) | 0.553 | <0.001** | <0.001*** |
| Alb (g/L) | 40.02±5.15 | 39.39±4.21 | 28.64±5.67 | 0.563 | <0.001** | <0.001*** |
| CRP (mg/L) | 4.35±4.19 | 5.81±4.77 | 23.39±28.88 | 0.164 | <0.001** | 0.002*** |
| ESR (mm/60 min) | 9.82±11.32 | 11.34±7.48 | 23.51±16.82 | 0.523 | <0.001** | <0.001*** |
| Fasting blood glucose (mmol/L) | 4.78±0.79 | 4.50±0.54 | 4.80±1.22 | 0.075 | 0.953 | 0.233 |
| TC (mmol/L) | 3.92±1.33 | 3.75±1.36 | 3.91±1.41 | 0.675 | 0.989 | 0.747 |
| TG (mmol/L) | 1.00±0.30 | 1.00±0.56 | 0.92±0.35 | 0.970 | 0.468 | 0.671 |
| HDL (mmol/L) | 1.25±0.46 | 1.37±0.38 | 1.20±0.44 | 0.345 | 0.754 | 0.250 |
| LDL (mmol/L) | 2.43±0.72 | 2.33±0.68 | 2.21±0.55 | 0.634 | 0.345 | 0.586 |
| ALT (IU/L) | 25.43±54.79 | 14.14±6.17 | 13.48±10.35 | 0.104 | 0.078 | 0.811 |
| AST (IU/L) | 16.80±6.39 | 15.48±4.48 | 14.94±11.45 | 0.163 | 0.189 | 0.978 |
| ALP (IU/L) | 65.87±27.07 | 62.96±16.61 | 62.84±16.49 | 0.610 | 0.574 | 0.914 |
| TBIL (µmol/L) | 12.83±7.4 | 10.21±4.19 | 10.05±6.81 | 0.053 | 0.090 | 0.685 |
| BUN (mmol/L) | 5.04±2.29 | 4.84±1.15 | 4.60±3.01 | 0.677 | 0.465 | 0.685 |
| Cr (µmol/L) | 59.86±14.53 | 55.47±11.19 | 63.88±49.86 | 0.168 | 0.655 | 0.377 |
| Hb (g/L) | 129.67±19.05 | 120.38±26.90 | 110.72±26.0 | 0.110 | 0.001** | 0.156 |
| PLT (×109/L) | 269.85±93.51 | 292.21±106.28 | 308.48±104.12 | 0.338 | 0.092 | 0.545 |
| WBC (×109/L) | 6.52±2.04 | 7.09±2.78 | 7.95±3.78 | 0.306 | 0.054 | 0.317 |
| Follow-up | ||||||
| Improvement | 22 | 10 | 7 | 0.327 | 0.023** | 0.243 |
| Changing treatment plans | 25 | 12 | 11 | 0.362 | 0.095 | 0.513 |
| Undergoing surgery | 1 | 6 | 16 | 0.010* | <0.001** | 0.022*** |
| Re-hospitalization due to recurrence | 2 | 7 | 16 | 0.008* | <0.001** | 0.048*** |
| Death | 0 | 1 | 6 | 0.377 | 0.003** | 0.109 |
Laboratory data were collected at baseline and based on a single measurement. *, control vs. pre-sarcopenia, P<0.05 was considered statistically significant; **, control vs. sarcopenia, P<0.05 was considered statistically significant; ***, pre-sarcopenia vs. sarcopenia, P<0.05 was considered statistically significant. BMI, body mass index; IBD, inflammatory bowel disease; Alb, albumin; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TC, total cholesterol; TG, total triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TBIL, total bilirubin; BUN, urea nitrogen; Cr, creatinine; Hb, hemoglobin; PLT, platelets; WBC, white blood cells.
Lower BMI and Alb levels as risk factors of developing sarcopenia among IBD patients
Statistically significant factors in the univariate analysis, including sex (P=0.022), BMI (P<0.001), history of alcohol consumption (P<0.001), parenteral manifestations (P<0.001), IBD-related complications (P<0.001), Alb levels (P<0.001), CRP levels (P=0.001), ESRs (P<0.001), and Hb levels (P=0.004), were used for in the multivariate logistic regression analysis. A lower BMI (β =−0.766; P=0.018; OR =0.449) and Alb level (β =−0.482; P=0.004; OR =0.608) were found to be risk factors for developing sarcopenia among patients with IBD (Table 2).
Table 2. Risk factors for sarcopenia in patients with IBD.
| Factors | Univariate logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|---|
| β | OR | P value | β | OR | P value | ||
| Sex | 0.979 | 2.662 | 0.022* | – | – | – | |
| BMI (kg/m2) | −0.498 | 0.607 | <0.001* | −0.766 | 0.449 | 0.018* | |
| Alcohol consumption history | 2.383 | 10.833 | <0.001* | – | – | – | |
| Gastrointestinal surgery history | 0.742 | 2.100 | 0.090 | – | – | – | |
| Parenteral manifestations | 2.582 | 13.222 | <0.001* | – | – | – | |
| IBD-related complications | 3.899 | 49.333 | <0.001* | – | – | – | |
| Alb (g/L) | −0.491 | 0.613 | <0.001* | −0.482 | 0.608 | 0.004* | |
| CRP (mg/L) | 0.111 | 1.117 | 0.001* | – | – | – | |
| ESR (mm/60 min) | 0.094 | 1.099 | <0.001* | – | – | – | |
| Hb (g/L) | −0.026 | 0.974 | 0.004* | – | – | – | |
*, P<0.05 was considered statistically significant. OR, odds ratio; β, regression coefficient; BMI, body mass index; IBD, inflammatory bowel disease; Alb, albumin; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin.
BIA of the UC and CD groups
The BIA-based body compositions of participants with UC and CD were compared. Results showed that those with CD had lower skeletal muscle mass (P<0.001) and left and right upper arm skeletal muscle mass (P=0.021, P=0.026) than those with UC (Table 3).
Table 3. Comparison of body compositions in UC and CD patients.
| Factors | UC patients | CD patients | P value |
|---|---|---|---|
| Protein (kg) | 10.7±2.7 | 10.0±2.5 | 0.261 |
| Fat (kg) | 16.5±5.8 | 15.7±5.3 | 0.523 |
| Skeletal muscle mass (kg) | 25.7±4.6 | 22.5±2.9 | <0.001* |
| Segmental muscle (kg) | |||
| Right arm | 2.7±0.8 | 2.3±0.5 | 0.026* |
| Left arm | 2.7±0.8 | 2.2±0.5 | 0.021* |
| Body | 21.2±3.9 | 20.1±2.9 | 0.181 |
| Right leg | 7.5±1.6 | 7.4±1.4 | 0.702 |
| Left leg | 7.5±1.5 | 7.3±1.3 | 0.587 |
| Segmental water (kg) | |||
| Right arm | 2.1±0.5 | 1.9±0.5 | 0.414 |
| Left arm | 2.1±0.5 | 1.9±0.5 | 0.432 |
| Body | 16.8±3.4 | 16.7±2.7 | 0.941 |
| Right leg | 6.2±1.2 | 6.2±1.1 | 0.957 |
| Left leg | 6.3±1.2 | 6.2±1.1 | 0.992 |
| Body cell mass (kg) | 30.3±5.0 | 29.9±4.3 | 0.688 |
| Waistline circumference (cm) | 78.1±9.9 | 74.5±6.9 | 0.099 |
| Visceral fat area (cm2) | 73.6±28.5 | 67.7±18.9 | 0.226 |
| Basal metabolic rate (kcal) | 1,391.6±165.7 | 1,402.5±182.7 | 0.778 |
| Bilateral upper arm girth (cm) | |||
| Left | 25.7±3.9 | 24.8±4.5 | 0.340 |
| Right | 25.3±3.8 | 24.0±4.6 | 0.176 |
| Skinfold thickness (mm) | |||
| Left | 14.1±3.5 | 13.5±2.8 | 0.397 |
| Right | 13.4±3.1 | 13.0±2.6 | 0.604 |
BIA was performed for patients with UC and CD, and body composition parameters, including protein, fat, skeletal muscle mass, segmental muscle and water, body cell mass, waistline circumference, visceral fat area, basal metabolic rate, and bilateral upper arm girth and skinfold thickness, were recorded. *, P<0.05 was considered statistically significant. UC, ulcerative colitis; CD, Crohn’s disease; BIA, bioelectrical impendence analysis.
Sarcopenia diagnosed according to the AWGS2019 criteria is associated with poor clinical outcomes in patients with IBD
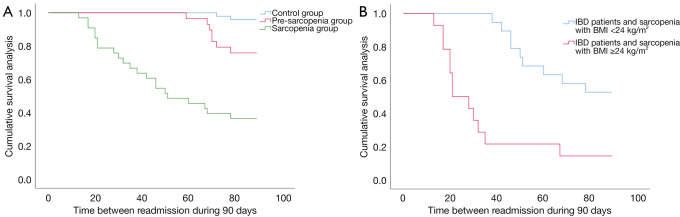
During the 90-day follow-up, participants in the pre-sarcopenia group underwent surgery more frequently (P=0.010; OR =12.261; 95% CI: 1.393–107.918) and had higher re-hospitalization rates (P=0.008; OR =7.318; 95% CI: 1.403–38.165) than those in the control group. Participants in the sarcopenia group also underwent surgery more frequently (P<0.001; OR =6.651; 95% CI: 2.333–18.959) and had higher re-hospitalization (P<0.001; OR =6.344; 95% CI: 2.874–14.003) and death rates (P=0.003) than those in the control group. In addition, participants in the sarcopenia group underwent surgery more frequently (P=0.022; OR =3.608; 95% CI: 1.167–11.151) and had higher re-hospitalization rates (P=0.048; OR =5.500; 95% CI: 1.817–16.646) than those in the pre-sarcopenia group, and participants with severe sarcopenia underwent surgery more frequently (P<0.001) and had higher re-hospitalization due to recurrence (P=0.012) than those with non-severe sarcopenia (Table 4). Time between re-hospitalization to hospital gradually decreased in the control group, pre-sarcopenia group and sarcopenia group during the follow-up (89 vs. 85 vs. 57 days, P<0.001) (Figure 3A).
Table 4. The relationship between sarcopenia diagnosed using the AWGS2019 criteria and surgery, re-hospitalization, and death.
| Subgroups | Surgery | Re-hospitalization | Death |
|---|---|---|---|
| Control group | 1 (2.08%) | 2 (4.17%) | 0 (0.0%) |
| Pre-sarcopenia group | 6 (20.69%) | 7 (24.14%) | 1 (3.44%) |
| P value | 0.010* | 0.008* | 0.377 |
| OR | 12.261 | 7.318 | – |
| 95% CI | 1.393–107.918 | 1.403–38.165 | – |
| Control group | 1 (2.08%) | 2 (4.17%) | 0 (0.0%) |
| Sarcopenia group | 16 (48.48%) | 16 (48.48%) | 6 (18.18%) |
| P value | <0.001* | <0.001* | 0.003* |
| OR | 6.651 | 6.344 | – |
| 95% CI | 2.333–18.959 | 2.874–14.003 | – |
| Pre-sarcopenia group | 6 (20.69%) | 7 (24.14%) | 1 (3.44%) |
| Sarcopenia group | 16 (48.48%) | 16 (48.48%) | 6 (18.18%) |
| P value | 0.022* | 0.048* | 0.109 |
| OR | 3.608 | 5.500 | 6.222 |
| 95% CI | 1.167–11.151 | 1.817–16.646 | 0.702–55.155 |
| Severe sarcopenia group | 9 (100%) | 9 (100%) | 3 (33.3%) |
| Non-severe sarcopenia group | 7 (29.17%) | 7 (29.17%) | 3 (12.5%) |
| P value | <0.001* | 0.012* | 0.188 |
| OR | – | – | 3.500 |
| 95% CI | – | – | 0.556–22.029 |
| Sarcopenic obesity group | 13 (92.86%) | 11 (78.57%) | 4 (28.57%) |
| Non-sarcopenic obesity group | 3 (15.79%) | 5 (26.32%) | 2 (10.53%) |
| P value | <0.001* | 0.003* | 0.192 |
| OR | 69.333 | 6.667 | – |
| 95% CI | 6.246–748.060 | 1.162–38.247 | – |
*, P<0.05 was considered significantly different. AWGS, Asian Working Group for Sarcopenia; OR, odds ratio; CI, confidence interval.
Figure 3.
Cumulative survival analyses of time between re-hospitalization in subgroups. (A) Cumulative survival analysis in the control group, pre-sarcopenia group and sarcopenia group. (B) Cumulative survival analysis of time in sarcopenic obesity group and non-sarcopenic obesity group. IBD, inflammatory bowel disease; BMI, body mass index.
Sarcopenic obesity diagnosed according to AWGS2019 criteria
In the sarcopenia group, there were 14 participants with sarcopenic obesity. During the 90-day follow-up, these participants underwent surgery more frequently (92.86%; P<0.001; OR =69.333; 95% CI: 6.246–748.060) and had higher rates of re-hospitalization due to recurrence (78.57%; P=0.003; OR =6.667; 95% CI: 1.162–38.247) than those in the group who did not have sarcopenic obesity (Table 4). Participants with sarcopenic obesity were readmitted to hospital due to IBD recurrence sooner than their counterparts without sarcopenic obesity (35 vs. 72 days, respectively, P<0.001) (Figure 3B).
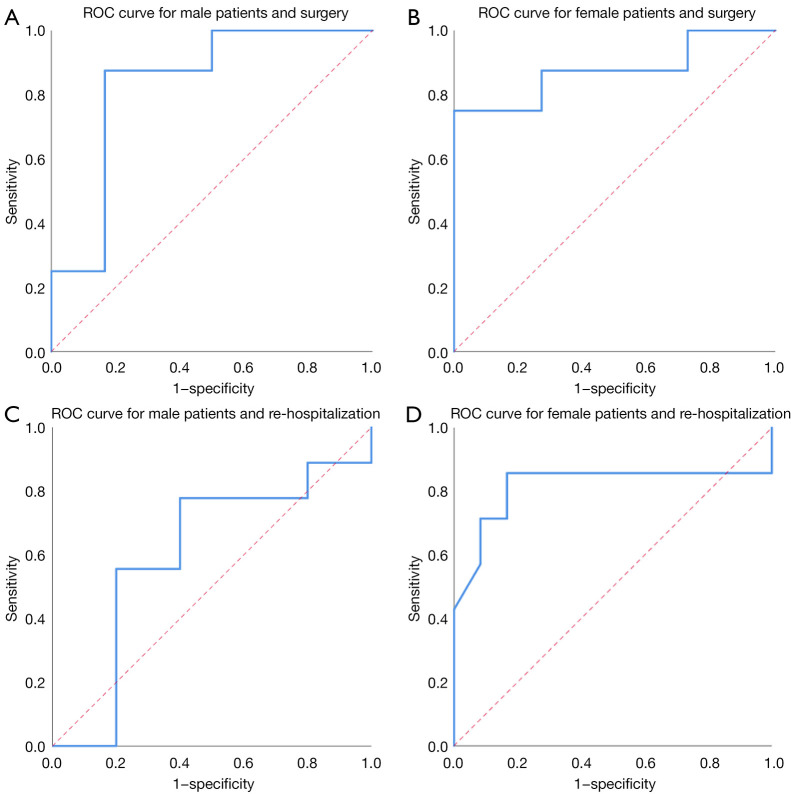
Based on the above results, receiver operating characteristic (ROC) curves were created to evaluate the predictive value of body fat percentage for surgery and re-hospitalization. For surgery, the area under the ROC curve was 0.833, with P=0.039, 95% CI: 0.590–1.000 and a cutoff value of 0.248 in men (Figure 4A), and 0.875, with P=0.006, 95% CI: 0.692–1.000 and a cutoff value of 0.320 in women (Figure 4B). For re-hospitalization, the area under the ROC curve was 0.600, with P=0.549 in men (Figure 4C), and 0.815, with a significance level of P=0.025, 95% CI: 0.556–1.000 and a cutoff value of 0.245 in women (Figure 4D). Patients with IBD whose body fat percentages were ≥24.8% (for males) and 32.0% (for females) were more likely to receive surgery. Female patients with a body fat percentage ≥24.5% were more likely to experience re-hospitalization due to recurrence.
Figure 4.
ROC curves for surgery, re-hospitalization in male and female patients. (A) ROC curve for male patients and surgery. (B) ROC curve for female patients and surgery. (C) ROC curve for male patients and re-hospitalization. (D) ROC curve for female patients and re-hospitalization. ROC, receiver operating characteristic.
Discussion
Sarcopenia was originally defined by Rosenberg as the loss of lean muscle coupled with a loss in muscle strength (8,9). The primary pathogenetic factor for sarcopenia is aging (18), while secondary pathogenetic factors include diseases such as IBD (19), osteoarthritis (2), long-term inactivity (20), and nonalcoholic fatty liver disease (21-24). In patients with IBD, sarcopenia may be caused by self-imposed food intake restrictions to prevent disease flare-ups, malabsorption, excessive loss of nutrients, or increased demand for nutrients (25). In this study, the prevalence of sarcopenia diagnosed according to the AWGS2019 criteria was 50.8% in 65 cases of suspected sarcopenia. Of those diagnosed with sarcopenia, 69.6% had poor outcomes. These results imply that sarcopenia is common among patients with IBD (25). Therefore, early screening, diagnosis, and intervention should be performed to improve the prognosis.
In clinical practice, early detection of sarcopenia based on common clinical data is not easy, suggesting a need for further evaluation in cases of suspected sarcopenia, using appropriate criteria (23,26). We used the AWGS2019 criteria, which emphasize the need to combine muscle mass and function in assessing sarcopenia (13). Previous studies have shown that sarcopenia may cause poor outcomes, including altered body composition (27,28), a higher proportion of complications after operations, longer hospital stays and higher costs, and a decreased quality of life in patients with IBD (2,4,24,29). Muscle function plays an important role in the prognosis for patients with IBD because it degrades more rapidly than muscle mass and may be used for early assessment and prevention of sarcopenia (22).
In this study, participants with sarcopenia, especially those with severe sarcopenia, had poorer clinical outcomes, including surgery, re-hospitalization due to recurrence, and death, than the control and pre-sarcopenia groups. We found that lower BMI and Alb levels were risk factors for developing sarcopenia. This is a novel finding compared with previous studies. Given that IBD is a chronic autoimmune disease, high CRP levels and ESRs may reflect more severe inflammatory responses (30,31), whereas low BMI and low Alb levels may imply poor nutritional status (22,32). Severe inflammatory responses and poor nutritional status may be caused by intestinal mucosal impairment, limited nutrient absorption, and a state of overconsumption, all of which can cause sarcopenia (4,17). These are all easily accessible clinical data, which can help clinicians to detect sarcopenia early in patients with IBD. This study showed that IBD patients with and without sarcopenia differed in many characteristics, including sex, BMI, and levels of Alb, CRP, ESR, and Hb, consistent with previous studies (1,19,29).
In this study, participants with sarcopenic obesity, especially those with body fat percentages ≥24.8% and 32.0% for males and females, respectively, were more likely to receive surgery, while female participants with a body fat percentage ≥24.5% were more likely to experience re-hospitalization due to recurrence. This is consistent with previous studies (1,26,33,34). Of note, the predictive value of BMI is limited to the evaluation of body fat (35). Patients with more body fat and low muscle mass may have a normal BMI but experience poor outcomes nonetheless. Thus, we used body fat percentage assessed by BIA rather than traditional BMI or nutritional screening methods to diagnose sarcopenic obesity. Based on our results, we believe it is necessary to include body composition analysis in clinical nutrition assessment and develop individualized therapeutic strategies to improve the prognosis of patients with IBD.
This study had several advantages. First, 110 patients with IBD were included in the study cohort, which is more than in previous studies (36-38). Second, this study used the AWGS2019 criteria to diagnose sarcopenia, which is novel, as few studies of sarcopenia have used this approach (36-40). Third, this study also found that low BMI and low Alb levels were risk factors for developing sarcopenia. Nevertheless, there also were some limitations. First, this was a single-center study and thus cannot represent most patients with IBD. Second, the follow-up time was not long. Multicenter studies with longer follow-up times are needed.
Conclusions
This study showed that sarcopenia, as defined by the AWGS2019 criteria, was common among patients with IBD. Those with sarcopenia and sarcopenic obesity had poorer clinical outcomes than those without sarcopenia. This finding implies that muscle function plays an important role in the outcomes of patients with IBD. The AWGS2019 criteria, which combine muscle mass and function, are a helpful and comprehensive guide to predicting clinical outcomes in patients with IBD.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Gastroenterology Collaborative Group. We thank LetPub (www.letpub.com) for linguistic assistance and an expert pre-submission review.
Funding: This work was supported by the China Postdoctoral Science Foundation (grant No. 2019M652332).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (No. QYFY WZLL 26290) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1126/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1126/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1126/coif). GM reports participation on Albireo PFIC and cholestatic liver disease Advisory board in January 2022 and Alexion Advisory Board Wilson-Webmeeting in December 2021. The other authors have no conflicts of interest to declare.
References
- 1.Adams DW, Gurwara S, Silver HJ, et al. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm Bowel Dis 2017;23:1182-6. 10.1097/MIB.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 2.Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28-36. 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan E, McNicholas D, Creavin B, et al. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm Bowel Dis 2019;25:67-73. 10.1093/ibd/izy212 [DOI] [PubMed] [Google Scholar]
- 4.Li S, Ney M, Eslamparast T, et al. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J Gastroenterol 2019;25:3823-37. 10.3748/wjg.v25.i28.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430-8. 10.1016/j.cgh.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006;81:1462-71. 10.4065/81.11.1462 [DOI] [PubMed] [Google Scholar]
- 7.Vatn MH, Sandvik AK. Inflammatory bowel disease. Scand J Gastroenterol 2015;50:748-62. 10.3109/00365521.2015.1033000 [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 2013;93:201-10. 10.1007/s00223-013-9757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S-1S. 10.1093/jn/127.5.990S [DOI] [PubMed] [Google Scholar]
- 10.Pedersen M, Cromwell J, Nau P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1867-72. 10.1097/MIB.0000000000001166 [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636-46. 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 12.Ünal NG, Oruç N, Tomey O, et al. Malnutrition and sarcopenia are prevalent among inflammatory bowel disease patients with clinical remission. Eur J Gastroenterol Hepatol 2021;33:1367-75. 10.1097/MEG.0000000000002044 [DOI] [PubMed] [Google Scholar]
- 13.Giallauria F, Cittadini A, Smart NA, et al. Resistance training and sarcopenia. Monaldi Arch Chest Dis 2016;84:738. 10.4081/monaldi.2015.738 [DOI] [PubMed] [Google Scholar]
- 14.Endo T, Akai K, Kijima T, et al. An association analysis between hypertension, dementia, and depression and the phases of pre-sarcopenia to sarcopenia: A cross-sectional analysis. PLoS One 2021;16:e0252784. 10.1371/journal.pone.0252784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-07.e2. 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437-48. 10.1111/j.1749-6632.2000.tb06498.x [DOI] [PubMed] [Google Scholar]
- 17.Erős A, Soós A, Hegyi P, et al. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: a meta-analysis. Surg Today 2020;50:1138-50. 10.1007/s00595-019-01893-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamba S, Sasaki M, Takaoka A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn's disease. PLoS One 2017;12:e0180036. 10.1371/journal.pone.0180036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang MK, Kim KO, Kim MC, et al. Sarcopenia Is a New Risk Factor of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Dig Dis 2020;38:507-14. 10.1159/000506938 [DOI] [PubMed] [Google Scholar]
- 21.Cawthon PM, Lui LY, Taylor BC, et al. Clinical Definitions of Sarcopenia and Risk of Hospitalization in Community-Dwelling Older Men: The Osteoporotic Fractures in Men Study. J Gerontol A Biol Sci Med Sci 2017;72:1383-9. 10.1093/gerona/glw327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balestrieri P, Ribolsi M, Guarino MPL, et al. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020;12:372. 10.3390/nu12020372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingworth TW, Oke SM, Patel H, et al. Getting to grips with sarcopenia: recent advances and practical management for the gastroenterologist. Frontline Gastroenterol 2021;12:53-61. 10.1136/flgastro-2019-101348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaap LA, van Schoor NM, Lips P, et al. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci 2018;73:1199-204. 10.1093/gerona/glx245 [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa H, Nakamura S, Miyazaki T, et al. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J Clin Med 2021;10:4214. 10.3390/jcm10184214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt DQ, Moore GT, Strauss BJ, et al. Visceral adiposity predicts post-operative Crohn's disease recurrence. Aliment Pharmacol Ther 2017;45:1255-64. 10.1111/apt.14018 [DOI] [PubMed] [Google Scholar]
- 27.Thangarajah D, Hyde MJ, Konteti VK, et al. Systematic review: Body composition in children with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:142-57. 10.1111/apt.13218 [DOI] [PubMed] [Google Scholar]
- 28.Tinsley A, Ehrlich OG, Hwang C, et al. Knowledge, Attitudes, and Beliefs Regarding the Role of Nutrition in IBD Among Patients and Providers. Inflamm Bowel Dis 2016;22:2474-81. 10.1097/MIB.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Cao L, Cao T, et al. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn's Disease Undergoing Bowel Resection. JPEN J Parenter Enteral Nutr 2017;41:592-600. 10.1177/0148607115612054 [DOI] [PubMed] [Google Scholar]
- 30.Dhaliwal A, Quinlan JI, Overthrow K, et al. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021;13:656. 10.3390/nu13020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An HJ, Tizaoui K, Terrazzino S, et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int J Mol Sci 2020;21:5678. 10.3390/ijms21165678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertani L, Ribaldone DG, Bellini M, et al. Inflammatory Bowel Diseases: Is There a Role for Nutritional Suggestions? Nutrients 2021;13:1387. 10.3390/nu13041387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamba S, Inatomi O, Takahashi K, et al. Assessment of Body Composition From CT Images at the Level of the Third Lumbar Vertebra in Inflammatory Bowel Disease. Inflamm Bowel Dis 2021;27:1435-42. 10.1093/ibd/izaa306 [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, Ding C, Xie T, et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin Nutr 2017;36:1586-92. 10.1016/j.clnu.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez MC, Correia MITD, Heymsfield SB. A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care 2017;20:314-21. 10.1097/MCO.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 36.Atlan L, Cohen S, Shiran S, et al. Sarcopenia is a Predictor for Adverse Clinical Outcome in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 2021;72:883-8. 10.1097/MPG.0000000000003091 [DOI] [PubMed] [Google Scholar]
- 37.Boparai G, Kedia S, Kandasamy D, et al. Combination of sarcopenia and high visceral fat predict poor outcomes in patients with Crohn's disease. Eur J Clin Nutr 2021;75:1491-8. 10.1038/s41430-021-00857-x [DOI] [PubMed] [Google Scholar]
- 38.Grillot J, D'Engremont C, Parmentier AL, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn's disease. Clin Nutr 2020;39:3024-30. 10.1016/j.clnu.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 39.Labarthe G, Dolores M, Verdalle-Cazes M, et al. Magnetic resonance imaging assessment of body composition parameters in Crohn's disease. Dig Liver Dis 2020;52:878-84. 10.1016/j.dld.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 40.Galata C, Hodapp J, Weiß C, et al. Skeletal Muscle Mass Index Predicts Postoperative Complications in Intestinal Surgery for Crohn's Disease. JPEN J Parenter Enteral Nutr 2020;44:714-21. 10.1002/jpen.1696 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as