We review our current understanding of adaptive immune responses to the fetoplacental allograft, emphasizing the divergent responses observed for trophoblast- versus fetal blood cell–derived antigens. We suggest these divergent responses arise from cell type–specific glycosylation programs that impart trophoblast-derived antigens with immunosuppressive properties.
Abstract
The paradox of fetomaternal tolerance has puzzled immunologists and reproductive biologists alike for almost 70 yr. Even the idea that the conceptus evokes a uniformly tolerogenic immune response in the mother is contradicted by the long-appreciated ability of pregnant women to mount robust antibody responses to paternal HLA molecules and RBC alloantigens such as Rh(D). Synthesizing these older observations with more recent work in mice, we discuss how the decision between tolerance or immunity to a given fetoplacental antigen appears to be a function of whether the antigen is trophoblast derived—and thus decorated with immunosuppressive glycans—or fetal blood cell derived.
Introduction
The conceptus, comprised of the placenta and the fetus proper, is not genetically identical to the mother yet fails to induce the rejection response traditionally observed for allogeneic organ transplants. Nearly 70 yr ago, Peter Medawar recognized this apparent paradox and posited three possible explanations: (1) the conceptus is kept physically separate from maternal immune cells; (2) the maternal immune system is generally suppressed; and (3) the conceptus does not express rejection antigens (Medawar, 1953). These ideas have since been largely disqualified for the following reasons. First, the main functional unit of the placenta, i.e., the villous tree in humans and the labyrinth in mice, positions placental epithelial cells (trophoblasts) directly in the maternal bloodstream (see Fig. 1 for the architecture of a human placental villus). Moreover, other populations of trophoblasts invade the uterine lining (the “decidua”), where they can locally interact with maternal immune cells. Second, maternal immune responses to pathogens and experimental foreign antigens are essentially intact across gestation, thus ruling out generalized immunosuppression. Third, trophoblasts secrete proteins into the maternal circulation, and various trophoblast subtypes express a variety of potential alloantigens, including nonclassic MHC class Ib molecules (HLA-E and HLA-G; Apps et al., 2009), oncofetal antigens (Jungbluth et al., 2007), cell type–specific proteins (Moore and Dveksler, 2014), and ubiquitously expressed minor histocompatibility antigens such as H-Y protein (Holland et al., 2012; Linscheid and Petroff, 2013). While human trophoblasts are uniformly negative for HLA class II, HLA-A, and HLA-B, some subtypes express HLA-C (Apps et al., 2009; Hiby et al., 2010; Proll et al., 1999), whose mismatch alone can trigger bone marrow graft failure (Petersdorf et al., 1997). In mice, some trophoblast subtypes express low levels of H-2K/D (Erlebacher et al., 2007; Redline and Lu, 1989), but even transgene-directed expression of allogeneic H-2K at high levels in all trophoblasts does not compromise pregnancy (Rogers et al., 1998; Shomer et al., 1998).
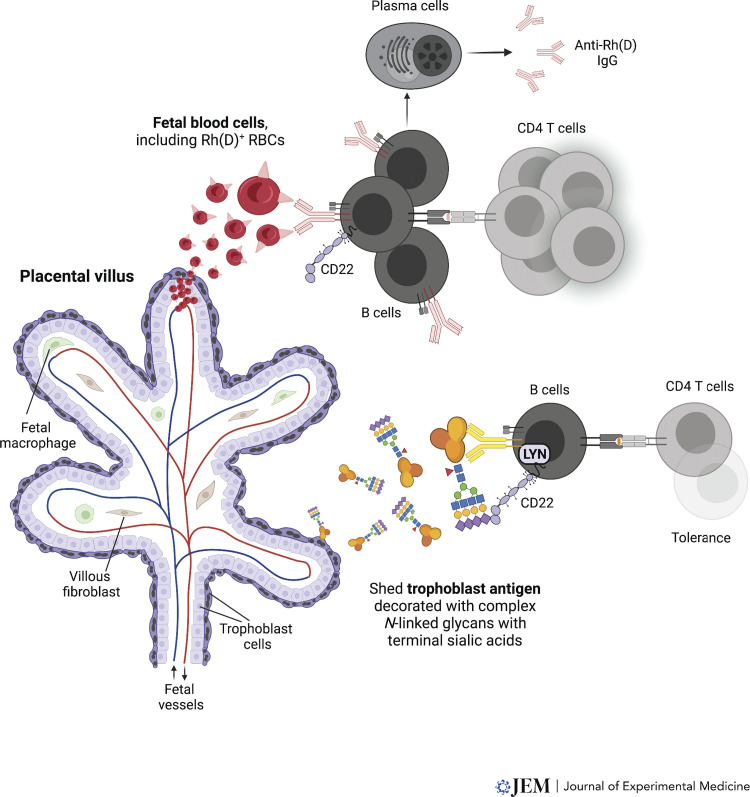
Figure 1.
Divergent responses to fetal blood cell antigens versus trophoblast antigens. Cartoon representation of a placental villus, showing that this is a trophoblast-lined structure that encases fetal blood vessels. The entire structure is bathed in maternal blood. During pregnancy, maternal immune cells encounter fetal blood cell antigens (upper, illustrating the specific case of Rh(D) antigen) and trophoblast-derived antigens (lower, illustrating a generic trophoblast antigen, modeled by t-mOVA in mice). Upper: Placental microhemorrhage releases Rh(D)+ fetal RBCs into the maternal circulation. The RBCs are then recognized by maternal Rh(D)-specific B cells, whose activation and differentiation into plasma cells likely involves cognate interaction with maternal Rh(D)-specific CD4 T helper cells, which presumably have also interacted with maternal DCs (not shown). Because Rh(D) antigen is not sialylated, B cell activation proceeds unimpeded. Ultimately, Rh disease results when the anti-Rh(D) IgG antibodies, transferred across the placenta by the neonatal Fc receptor, bind to the Rh(D)+ fetal RBCs and induce their lysis (not depicted). Although less well studied, the maternal antibody response to paternal HLA might occur similarly when placental microhemorrhage allows maternal B cells to encounter fetal white blood cells (not shown). Lower: Sialylated t-mOVA is shed into maternal circulation and is presented to CD4 T cells exclusively by antigen-specific B cells, whose activation is suppressed by concomitant engagement of CD22. CD22 recognizes α2,6-linked sialic acids and requires LYN kinase for signaling. Because B cell activation is suppressed, cognate CD4 T cell activation is also suppressed.
Thus, the paradox of fetomaternal tolerance has become focused on the more refined question of how an allograft that does express rejection antigens and does interact extensively with immune cells in an immune-competent host fails to elicit a traditional rejection response. Here we review our current understanding of systemic maternal B and T cell responses to the fetoplacental allograft, emphasizing the divergence in response apparent for antigens expressed by trophoblasts versus fetal blood cells and the potential role of cell type–specific protein glycosylation in explaining this divergence.
Maternal B and T cell responses to fetal blood cell antigens
Despite a varied architecture across mammals, the placenta forms an anatomic barrier between maternal and fetal circulation that separates fetal cells of nontrophoblast origin from the maternal immune system. However, in both humans and mice, antigenic quantities of fetal blood cells can enter maternal circulation and trigger maternal alloimmunization (Masson et al., 2013; Porrett, 2018; Urbaniak and Greiss, 2000). In both species, this exposure can occur during delivery, and in humans it can also occur during the third trimester as a result of occult placental microhemorrhage.
The classic example of a situation in which the mother becomes immunized to a fetal blood cell antigen is the clinical condition known as hemolytic disease of the fetus and newborn (HDFN), which is caused by maternal B cells that have reacted to alloantigens expressed by fetal RBCs (Webb and Delaney, 2018). Most cases of alloimmunization occur in women lacking the integral RBC membrane protein Rhesus (Rh)D antigen (Rh(D)− women) who are exposed to Rh(D)+ fetal blood during late gestation or delivery. HDFN, or Rh disease, as it is known when Rh(D) is the inciting antigen, can then occur when anti-Rh(D) IgG antibodies are transported across the placenta by the same pathway that provides protective passive immunization to the developing fetus. Maternal anti-Rh(D) antibodies then bind to fetal RBCs, which subjects them to antibody-dependent lysis. The clinical outcome is variable and ranges from mild anemia to fetal or neonatal death. Before the advent of preventive therapy, clinically significant HDFN occurred in ∼16% of gestations with maternal/fetal Rh incompatibility, or 1 in 1,000 total pregnancies, attesting to the pathogenicity of the response (Tovey, 1992; Urbaniak and Greiss, 2000). Although mice lack Rh(D), a mouse model of Rh disease was developed by Stowell et al. (2013), who expressed the human RBC KEL antigen on fetal RBCs that were otherwise syngeneic to the mother. Antigenic quantities of fetal blood cells enter the maternal circulation of mice only during delivery, and thus anti-KEL antibodies become measurable in the postpartum period and elicit a disease resembling HDFN in subsequent KEL+ gestations (Stowell et al., 2013).
Like Rh(D) antigen, paternal HLA molecules can also trigger maternal B cell responses. Anti-paternal HLA antibodies directed against the full set of paternal HLA molecules (HLA-A, -B, -C, and -DR) are identified in most multiparous women and are sometimes detectable in maternal plasma during the third trimester of a first pregnancy. This suggests that, in analogous fashion to the response to Rh(D), the anti-HLA response is driven by exposure to antigenic quantities of blood-borne, HLA-expressing fetal white blood cells as the result of placental microhemorrhage and/or delivery (Honger et al., 2013; Regan et al., 1991; Van Rood et al., 1958). It is also possible that paternal HLA molecules reach the maternal circulation via the transport of fetal cell–derived exosomes across the placenta. However, unlike anti-Rh(D) antibodies, anti-HLA antibodies do not overtly compromise fetal or placental health. Currently, we have no obvious explanation for why this is the case, especially since the same anti-HLA antibodies can mediate transfusion reactions and compromise organ transplantation in multiparous women (Durgam et al., 2022; Porrett, 2018). An intriguing possibility raised by older literature is that placental HLA molecules, either the restricted set expressed by trophoblasts or the more extensive set expressed by placental fibroblasts and macrophages that populate the villous stroma, serve as an “immunoabsorbent” that prevents these antibodies from reaching the fetal circulation (Wegmann, 1981). Specifically, it was found that anti-paternal HLA antibodies could be detected in human placental eluents but not in fetal umbilical cord blood (Doughty and Gelsthorpe, 1974; Tongio et al., 1975; Tongio et al., 1983), and that injection of pregnant mice with radiolabeled allospecific anti–H-2 antibodies, as well as Fab fragments derived therefrom, accumulated in placentas expressing the H-2 target (Wegmann et al., 1979a; Wegmann et al., 1979b). Immunoabsorption per se, of course, does not explain why the antibodies do not damage placental cells (and as we discuss below, perhaps they sometimes do), but we note that both syncytiotrophoblasts in humans and the analogous cells in mice express high levels of regulator proteins that prevent local complement activation (Girardi et al., 2006; Tedesco et al., 1993; Xu et al., 2000).
Somewhat surprisingly, the nature of the maternal CD4 T cell response to Rh(D) and fetal HLA antigens has not been thoroughly addressed. Because maternal anti-Rh(D) and anti-HLA antibodies are class-switched, it has been presumed that maternal CD4 helper T cell responses are also mounted to Rh(D) or a physically associated RBC antigen (since Rh(D) is a component of an integral membrane protein complex). Such CD4 T cell responses are also consistent with the correlation between the number of paternal HLA-derived epitopes predicted to be available to stimulate CD4 T cells during pregnancy and the probability of generating anti-HLA antibodies (Geneugelijk et al., 2015). Moreover, CD8 T cells with reactivity towards paternal class I HLA have been detected in women with anti-paternal HLA antibodies (Bouma et al., 1996), suggesting the generation of T cell help that supports both CD8 T cell and B cell responses. In contrast, recent work showing that T cell help is dispensable for the generation of IgG antibodies to KEL antigen suggests that CD4 T cell–independent, class-switched responses may also occur (Mener et al., 2018).
Maternal B cell responses to trophoblast antigens
Considering the grave pathogenicity of maternal B cells with specificity toward fetal RBC antigens, the field naturally wondered about the nature of B cell responses to trophoblast-expressed antigens. Experiments performed around 1980 uncovered the presence of “noncytotoxic” anti-paternal IgG1 antibodies in multiparous mice, but closer examination revealed that they arose only in some strain mating combinations and only after multiple gestations (Bell and Billington, 1980; Bell and Billington, 1981). Similar, noncytotoxic anti-paternal IgG1 antibodies could be generated by vaccinating virgin females with crude placental but not fetal extracts (which instead induced a cytotoxic antibody response; Bell and Billington, 1986), suggesting that the IgG1 antibodies might be specific for trophoblast antigens. Along these lines, recently work by Suah et al. (2021) revealed the de novo production of antibodies reactive against paternal-strain spleen cells in the latter half of a first pregnancy in mice. As with the older work, levels of these antibodies were variable and even undetectable in some mice, and all responses were lower than those induced by skin grafting (Suah et al., 2021). Moreover, germinal center phenotype B cells were not detected, suggesting that the antibodies were produced by extrafollicular plasma cells, which canonically yield antibodies of lower affinity. Nonetheless, since mice do not mount responses to fetal RBCs until after delivery (as described above), the antigenic target cell in this case was most likely a trophoblast.
The behavior of maternal B cells with specificity toward a paternal alloantigen known to be expressed by trophoblasts was first directly examined in experiments using female mice bearing BCR transgenes encoding an IgM molecule with relative high affinity for a paternal strain H-2K (Ait-Azzouzene et al., 2001; Ait-Azzouzene et al., 1998). Although results should be interpreted with caution because of the supraphysiologic precursor frequency of monoclonal cells with such a relatively high affinity receptor, a 60–70% deletion of transgenic B cells was observed in maternal spleen, blood, and bone marrow at midgestation in antigen-matched matings but not in syngeneic or third-party matings (Ait-Azzouzene et al., 1998). Interestingly, when expression of the allo–H-2K molecule was restricted to trophoblast giant cells, deletion was observed for developing bone marrow B cells but not peripheral B cells (Ait-Azzouzene et al., 2001). Together, these findings suggest that cells expressing a BCR with high affinity for trophoblast antigen might be subject to deletion in the bone marrow, an obvious safeguard against the generation of high-affinity anti-trophoblast antibodies.
Our laboratory has recently examined the fate of the endogenous repertoire of peripheral B cells specific for a trophoblast antigen (Rizzuto et al., 2022). This work used a pregnancy model previously used to study T cell responses in which a paternally inherited transgene directs expression of a transmembrane form of chicken OVA (mOVA; Ehst et al., 2003) throughout the conceptus, with particularly high expression by trophoblasts positioned within the maternal bloodstream (Erlebacher et al., 2007). By using fluorescently labeled antigen tetramers to visualize OVA-specific B cells in the spleen (Taylor et al., 2012), we found that such B cells sensed trophoblast mOVA (t-mOVA) starting around midgestation, when the antigen starts being shed into the maternal circulation. Even in the presence of systemic adjuvant and T cell help, the OVA-specific B cells failed to expand and did not differentiate into germinal center cells. Strikingly, mOVA mating also rendered OVA-specific B cells unable to respond to vaccination with chicken-derived OVA (c-OVA), demonstrating that t-mOVA induced the suppression of antigen-specific B cells.
Because the protein coding sequence of c-OVA and trophoblast-derived OVA is identical, we evaluated whether a trophoblast-specific posttranslational modification could explain the divergent response of B cells to the two forms of the protein. Indeed, while both sources of OVA were decorated with N-linked glycans, a biochemical analysis revealed that t-OVA was more heavily glycosylated and that its glycans were terminated with α2,6-linked and α2,3-linked sialic acid residues (Rizzuto et al., 2022). These sialic acids can serve as “self” ligands by binding to sialic acid–binding Ig-like lectins (Siglecs), a family of transmembrane proteins that typically inhibit immune cell activation (Laubli and Varki, 2020). Accordingly, we found that OVA-specific B cells were no longer suppressed in mice lacking the B cell–specific inhibitory Siglec, CD22, which recognizes α2,6-linked sialic acids, and that the maternal B cell response to t-mOVA was fully unleashed in pregnant mice lacking LYN, the Src family tyrosine kinase member uniquely required for CD22 inhibitory activity (Rizzuto et al., 2022; Smith et al., 1998). That the response to trophoblast antigen was more dramatic in Lyn−/− versus Cd22−/− pregnant females hints at engagement of additional inhibitory receptor(s) upstream of LYN, such as Siglec-G (Nitschke, 2014).
These observations suggest a critical role for protein glycosylation in suppressing antigen-specific maternal B cell responses to trophoblast antigens. Intriguingly, this idea was foreshadowed by both an old hypothesis stating that trophoblast antigens are masked by a “sialomucin coat” (Beer and Sio, 1982), and by experiments in the 1960s, which, although controversial at the time, showed that neuraminidase treatment unmasks immunogenicity of crude mouse placenta homogenates (Currie et al., 1968; Taylor et al., 1979). While sialylated glycans decorate most mammalian cell surface proteins, an identical protein that is expressed in different cell types (or by the same cell type under various conditions) is often differently glycosylated because of the cells’ differential expression of sugar transporters, glycosyltransferases, or deglycosylases or as the result of dissimilar transit speeds through the ER/Golgi processing pathway (Varki et al., 2022). Accordingly, as compared with mOVA expressed by nonplacental organs such as the adult skin (where mOVA serves as a rejection antigen; Ehst et al., 2003), mOVA in maternal plasma was more sialylated as well as more glycosylated in general (Rizzuto et al., 2022). Moreover, its levels of α2,6-sialylation and overall glycosylation matched that of mOVA from placental tissue but not the fetus proper, confirming that its source was indeed the placenta, where, as mentioned, the protein is expressed at particularly high levels by trophoblasts bathed in maternal blood. We also found that glycoproteins of known or likely trophoblast origin bearing α2,6-linked sialic acids are present in pregnant plasma of mice and humans, indicating that relevant sialylation pathways also apply to bona fide trophoblast antigens. Differential glycosylation, including the extent of sialylation, thus provides an explanation for how antigens expressed by trophoblasts can be uniquely tolerogenic. Indeed, we suggest that differential glycosylation of fetal nontrophoblast versus trophoblast proteins could at least in part explain the differences in maternal B cell reactivity toward fetal blood antigens versus trophoblast antigens (Fig. 1). Consistent with this idea, Rh(D) antigen appears to be entirely devoid of associated glycans, unlike nearly all other mammalian cell surface proteins (Gahmberg, 1983; Moore and Green, 1987).
Maternal T cell responses to trophoblast antigens
Experiments in mice, again largely using the mOVA system described above, have revealed several distinct mechanisms that limit maternal CD4 T cell responses to trophoblast antigens. One mechanism documented for early gestation is the entrapment of migratory dendritic cells (DCs) within the decidua, which precludes input from these potent APCs in the uterine draining lymph nodes (Collins et al., 2009). Instead, t-mOVA undergoes cell-free transport within myometrial lymphatic vessels, akin to its cell-free transport within the maternal bloodstream described above. Accordingly, the antigen is taken up and presented by spleen- and lymph node–resident APCs. Since these APCs are of maternal origin, maternal T cell awareness of the fetoplacental allograft thus occurs entirely via the “indirect” allorecognition pathway and not the “direct” or “semidirect” pathways that would involve, respectively, antigen presentation by fetal APCs or the transfer of intact fetal MHC–peptide complexes to maternal APCs (Erlebacher et al., 2007). Sole reliance on the indirect allorecognition pathway might itself be considered a mechanism of fetomaternal tolerance, since it substantially reduces the number of T cells participating in the rejection response. However, indirect allorecognition is sufficient to trigger organ rejection (Marino et al., 2016), and in fact expression of the mOVA antigen alone is sufficient to elicit the rejection of otherwise syngeneic skin grafts (Ehst et al., 2003). Thus, an outstanding question has been the exact nature of maternal T cell responses to trophoblast antigens and how they might differ from responses to pathogen-encoded antigens or minor histocompatibility antigens encountered following organ transplantation, which are similarly presented by the indirect allorecognition pathway.
The fate of trophoblast antigen-specific CD4 T cells has been investigated using the mOVA system in conjunction with T cell adoptive transfers, as well as a modified version of this system in which a model antigenic peptide, 2W1S (Rees et al., 1999), is incorporated into mOVA construct (generating 2W1S-mOVA). This latter approach allows for the visualization of the endogenous repertoire of CD4 T cells using 2W1S-MHCII tetramers. Perhaps not surprisingly, given that pregnancy is not an inflammatory state that induces the systemic activation of maternal APCs, CD4 T cells responding to t-mOVA or trophoblast 2W1S do not differentiate into T helper 1 (TH1) cells (Rizzuto et al., 2022; Rowe et al., 2012). However, OVA-specific CD4 T cells fail to differentiate into TH1 cells even when the mice are given adjuvants, and OVA- and 2W1S-specific CD4 T cells both fail to differentiate into TH1 cells when the mice are respectively injected with c-OVA plus adjuvants or infected with 2W1S-expressing Listeria monocytogenes, two manipulations that generate strong TH1 responses when mice do not bear mOVA+ concepti (Rizzuto et al., 2022; Rowe et al., 2012). These observations indicate that trophoblast antigen-specific CD4 T cells experience dominant immune suppression, and the field has largely focused on the potential role of regulatory T cells in mediating this suppression. Accordingly, 2W1S-specific regulatory T cells (Tregs) expand concurrently with the release of 2W1S-mOVA antigen from the placenta beginning at midgestation (Kalekar et al., 2016; Rowe et al., 2012; Suah et al., 2021), and partial depletion of these cells disinhibits IFNγ production by the non-converted population of 2W1S-specific CD4 T cells (Rowe et al., 2012). The idea that Tregs are important for pregnancy is also supported by observations that systemic Treg cell frequencies increase at midgestation, and more so in allogeneic than syngeneic pregnancies (Aluvihare et al., 2004; Rowe et al., 2011; Thuere et al., 2007; Zhao et al., 2007). In addition, partial Treg cell depletion beginning at midgestation, achieved by administration of diphtheria toxin to mice that express the diphteria toxin receptor from the Foxp3 locus, results in a significant rate of fetal loss following allogeneic but not syngeneic mating (Chaturvedi et al., 2015; Rowe et al., 2012). A similar, but lesser degree of fetal loss is evident in mice lacking the Foxp3 locus enhancer element called conserved noncoding sequence 1 that is required for peripheral conversion of naive CD4 T cells to induced Tregs (Samstein et al., 2012). Provocatively, the conserved noncoding sequence 1 element is uniquely found within placental mammals (Andersen et al., 2012), thus linking the evolution of the placenta with placental-specific induced Tregs that may foster tolerance to trophoblast antigens by suppressing effector T cell responses.
Surprisingly, our recent work on trophoblast glycans revealed that OVA-specific CD4 T cells are not disinhibited when Tregs are depleted during mOVA pregnancies, even though these pregnancies cause OVA-specific CD4 T cells to convert to Treg cells to some extent (Rizzuto et al., 2022). We also unexpectedly found that immunodominant t-mOVA peptides are presented to splenic CD4 T cells by OVA-specific B cells, and not DCs. Such B cell–exclusive antigen presentation, which we speculate is a consequence of how trophoblast antigen glycans influence antigen transport within the spleen, might thus also be considered a mechanism of fetomaternal tolerance, since strong CD4 T cell responses usually require input from DCs (Archambault et al., 2013). Moreover, we found that the glycan-mediated suppression of the OVA-specific B cells contributes to the suppression of OVA-specific CD4 T cells, since the CD4 T cell suppression is partially reversed in pregnant LYN-deficient mice whose B cells can no longer signal via inhibitory Siglecs, and which show robust B cell responses to t-mOVA (Rizzuto et al., 2022). It is likely that these results will be relevant to CD4 T cell responses to the 2W1S antigen, since the 2W1S-mOVA protein is only 14 amino acids longer than the mOVA protein and is presumably decorated by trophoblasts with the same immunosuppressive glycans as t-mOVA. The intersection of glycan-suppressed B cells and the generation of antigen-specific Tregs currently remains unclear, although B cell–deficient μMT mice intriguingly show blunted midgestational expansion of maternal Tregs (Busse et al., 2019). Thus, B cell–mediated presentation of trophoblast antigens may explain why trophoblast antigen-specific Tregs expand during pregnancy, but with the ultimate importance of Tregs in maintaining fetomaternal tolerance depending on the specific antigen in question.
In contrast to the more classically tolerance-like maternal CD4 T cell response to trophoblast-derived antigens, the CD8 T cell response is best described as nonimmunogenic, since this response is characterized by neither tolerance nor immunity. Thus, while CD8 T cells do not become activated to t-mOVA, even when the mice are given adjuvants, mOVA-mated mice show robust CD8 T cell responses to OVA vaccination (Tay et al., 2013). Moreover, trophoblast antigen-specific CD8 T cells persist postpartum in an antigen-experienced/quasi-memory state and can participate in the antigen-specific rejection of skin grafts and tumor cells, albeit with reduced effector capacity (Barton et al., 2017; Jasti et al., 2017; Kinder et al., 2020; Lewis et al., 2022). It currently remains unclear the extent to which this response can be explained by differences in the APC type that present trophoblast antigens to CD4 and CD8 T cells (B cells versus DCs in the case of t-mOVA; Rizzuto et al., 2022), or whether the sialylation of trophoblast antigens influences the CD8 T cell response.
Are there any instances of antigen-specific placental rejection?
Although not the topic of this review article, mechanisms also exist to protect the conceptus from attack by T cells, should they happen to become activated, as well as mechanisms that prevent antibodies from damaging trophoblasts. These mechanisms rely on the unique tissue characteristics and immunology of the maternal–fetal interface (reviewed in Erlebacher [2013]), as well as the high expression of complement regulatory proteins by trophoblasts, as alluded to above. Given this redundancy, it is perhaps not surprising that manipulations that induce what we would consider to be true placental rejection have not yet been described. We acknowledge that many perturbations can trigger fetal loss in mice. However, some of these, such as low-dose LPS or systemic maternal B cell and DC activation with anti-CD40 antibodies in the peri-implantation period, induce fetal loss via nonspecific inflammation that does not involve an antigen-specific component and thus cannot be considered ruptures in fetomaternal tolerance (Erlebacher et al., 2004; Gendron et al., 1990). Other manipulations, including maternal Treg cell depletion (Rowe et al., 2011), PD-L1/PD-1 blockade (Guleria et al., 2005), myeloid-derived suppressor cell depletion (Ostrand-Rosenberg et al., 2017), and indoleamine 2,3-dioxygenase inhibition (Munn et al., 1998), show greater loss of allogeneic versus syngeneic concepti and are thus more indicative of antigen-specific rejection. However, in some of these models, the fetal loss was no longer observed in the genetic knockout (Baban et al., 2004; Taglauer et al., 2009), and in all mouse models of fetal loss to date, antigen-specific T cells have not been observed to accumulate in an appreciable way at the maternal–fetal interface. This situation thus raises questions about exact effector mechanisms, since it contrasts with the robust T cell infiltration observed during organ and tumor rejection. Remarkably, several case reports now show that pregnancy is unharmed in women with cancer who received checkpoint blockade inhibitors in early gestation, thus suggesting that these pathways are not singularly required for fetomaternal tolerance (Xu et al., 2019).
A single clinical situation, which like many pregnancy complications is currently of unclear pathogenesis, may in fact represent coordinated T cell– and antibody-mediated attack of the placenta. Villitis of unknown etiology (VUE) is diagnosed when, in the absence of infection, histologic examination of the placenta reveals a lymphocyte infiltrate in the stroma of the trophoblast-lined placental villi (Kim et al., 2015; Redline and Patterson, 1993; Tamblyn et al., 2013). This infiltrate predominantly consists of maternal CD8 T cells, and affected villi show expression of T cell–recruiting chemokines with upregulation of ICAM-1 and the deposition of complement components on a damaged syncytiotrophoblast layer (Ito et al., 2015; Lee et al., 2013; Kim et al., 2008; Kim et al., 2009; Rudzinski et al., 2013). Cases of VUE are designated as mild or severe based on the extent of villus involvement. Mild VUE is focally limited, is not associated with adverse pregnancy outcomes, and is observed in upwards of a third of all mature placentas. Severe VUE, on the other hand, is characterized by diffuse infiltration of placental villi and is associated with a mild increase in the risk of stillbirth and the presence of maternal T cells within the fetus (Redline, 2007). Consistent with an adaptive immune etiology, severe cases show a high recurrence risk in subsequent pregnancies (Redline and Abramowsky, 1985). Moreover, there is an association between VUE and the detection of maternal anti-HLA antibodies in serum (Lee et al., 2011).
Drawing on the divergence in maternal responses to trophoblast versus fetal blood cell antigens outlined above, we propose the following model for VUE pathogenesis that is based on the priming of maternal T and B cells specific for fetal blood cell antigens rather than trophoblast antigens. First, placental microhemorrhage exposes maternal T cells to fetal blood cells that express paternal allogeneic HLA. Maternal CD4 and CD8 T cells are then activated via the direct allorecognition pathway, with CD4 T cells also providing help for a B cell response. Second, there is focal damage to the usually impenetrable syncytiotrophoblast layer, perhaps via alloantibody binding and complement activation, and potentially involving the killing of trophoblasts. This allows CD8 T cells to gain access to the underlying villous stroma, where they encounter fetal fibroblasts, macrophages, and endothelial cells, which express the full set of paternal class I HLA, thus vigorously activating the T cells and reinforcing the inflammatory reaction. It is also possible that T cells gain entry to the villous stroma in focal areas where the syncytiotrophoblast layer has been damaged by a pathogen, or merely by compromised blood flow.
Summary and future prospects
Now is a very exciting time in reproductive immunology, as newly developed tools are allowing us to thoroughly dissect adaptive immune responses to the fetoplacental allograft. Based on the current literature, we have tried here to articulate a framework that explains why these responses appear to be so divergent, with trophoblast antigens inducing tolerance and fetal blood cell antigens driving immunity (Fig. 1). This framework relies on clinical observation and the study of a limited number of “model” antigens in mice (such as mOVA, 2W1S-mOVA, and KEL), and thus an important next step is to determine the extent to which the same principles we articulate here apply to bona fide trophoblasts antigens, as well as to fetal proteins that are transported across the placenta into the maternal bloodstream. It is also unclear the extent to which these principles apply to CD8 T cell responses to fetal and placental antigens. Indeed, many minor histocompatibility antigens, including those encoded by the Y-chromosome and that appear to elicit maternal CD8 T cell responses (James et al., 2003; Lissauer et al., 2012; van Kampen et al., 2001; Verdijk et al., 2004), show both placental and fetal expression, and it is unclear whether they reflect exposure to trophoblasts versus fetal blood cells. It will also be crucial to determine the extent to which the maternal immune system is even aware of the non–plasma membrane proteins of trophoblasts, noting that cytoplasmic proteins are much less likely to be modified with glycans of any kind.
Mechanisms of increased trophoblast membrane protein sialylation are also an open area of research. As alluded to above, these mechanisms might include increased activity of sialyltransferases and sialic acid transporters, decreased activity of sialidases, and altered transit through the ER/Golgi processing pathway. Trophoblast antigen sialylation (and glycosylation more generally) might also be regulated in subtype-specific fashion and under the influence of paracrine factors. Indeed, recent evidence suggests that the glycan pattern of extravillous trophoblasts located in the uterine decidua might in part be regulated by cross talk with uterine natural killer cells and DCs (Borowski et al., 2020). There may also be important, perhaps hormonally driven, changes in the glycosylation of maternally expressed proteins during pregnancy. For example, maternal IgG species are well documented to become more galactosylated and sialylated during pregnancy, and the extent of this increase shows a striking association with the amelioration of rheumatoid arthritis that is often observed in latter gestation (Bondt et al., 2018; van de Geijn et al., 2009). Lastly, we note that patients with lupus, an autoimmune disease characterized by B cell hyperactivity, show decreased protein levels of LYN (Brodie et al., 2018) and high rates of pregnancy complications (Buyon et al., 2015). These observations raise the possibility that changes in the glycosylation of trophoblast antigens, or in how maternal immune cells respond to trophoblast antigen-associated glycans, can cause these antigens to become pathologically immunogenic in some patients.
Acknowledgments
G. Rizzuto is supported by National Institutes of Health grant K08AI137209. Work in the Erlebacher lab is supported by National Institutes of Health grants R01AI143187 and R01AI150191, a grant from the March of Dimes Foundation (#6-FY-798), and a grant from the Burroughs Wellcome Fund (1019789).
Author contributions: Conceptualization: G. Rizzuto and A. Erlebacher; Visualization: G. Rizzuto and A. Erlebacher; Writing – original draft: G. Rizzuto; Writing – review & editing: G. Rizzuto and A. Erlebacher.
References
- Ait-Azzouzene, D., Gendron M.C., Houdayer M., Langkopf A., Burki K., Nemazee D., and Kanellopoulos-Langevin C.. 1998. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J. Immunol. 161:2677–2683. [PubMed] [Google Scholar]
- Ait-Azzouzene, D., Caucheteux S., Tchang F., Wantyghem J., Moutier R., Langkopf A., Gendron M.C., and Kanellopoulos-Langevin C.. 2001. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol. Reprod. 65:337–344. 10.1095/biolreprod65.2.337 [DOI] [PubMed] [Google Scholar]
- Aluvihare, V.R., Kallikourdis M., and Betz A.G.. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5:266–271. 10.1038/ni1037 [DOI] [PubMed] [Google Scholar]
- Andersen, K.G., Nissen J.K., and Betz A.G.. 2012. Comparative genomics reveals key gain-of-function events in Foxp3 during regulatory T cell evolution. Front. Immunol. 3:113. 10.3389/fimmu.2012.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps, R., Murphy S.P., Fernando R., Gardner L., Ahad T., and Moffett A.. 2009. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 127:26–39. 10.1111/j.1365-2567.2008.03019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault, A.S., Carrero J.A., Barnett L.G., McGee N.G., Sim J., Wright J.O., Raabe T., Chen P., Ding H., Allenspach E.J., et al. 2013. Cutting edge: Conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J. Immunol. 191:545–550. 10.4049/jimmunol.1201598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban, B., Chandler P., McCool D., Marshall B., Munn D.H., and Mellor A.L.. 2004. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61:67–77. 10.1016/j.jri.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Barton, B.M., Xu R., Wherry E.J., and Porrett P.M.. 2017. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J. Leukoc. Biol. 101:975–987. 10.1189/jlb.1A0316-135R [DOI] [PubMed] [Google Scholar]
- Beer, A.E., and Sio J.O.. 1982. Placenta as an immunological barrier. Biol. Reprod. 26:15–27. 10.1095/biolreprod26.1.15 [DOI] [PubMed] [Google Scholar]
- Bell, S.C., and Billington W.D.. 1980. Major anti-paternal alloantibody induced by murine pregnancy is non-complement-fixing IgG1. Nature. 288:387–388. 10.1038/288387a0 [DOI] [PubMed] [Google Scholar]
- Bell, S.C., and Billington W.D.. 1981. Humoral immune responses in murine pregnancy. I. Anti-paternal alloantibody levels in maternal serum. J. Reprod. Immunol. 3:3–13. 10.1016/0165-0378(81)90024-3 [DOI] [PubMed] [Google Scholar]
- Bell, S.C., and Billington W.D.. 1986. Humoral immune responses in murine pregnancy. V. Relationship to the differential immunogenicity of placental and fetal tissues. J. Reprod. Immunol. 9:289–302. 10.1016/0165-0378(86)90030-6 [DOI] [PubMed] [Google Scholar]
- Bondt, A., Hafkenscheid L., Falck D., Kuijper T.M., Rombouts Y., Hazes J.M.W., Wuhrer M., and Dolhain R.J.E.M.. 2018. ACPA IgG galactosylation associates with disease activity in pregnant patients with rheumatoid arthritis. Ann. Rheum. Dis. 77:1130–1136. 10.1136/annrheumdis-2018-212946 [DOI] [PubMed] [Google Scholar]
- Borowski, S., Tirado-Gonzalez I., Freitag N., Garcia M.G., Barrientos G., and Blois S.M.. 2020. Altered glycosylation contributes to placental dysfunction upon early disruption of the NK cell-DC dynamics. Front. Immunol. 11:1316. 10.3389/fimmu.2020.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma, G.J., van Caubergh P., van Bree S.P., Castelli-Visser R.M., Witvliet M.D., van der Meer-Prins E.M., van Rood J.J., and Claas F.H.. 1996. Pregnancy can induce priming of cytotoxic T lymphocytes specific for paternal HLA antigens that is associated with antibody formation. Transplantation. 62:672–678. 10.1097/00007890-199609150-00023 [DOI] [PubMed] [Google Scholar]
- Brodie, E.J., Infantino S., Low M.S.Y., and Tarlinton D.M.. 2018. Lyn, Lupus, and (B) Lymphocytes, a lesson on the critical balance of kinase signaling in immunity. Front. Immunol. 9:401. 10.3389/fimmu.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse, M., Jutta Campe K.-N., Nowak D., Schumacher A., Plenagl S., Langwisch S., Tiegs G., Reinhold A., and Zenclussen A.C.. 2019. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 9:9335. 10.1038/s41598-019-45860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyon, J.P., Kim M.Y., Guerra M.M., Laskin C.A., Petri M., Lockshin M.D., Sammaritano L., Branch D.W., Porter T.F., Sawitzke A., et al. 2015. Predictors of pregnancy outcomes in patients with Lupus: A cohort study. Ann. Intern. Med. 163:153–163. 10.7326/M14-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi, V., Ertelt J.M., Jiang T.T., Kinder J.M., Xin L., Owens K.J., Jones H.N., and Way S.S.. 2015. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J. Clin. Invest. 125:1713–1725. 10.1172/JCI78578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, M.K., Tay C.-S., and Erlebacher A.. 2009. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119:2062–2073. 10.1172/jci38714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie, G.A., Van Doorninck W., and Bagshawe K.D.. 1968. Effect of neuraminidase on the immunogenicity of early mouse trophoblast. Nature. 219:191–192. 10.1038/219191a0 [DOI] [PubMed] [Google Scholar]
- Doughty, R.W., and Gelsthorpe K.. 1974. An initial investigation of lymphocyte antibody activity through pregnancy and in eluates prepared from placental material. Tissue Antigens. 4:291–298. 10.1111/j.1399-0039.1974.tb00255.x [DOI] [PubMed] [Google Scholar]
- Durgam, S.S., Alegre M.L., and Chong A.S.. 2022. Lessons learned from pregnancy for immune tolerance and transplantation. J. Exp. Med. [Google Scholar]
- Ehst, B.D., Ingulli E., and Jenkins M.K.. 2003. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transpl. 3:1355–1362. 10.1046/j.1600-6135.2003.00246.x [DOI] [PubMed] [Google Scholar]
- Erlebacher, A. 2013. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 31:387–411. 10.1146/annurev-immunolan032712-100003 [DOI] [PubMed] [Google Scholar]
- Erlebacher, A., Zhang D., Parlow A.F., and Glimcher L.H.. 2004. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 114:39–48. 10.1172/JCI20645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher, A., Vencato D., Price K.A., Zhang D., and Glimcher L.H.. 2007. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117:1399–1411. 10.1172/JCI28214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg, C.G. 1983. Molecular characterization of the human red cell Rho(D) antigen. EMBO J. 2:223–227. 10.1002/j.1460-2075.1983.tb01409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron, R.L., Nestel F.P., Lapp W.S., and Baines M.G.. 1990. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-alpha. J. Reprod. Fertil. 90:395–402. 10.1530/jrf.0.0900395 [DOI] [PubMed] [Google Scholar]
- Geneugelijk, K., Honger G., van Deutekom H.W.M., Thus K.A., Kesmir C., Hosli I., Schaub S., and Spierings E.. 2015. Predicted indirectly recognizable HLA epitopes presented by HLA-DRB1 are related to HLA antibody formation during pregnancy. Am. J. Transpl. 15:3112–3122. 10.1111/ajt.13508 [DOI] [PubMed] [Google Scholar]
- Girardi, G., Bulla R., Salmon J.E., and Tedesco F.. 2006. The complement system in the pathophysiology of pregnancy. Mol. Immunol. 43:68–77. 10.1016/j.molimm.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Guleria, I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., and Sayegh M.H.. 2005. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202:231–237. 10.1084/jem.20050019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby, S.E., Apps R., Sharkey A.M., Farrell L.E., Gardner L., Mulder A., Claas F.H., Walker J.J., Redman C.W., Morgan L., et al. 2010. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120:4102–4110. 10.1172/JCI43998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, O.J., Linscheid C., Hodes H.C., Nauser T.L., Gilliam M., Stone P., Chamley L.W., and Petroff M.G.. 2012. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: Implications for maternal alloreactivity to the fetus. Am. J. Pathol. 180:256–266. 10.1016/j.ajpath.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honger, G., Fornaro I., Granado C., Tiercy J.M., Hosli I., and Schaub S.. 2013. Frequency and determinants of pregnancy-induced child-specific sensitization. Am. J. Transpl. 13:746–753. 10.1111/ajt.12048 [DOI] [PubMed] [Google Scholar]
- Ito, Y., Matsuoka K., Uesato T., Sago H., Okamoto A., Nakazawa A., and Hata K.. 2015. Increased expression of perforin, granzyme B, and C5b-9 in villitis of unknown etiology. Placenta. 36:531–537. 10.1016/j.placenta.2015.02.004 [DOI] [PubMed] [Google Scholar]
- James, E., Chai J.G., Dewchand H., Macchiarulo E., Dazzi F., and Simpson E.. 2003. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 102:388–393. 10.1182/blood-2002blo10-3170 [DOI] [PubMed] [Google Scholar]
- Jasti, S., Farahbakhsh M., Nguyen S., Petroff B.K., and Petroff M.G.. 2017. Immune response to a model shared placenta/tumor-associated antigen reduces cancer risk in parous mice. Biol. Reprod. 96:134–144. 10.1095/biolreprod.116.144907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth, A.A., Silva W.A. Jr., Iversen K., Frosina D., Zaidi B., Coplan K., Eastlake-Wade S.K., Castelli S.B., Spagnoli G.C., Old L.J., and Vogel M.. 2007. Expression of cancer-testis (CT) antigens in placenta. Cancer Immun. 7:15. [PMC free article] [PubMed] [Google Scholar]
- Kalekar, L.A., Schmiel S.E., Nandiwada S.L., Lam W.Y., Barsness L.O., Zhang N., Stritesky G.L., Malhotra D., Pauken K.E., Linehan J.L., et al. 2016. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 17:304–314. 10.1038/ni.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.S., Romero R., Kim M.R., Kim Y.M., Friel L., Espinoza J., and Kim C.J.. 2008. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 52:457–464. 10.1111/j.1365-2559.2008.02964.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.J., Romero R., Kim C.J., Tarca A.L., Chhauy S., LaJeunesse C., Lee D.C., Draghici S., Gotsch F., Kusanovic J.P., et al. 2009. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: Implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J. Immunol. 182:3919–3927. 10.4049/jimmunol.0803834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C.J., Romero R., Chaemsaithong P., and Kim J.S.. 2015. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 213:S53–S69. 10.1016/j.ajog.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder, J.M., Turner L.H., Stelzer I.A., Miller-Handley H., Burg A., Shao T.Y., Pham G., and Way S.S.. 2020. CD8(+) T cell functional exhaustion overrides pregnancy-induced fetal antigen alloimmunization. Cell Rep. 31:107784. 10.1016/j.celrep.2020.107784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubli, H., and Varki A.. 2020. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell. Mol. Life Sci. 77:593–605. 10.1007/s00018-019s0003288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., Romero R., Xu Y., Kim J.S., Topping V., Yoo W., Kusanovic J.P., Chaiworapongsa T., Hassan S.S., Yoon B.H., and Kim C.J.. 2011. A signature of maternal anti-fetal rejection in spontaneous preterm birth: Chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 6:e16806. 10.1371/journal.pone.0016806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.A., Kim Y.W., Shim J.Y., Won H.S., Lee P.R., Kim A., and Kim C.J.. 2013. Distinct patterns of C4d immunoreactivity in placentas with villitis of unknown etiology, cytomegaloviral placentitis, and infarct. Placenta. 34:432–435. 10.1016/j.placenta.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Lewis, E.L., Xu R., Beltra J.-C., Ngiow S.F., Cohen J., Telange R., Crane A., Sawinski D., Wherry E.J., and Porrett P.M.. 2022. NFAT-dependent and -independent exhaustion circuits program maternal CD8 T cell hypofunction in pregnancy. J. Exp. Med. 219:e20201599. 10.1084/jem.20201599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscheid, C., and Petroff M.G.. 2013. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am. J. Reprod. Immunol. 69:304–314. 10.1111/aji.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer, D., Piper K., Goodyear O., Kilby M.D., and Moss P.A.H.. 2012. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 189:1072–1080. 10.4049/jimmunol.1200544 [DOI] [PubMed] [Google Scholar]
- Marino, J., Paster J., and Benichou G.. 2016. Allorecognition by T Lymphocytes and allograft rejection. Front. Immunol. 7:582. 10.3389/fimmu.2016.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson, E., Vidal C., Deschamps M., Bongain S., Thevenin C., Dupont I., Rietmulher D., Pouthier F., Mongaillard G., Chabod J., et al. 2013. Incidence and risk factors of anti-HLA immunization after pregnancy. Hum. Immunol. 74:946–951. 10.1016/j.humimm.2013.04.025 [DOI] [PubMed] [Google Scholar]
- Medawar, P.B. 1953. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7:320–338. [Google Scholar]
- Mener, A., Patel S.R., Arthur C.M., Chonat S., Wieland A., Santhanakrishnan M., Liu J., Maier C.L., Jajosky R.P., Girard-Pierce K., et al. 2018. Complement serves as a switch between CD4+ T cell-independent and -dependent RBC antibody responses. JCI Insight. 3:e121631. 10.1172/jci.insight.121631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, T., and Dveksler G.S.. 2014. Pregnancy-specific glycoproteins: Complex gene families regulating maternal-fetal interactions. Int. J. Dev. Biol. 58:273–280. 10.1387/ijdb.130329gd [DOI] [PubMed] [Google Scholar]
- Moore, S., and Green C.. 1987. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem. J. 244:735–741. 10.1042/bj2440735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., and Mellor A.L.. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 281:1191–1193. 10.1126/science.281.5380.1191 [DOI] [PubMed] [Google Scholar]
- Nitschke, L. 2014. CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance. Glycobiology. 24:807–817. 10.1093/glycob/cwu066 [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg, S., Sinha P., Figley C., Long R., Park D., Carter D., and Clements V.K.. 2017. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J. Leukoc. Biol. 101:1091–1101. 10.1189/jlb.1HI1016-306RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersdorf, E.W., Longton G.M., Anasetti C., Mickelson E.M., McKinney S.K., Smith A.G., Martin P.J., and Hansen J.A.. 1997. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 89:1818–1823. [PubMed] [Google Scholar]
- Porrett, P.M. 2018. Biologic mechanisms and clinical consequences of pregnancy alloimmunization. Am. J. Transpl. 18:1059–1067. 10.1111/ajt.14673 [DOI] [PubMed] [Google Scholar]
- Proll, J., Blaschitz A., Hutter H., and Dohr G.. 1999. First trimester human endovascular trophoblast cells express both HLA-C and HLA-G. Am. J. Reprod. Immunol. 42:30–36. 10.1111/j.1600-0897.1999.tb00462.x [DOI] [PubMed] [Google Scholar]
- Redline, R.W. 2007. Villitis of unknown etiology: Noninfectious chronic villitis in the placenta. Hum. Pathol. 38:1439–1446. 10.1016/j.humpath.2007.05.025 [DOI] [PubMed] [Google Scholar]
- Redline, R.W., and Abramowsky C.R.. 1985. Clinical and pathologic aspects of recurrent placental villitis. Hum. Pathol. 16:727–731. 10.1016/s0046-8177(85)80159-3 [DOI] [PubMed] [Google Scholar]
- Redline, R.W., and Lu C.Y.. 1989. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab. Invest. 61:27–36. [PubMed] [Google Scholar]
- Redline, R.W., and Patterson P.. 1993. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am. J. Pathol. 143:473–479. [PMC free article] [PubMed] [Google Scholar]
- Rees, W., Bender J., Teague T.K., Kedl R.M., Crawford F., Marrack P., and Kappler J.. 1999. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 96:9781–9786. 10.1073/pnas.96.17.9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan, L., Braude P.R., and Hill D.P.. 1991. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum. Reprod. 6:294–298. 10.1093/oxfordjournals.humrep.a137325 [DOI] [PubMed] [Google Scholar]
- Rizzuto, G., Brooks J.F., Tuomivaara S.T., McIntyre T.I., Ma S., Rideaux D., Zikherman J., Fisher S.J., and Erlebacher A.. 2022. Establishment of fetomaternal tolerance through glycan-mediated B cell suppression. Nature. 603:497–502. 10.1038/s41586-022-04471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, A.M., Boime I., Connolly J., Cook J.R., and Russell J.H.. 1998. Maternal-fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur. J. Immunol. 28:3479–3487. [DOI] [PubMed] [Google Scholar]
- Rowe, J.H., Ertelt J.M., Aguilera M.N., Farrar M.A., and Way S.S.. 2011. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 10:54–64. 10.1016/j.chom.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, J.H., Ertelt J.M., Xin L., and Way S.S.. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 490:102–106. 10.1038/nature11462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinski, E., Gilroy M., Newbill C., and Morgan T.. 2013. Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr. Dev. Pathol. 16:7–13. 10.2350/12-051195-OA.1 [DOI] [PubMed] [Google Scholar]
- Samstein, R.M., Josefowicz S.Z., Arvey A., Treuting P.M., and Rudensky A.Y.. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 150:29–38. 10.1016/j.cell.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomer, B., Toder V., Egorov I., and Ehrlich R.. 1998. Expression of allogeneic MHC class I antigens by transgenic mouse trophoblast does not interfere with the normal course of pregnancy. Transgenic Res. 7:343–355. 10.1023/a:1008897308025 [DOI] [PubMed] [Google Scholar]
- Smith, K.G., Tarlinton D.M., Doody G.M., Hibbs M.L., and Fearon D.T.. 1998. Inhibition of the B cell by CD22: A requirement for Lyn. J. Exp. Med. 187:807–811. 10.1084/jem.187.5.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell, S.R., Henry K.L., Smith N.H., Hudson K.E., Halverson G.R., Park J.C., Bennett A.M., Girard-Pierce K.R., Arthur C.M., Bunting S.T., et al. 2013. Alloantibodies to a paternally derived RBC KEL antigen lead to hemolytic disease of the fetus/newborn in a murine model. Blood. 122:1494–1504. 10.1182/blood-2013b03-488874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suah, A.N., Tran D.-K.V., Khiew S.H., Andrade M.S., Pollard J.M., Jain D., Young J.S., Yin D., Chalasani G., Alegre M.L., and Chong A.S.. 2021. Pregnancy-induced humoral sensitization overrides T cell tolerance to fetus-matched allografts in mice. J. Clin. Invest. 131:e140715. 10.1172/JCI140715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglauer, E.S., Yankee T.M., and Petroff M.G.. 2009. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 80:12–21. 10.1016/j.jri.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamblyn, J.A., Lissauer D.M., Powell R., Cox P., and Kilby M.D.. 2013. The immunological basis of villitis of unknown etiology - review. Placenta. 34:846–855. 10.1016/j.placenta.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Tay, C.S., Tagliani E., Collins M.K., and Erlebacher A.. 2013. Cis-acting pathways selectively enforce the non-immunogenicity of shed placental antigen for maternal CD8 T cells. PLoS One. 8:e84064. 10.1371/journal.pone.0084064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P.V., Hancock K.W., and Gowland G.. 1979. Effect of neuraminidase on immunogenicity of early mouse trophoblast. Transplantation. 28:256–257. 10.1097/00007890-197909000-00020 [DOI] [PubMed] [Google Scholar]
- Taylor, J.J., Martinez R.J., Titcombe P.J., Barsness L.O., Thomas S.R., Zhang N., Katzman S.D., Jenkins M.K., and Mueller D.L.. 2012. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J. Exp. Med. 209:2065–2077. 10.1084/jem.20112272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco, F., Narchi G., Radillo O., Meri S., Ferrone S., and Betterle C.. 1993. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J. Immunol. 151:1562–1570 [PubMed] [Google Scholar]
- Thuere, C., Zenclussen M.L., Schumacher A., Langwisch S., Schulte-Wrede U., Teles A., Paeschke S., Volk H.D., and Zenclussen A.C.. 2007. Kinetics of regulatory T cells during murine pregnancy. Am. J. Reprod. Immunol. 58:514–523. 10.1111/j.1600-0897.2007.00538.x [DOI] [PubMed] [Google Scholar]
- Tongio, M.M., Mayer S., and Lebec A.. 1975. Transfer of HL-A antibodies from the mother to the child. Complement of information. Transplantation. 20:163–166. 10.1097/00007890-197508000-00011 [DOI] [PubMed] [Google Scholar]
- Tongio, M.M., Werneburg B., and Mayer S.. 1983. Transfer of anti-HLA-DR antibodies from the mother to the child. Are DR antigens expressed on the placenta? Tissue Antigens. 22:24–28. 10.1111/j.1399-0039.1983.tb01160.x [DOI] [PubMed] [Google Scholar]
- Tovey, L.A. 1992. Oliver memorial lecture. Towards the conquest of Rh haemolytic disease: Britain’s contribution and the role of serendipity. Transfus. Med. 2:99–109. 10.1111/j.1365-3148.1992.tb00142.x [DOI] [PubMed] [Google Scholar]
- Urbaniak, S.J., and Greiss M.A.. 2000. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 14:44–61. 10.1054/blre.1999.0123 [DOI] [PubMed] [Google Scholar]
- van de Geijn, F.E., Wuhrer M., Selman M.H., Willemsen S.P., de Man Y.A., Deelder A.M., Hazes J.M., and Dolhain R.J.. 2009. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res. Ther. 11:R193. 10.1186/ar2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kampen, C.A., Versteeg-van der Voort Maarschalk M.F., Langerak-Langerak J., van Beelen E., Roelen D.L., and Claas F.H.. 2001. Pregnancy can induce long-persisting primed CTLs specific for inherited paternal HLA antigens. Hum. Immunol. 62:201–207. 10.1016/s0198-8859(01)00209-9 [DOI] [PubMed] [Google Scholar]
- Van Rood, J.J., Eernisse J.G., and Van Leeuwen A.. 1958. Leucocyte antibodies in sera from pregnant women. Nature. 181:1735–1736. 10.1038/1811735a0 [DOI] [PubMed] [Google Scholar]
- Varki, A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al. 2022. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [PubMed] [Google Scholar]
- Verdijk, R.M., Kloosterman A., Pool J., van de Keur M., Naipal A.M.I.H., van Halteren A.G.S., Brand A., Mutis T., and Goulmy E.. 2004. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: Implications for stem cell transplantation and immunotherapy. Blood. 103:1961–1964. 10.1182/blood-2003blo05-1625 [DOI] [PubMed] [Google Scholar]
- Webb, J., and Delaney M.. 2018. Red blood cell alloimmunization in the pregnant patient. Transfus. Med. Rev. 32:213–219. 10.1016/j.tmrv.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Wegmann, T.G. 1981. The presence of class 1 MHC antigens at the maternal-fetal interface and hypotheses concerning the survival of the murine fetal allograft. J. Reprod. Immunol. 3:267–270. 10.1016/0165-0378(81)90034-6 [DOI] [PubMed] [Google Scholar]
- Wegmann, T.G., Mosmann T.R., Carlson G.A., Olijnyk O., and Singh B.. 1979a. The ability of the murine placenta to absorb monoclonal anti-fetal H-2K antibody from the maternal circulation. J. Immunol. 123:1020–1023 [PubMed] [Google Scholar]
- Wegmann, T.G., Singh B., and Carlson G.A.. 1979b. Allogeneic placenta is a paternal strain antigen immunoabsorbent. J. Immunol. 122:270–274 [PubMed] [Google Scholar]
- Xu, C., Mao D., Holers V.M., Palanca B., Cheng A.M., and Molina H.. 2000. A critical role for murine complement regulator Crry in fetomaternal tolerance. Science. 287:498–501. 10.1126/science.287.5452.498 [DOI] [PubMed] [Google Scholar]
- Xu, W., Moor R.J., Walpole E.T., and Atkinson V.G.. 2019. Pregnancy with successful foetal and maternal outcome in a melanoma patient treated with nivolumab in the first trimester: Case report and review of the literature. Melanoma Res. 29:333–337. 10.1097/cmr.0000000000000586 [DOI] [PubMed] [Google Scholar]
- Zhao, J.X., Zeng Y.Y., and Liu Y.. 2007. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J. Reprod. Immunol. 75:71–81. 10.1016/j.jri.2007.06.052 [DOI] [PubMed] [Google Scholar]