Abstract
Purpose:
To determine if treatment with a photobiomodulation (PBM) device results in greater improvement in central subfield thickness (CST) than placebo in eyes with center-involved diabetic macular edema (CI-DME) and good vision.
Design:
Phase 2 randomized clinical trial.
Participants:
Participants had CI-DME and visual acuity (VA) 20/25 or better in the study eye and were recruited from 23 clinical sites in the United States.
Methods:
One eye of each participant was randomly assigned 1:1 to a 670-nm light-emitting PBM eye patch or an identical device emitting broad-spectrum white light at low power. Treatment was applied for 90 seconds twice daily for 4 months.
Main Outcome Measures:
Change in CST on spectral-domain OCT at 4 months.
Results:
From April 2019 to February 2020, 135 adults were randomly assigned to either PBM (n = 69) or placebo (n = 66); median age was 62 years, 37% were women, and 82% were White. The median device compliance was 92% with PBM and 95% with placebo. OCT CST increased from baseline to 4 months by a mean (SD) of 13 (53) μm in PBM eyes and 15 (57) μm in placebo eyes, with the mean difference (95% confidence interval [CI]) being −2 (−20 to 16) μm (P = 0.84). CI-DME, based on DRCR Retina Network sex- and machine-based thresholds, was present in 61 (90%) PBM eyes and 57 (86%) placebo eyes at 4 months (adjusted odds ratio [95% CI] = 1.30 (0.44–3.83); P = 0.63). VA decreased by a mean (SD) of −0.2 (5.5) letters and −0.6 (4.6) letters in the PBM and placebo groups, respectively (difference [95% CI] = 0.4 (−1.3 to 2.0) letters; P = 0.64). There were 8 adverse events possibly related to the PBM device and 2 adverse events possibly related to the placebo device. None were serious.
Conclusions:
PBM as given in this study, although safe and well-tolerated, was not found to be effective for the treatment of CI-DME in eyes with good vision.
Keywords: Diabetic macular edema, DRCR Retina Network, photobiomodulation
The rapidly expanding global epidemic of diabetes will place nearly 700 million individuals worldwide at risk of vision loss from diabetic eye complications by the year 2045.1 Among those with diabetes, diabetic macular edema (DME) is a leading cause of vision loss.2 Treatments such as intravitreal anti-VEGF, intravitreal steroid, and macular laser photocoagulation have been demonstrated to improve visual outcomes in patients with center-involved DME (CI-DME).3–7 However, intravitreal injections have disadvantages, including the need for repeat injections, risk of endophthalmitis, and high cost of some of the medications. Macular focal/grid laser photocoagulation offers less chance of visual gain compared with anti-VEGF injections, and the resultant retinal scarring can lead to central or paracentral scotomas. Furthermore, all these treatments require access to specialized retinal care. Therefore, novel therapies for CI-DME that are effective, safe, affordable, and scalable for global use are needed. In addition, if treatment could be performed at home, rather than at physicians’ offices, it might dramatically improve access to care and reduce the treatment burden.
Recent studies have suggested that photobiomodulation (PBM), or irradiation using light in the far-red to near-infrared region of the spectrum (630–900 nm), may have a beneficial effect in eyes with DME through the amelioration of oxidative stress and reduced expression of proinflammatory proteins in the retina.8,9 Preclinical studies in rodent models demonstrated that PBM inhibits diabetes-related retinal ganglion cell apoptosis, leukostasis, oxidative stress, and functional abnormalities.10 Daily application of PBM over 8 months in a diabetic mouse model resulted in the reduction of capillary degeneration and leakage, while improving visual function.11 An initial, nonrandomized human study treating 1 eye of 4 patients through the closed eyelid with bilateral non–CI-DME with PBM for 160 seconds per day at 9 J/cm2 for 2–9 months demonstrated greater reduction in retinal thickening in the eyes treated with PBM than in untreated fellow eyes.9 No adverse events were reported in association with PBM treatment, and the doses of light used in these studies constituted a “nonsignificant risk” per US Food and Drug Administration guidelines.
Considering the potential large positive impact on public health of this novel, noninvasive treatment, the DRCR Retina Network (DRCR.net) conducted a pilot study (Protocol AE) to compare the short-term effects of PBM delivered at home using a light-emitting eye patch with a placebo treatment, on improving CI-DME in eyes with good vision. The study goal was to determine whether the conduct of a subsequent pivotal trial was warranted and to provide information needed to design a pivotal trial.
Methods
This study adhered to the tenets of the Declaration of Helsinki. A central institutional review board of the Jaeb Center for Health Research provided approval for each site. Study participants provided written informed consent. An independent data and safety monitoring board provided oversight. The complete study protocol and statistical analysis plan are available on the study website (www.drcr.net).
Study Population
Protocol AE recruited adults with type 1 or type 2 diabetes at 23 clinical sites in the United States. Study eyes had best-corrected visual acuity (VA) letter score ≥79 letters (Snellen equivalent 20/25 or better) and evidence of CI-DME on clinical examination, confirmed by central subfield thickness (CST) on spectral-domain OCT (Zeiss Cirrus: ≥290 μm in women, and ≥305 μm in men; Heidelberg Spectralis: ≥305 μm in women, and ≥320 μm in men). Each participant could have only 1 study eye. Exclusion criteria included macular edema due to a cause other than DME, any ocular condition other than DME that might affect VA, an anticipated need to treat DME or diabetic retinopathy, any major ocular surgery within 4 months before enrollment, and any treatment for DME or diabetic retinopathy within 12 months before enrollment. Eyes with previous treatment could not have received >4 prior intraocular injections. No more than 15% of the cohort was permitted to have prior anti-VEGF injections or panretinal photocoagulation.
Study Design
Eyes were assigned randomly (1:1) to the PBM (670-nm wave-length) or placebo (broad-spectrum white light) device. Randomization was stratified by site and recent (within 4 months) or planned intravitreous treatment (anti-VEGF or steroid) in the nonstudy eye. An optional second randomization (stratified by treatment group) was performed for participants who consented to receive text message reminders to use the device. Those randomized to text message received reminders daily for 2 weeks and then weekly thereafter. Randomization was completed on the DRCR.net website.
There were 2 phases of the study, the primary outcome phase (phase 1, first 4 months) and the post-outcome phase (phase 2, second 4 months). Visits occurred at monthly intervals in phase 1 and every other month during phase 2. Network-certified technicians obtained E-ETDRS best-corrected VA and spectral-domain OCT scans (Zeiss Cirrus or Heidelberg Spectralis) in both eyes at each visit. At the primary outcome 4-month visit, participants returned the original device received at randomization and received the alternative treatment group device. Phase 2 was exploratory and designed to allow the initial placebo group to receive PBM treatment (the study was not a crossover design) while assessing the duration of the treatment effect, if any, in the treatment group once the treatment was stopped. Treatment group assignment was masked to study participants, investigators, OCT technicians, and VA testers. Study coordinators who performed device training were unmasked.
Modifications were made to the study protocol due to the COVID-19 pandemic to minimize risks associated with in-person clinic visits. Beginning April 16, 2020, the participants were unmasked to their original treatment group assignment after the completion of the primary outcome visit. Participants randomized to placebo had the option to receive the PBM device for phase 2, whereas the PBM group participants discontinued placebo device use during phase 2. The participants in both groups who were not using a device during phase 2 had the option of remaining in the study for follow-up and evaluation or ending participation early.
Study Treatment
The PBM device that was used in this study was the Retilux Eye Patch developed by PhotoOptx, LLC, Solon, OH. The device was specifically manufactured in collaboration with the Network to deliver the same dose at the retina as a device (WARP-10, Quantum Devices, Inc., Barneveld, WI) used in prior clinical studies that had results consistent with a possible biological effect.8,9 The device is worn as a single eye patch to direct the treatment effect to the study eye (Fig 1). The active treatment patch emits red light of 670 nm at a dose of 4.5 J/cm2 with an irradiance not >50 mW/cm2. The placebo device seems identical to the active device, except that a broad-spectrum, low-power white light, believed not to have any biologic effect, is emitted. The participants were told that the study was comparing an inactive light with an active light but were masked as to which color light was active.
Figure 1.
Photobiomodulation ophthalmic treatment device. Image credit: https://photooptx.com/retilux.
Clinical site coordinators instructed the participants on proper device use, and the participants were required to demonstrate the initial treatment in the office. The participants were provided with instructions to take the device home and asked to complete the second treatment that day before bed. After that, the participants used the device twice daily for 90 seconds (in the morning and before bed). The participant started the device, which stopped automatically after 90 seconds. The length of dosing was chosen based on these previous studies, with twice daily frequency to ensure that if a session was missed, the participant could still receive daily treatment.8,9 The time of usage and light reflectance back to the patch during usage was stored on the device and was downloaded at each visit to assess compliance. The reflectance of 100 or less was considered indicative of the user not wearing the patch during the treatment session.
Investigators could provide an alternative treatment for CI-DME if there were a vision loss presumed to be from DME of at least 10 letters at a single visit or of 5 to 9 letters at 2 consecutive visits, at least 21 days apart. Once the criteria were met, alternative treatment was at the investigator’s discretion as a part of the usual care. Eyes that received alternative treatment discontinued study device use and discontinued participation in the study after the 4-month visit (or next study visit, if after 4 months).
Study Outcomes
The primary outcome was the change in OCT CST from baseline to 4 months. The secondary outcomes included the change in OCT retinal volume, percentage of eyes with CI-DME at 4 months, and percentage of eyes with a 5-letter loss in VA from baseline to 4 months.
Statistical Analyses
The study planned to enroll 134 eyes to provide 90% power to reject the null hypothesis of no treatment group difference in mean CST change, assuming a true mean difference of at least 30 μm and standard deviation of 50 μm, accounting for 10% lost to follow-up.
Descriptive statistics are reported using observed data. For eyes that received alternative treatment for DME before the primary outcome visit (3 PBM, 1 placebo), the last measurements taken before DME treatment was initiated were the prespecified outcome data. The missing 4-month outcome data (CST, OCT volume, VA, and compliance) were imputed using multiple imputation (Markov chain Monte Carlo method) stratified by the treatment group.
Treatment group differences were estimated using linear or logistic regression as appropriate based on outcome type. Treatment group risk differences were estimated using the marginal probabilities from a counterfactual model with confidence intervals (CIs) constructed using bootstrap resampling. Regression equations included adjustment for baseline levels of the outcome and recent or planned intravitreal treatment in the nonstudy eye. Only descriptive statistics are reported for phase 2. All P values are 2-sided, and CIs are at the 95% significance level. Analyses were completed using SAS software version 9.4 (SAS Institute Inc).
Results
The participants were enrolled from April 2019 to February 2020 and randomly assigned to PBM (n = 69) or placebo (n = 66) (Fig 2). Overall, the median (interquartile range [IQR]) age was 62 (56–68) years; 37% were women; 82% were White, 9% Hispanic or Latino, and 8% Black/African American. The median (IQR) baseline CST (Spectralis equivalent) was 354 (335–378) μm, and median (IQR) baseline VA was 84 (81–87) letters (median Snellen equivalent of 20/20). Excluding 1 death in the PBM group, 100% of participants completed the primary outcome visit. The participant and study eye characteristics by the treatment group are shown in Table 1.
Figure 2.
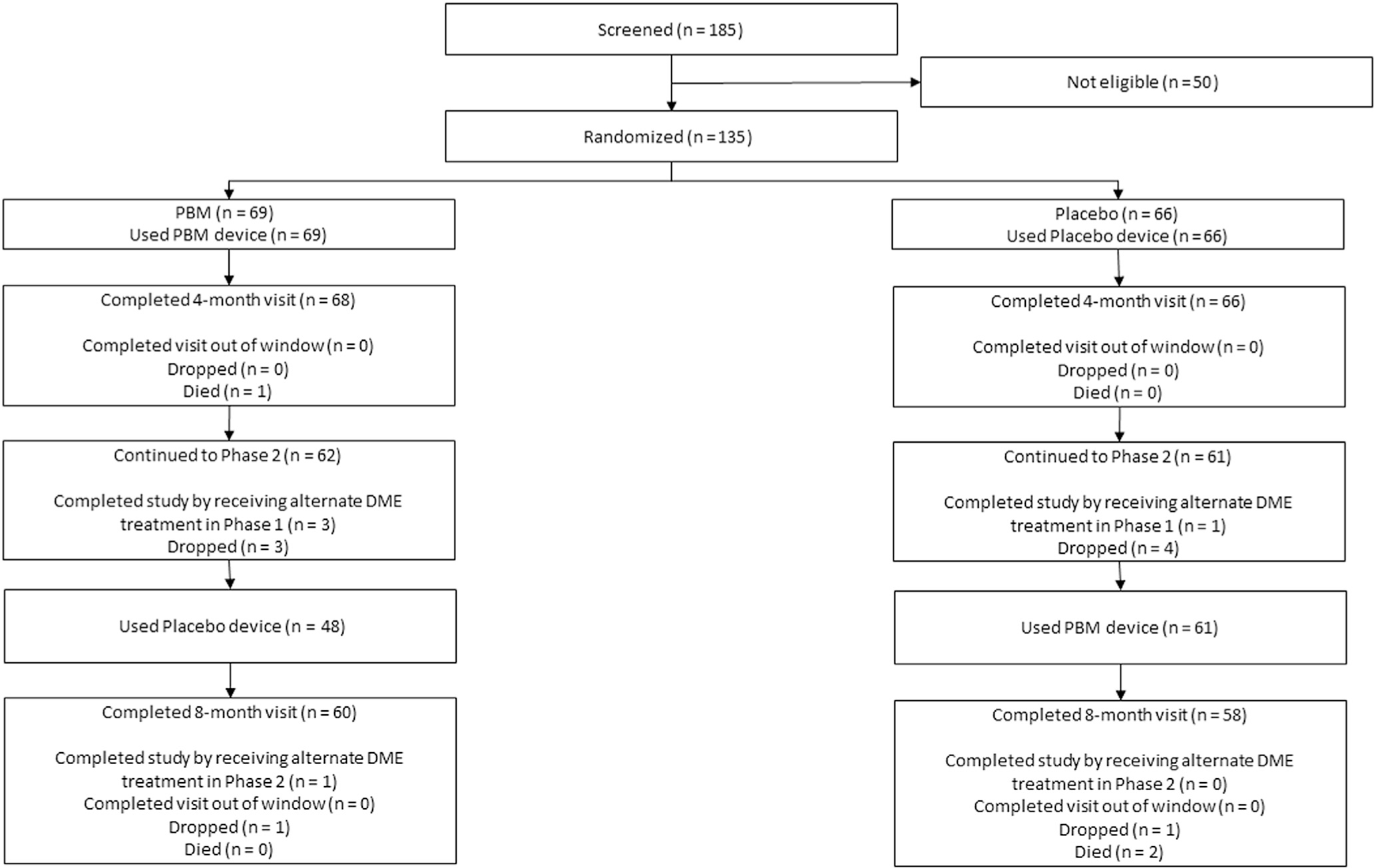
Study flow diagram. Participants were not formally screened before obtaining informed consent. Reasons for ineligibility were not systematically collected. Visit completion at 4 months was prespecified as the completion of any study visit from 12 to 24 weeks, but due to the COVID-19 pandemic, the window was extended to 32 weeks, and participants in phase 2 could also discontinue the device use. DME = diabetic macular edema; PBM = photobiomodulation.
Table 1.
Baseline Participant and Study Eye Characteristics
| Treatment Group |
||
|---|---|---|
| Baseline Characteristics | PBM (n = 69) | Placebo (n = 66) |
|
| ||
| Participant characteristics | ||
| Age (yrs), Median (IQR) | 63 (55, 66) | 62 (58, 69) |
| Sex, n (%) | ||
| Female | 22 (32%) | 28 (42%) |
| Male | 47 (68%) | 38 (58%) |
| Race/ethnicity, n (%) | ||
| Asian | 1 (1%) | 0 |
| Black/African American | 4 (6%) | 7 (11%) |
| Hispanic or Latino | 5 (7%) | 7 (11%) |
| White | 59 (86%) | 52 (79%) |
| Diabetes type, n (%) | ||
| Type 1 | 5 (7%) | 10 (15%) |
| Type 2 | 64 (93%) | 56 (85%) |
| Duration of diabetes, yrs | ||
| Median (IQR) | 16.3 (12.3, 24.0) | 20.0 (13.5, 24.0) |
| Insulin used | ||
| n (%) | 46 (67%) | 44 (67%) |
| Glycosylated hemoglobin, %* | ||
| Median (IQR) | 7.5 (7.1, 8.6) | 7.9 (6.9, 8.9) |
| Glycosylated hemoglobin, n (%) | ||
| <7.5% | 33 (49%) | 23 (36%) |
| ≥7.5% | 34 (51%) | 41 (64%) |
| Body mass index, kg/m2¶ | ||
| Median (IQR) | 34.0 (27.0, 38.9) | 31.0 (28.2, 35.0) |
| Mean arterial blood pressure, mmHg | ||
| Median (IQR) | 95.7 (90.3, 104.7) | 97.5 (87.3, 106.3) |
| Prior myocardial infarction | ||
| n (%) | 4 (6%) | 9 (14%) |
| Prior stroke | ||
| n (%) | 5 (7%) | 1 (2%) |
| Study eye characteristics | ||
| Visual acuity, letters | ||
| Median (IQR) | 84.0 (81.0, 87.0) | 85.0 (82.0, 88.0) |
| Visual acuity, Snellen equivalent | ||
| Median (IQR) | 20/20 (20/25, 20/20) | 20/20 (20/25, 20/20) |
| OCT CST, mm (Spectralis equivalent)† | ||
| Median (IQR) | 352 (335, 387) | 355 (337, 374) |
| OCT retinal volume, mm3 (Stratus equivalent) ‡,§ | ||
| Median (IQR) | 7.72 (7.19, 8.15) | 7.68 (7.12, 8.22) |
| Lens status, n (%) | ||
| PC IOL | 19 (28%) | 15 (23%) |
| Phakic | 50 (72%) | 51 (77%) |
| Iris color, n (%) | ||
| Blue | 19 (28%) | 28 (42%) |
| Brown | 31 (45%) | 27 (41%) |
| Other | 19 (28%) | 11 (17%) |
| Diabetic retinopathy severity,|| n (%) | ||
| Microaneurysms only | 0 | 1 (2%) |
| Mild/moderate NPDR | 58 (84%) | 48 (73%) |
| Severe NPDR | 7 (10%) | 11 (17%) |
| PDR and/or prior scatter | 4 (6%) | 6 (9%) |
| Center-involved DME present at the participant’s first visit to office | ||
| n (%) | 33 (55%) | 40 (73%) |
| At least 1 prior DME treatment | ||
| n (%) | 15 (22%) | 12 (18%) |
| Prior macular laser photocoagulation | ||
| n (%) | 7 (10%) | 9 (14%) |
| Prior intravitreal corticosteroid | ||
| n (%) | 1 (1%) | 0 |
| Prior peribulbar corticosteroid | ||
| n (%) | 1 (1%) | 0 |
| Prior vitrectomy | ||
| n (%) | 0 | 0 |
| Prior intravitreal anti-VEGF | ||
| n (%) | 11 (16%) | 4 (6%) |
| Prior panretinal scatter photocoagulation | ||
| n (%) | 4 (6%) | 5 (8%) |
| Recent or planned intravitreous (anti-VEGF or steroid) DME treatment in the nonstudy eye | ||
| n (%) | 11 (16%) | 9 (14%) |
Abbreviations: CST = central subfield thickness; DME = diabetic macular edema; IQR = interquartile range; NPDR = nonproliferative diabetic retinopathy; PBM = photobiomodulation; PC IOL = Posterior Chamber Intraocular Lenses; PDR = proliferative diabetic retinopathy.
Glycosylated hemoglobin data were missing for 2 PBM and 2 placebo participants.
OCT retinal volume was missing for 4 PBM and 3 placebo eyes.
Cirrus measurements were converted to Spectralis equivalents using the following formula: Spectralis = 40.78 + 0.95 × Cirrus.
All retinal volume measurements were converted to Stratus equivalents using the following formulas: Stratus = −43.12 + 1.01 × Cirrus; Stratus = −72.76 + 1.03 × Spectralis.13
Diabetic retinopathy severity was judged by the investigator based on clinical examination, including any retinal images taken for standard clinical care. Fundus photographs were not performed as a part of the study.
Body mass index was missing for 19 PBM and 21 placebo participants.
Treatment Group Effect
OCT CST increased from baseline to 4 months by a mean (SD) of 13 (53) μm in PBM eyes and 15 (57) μm in placebo eyes (PBM vs. placebo adjusted mean difference [95% CI] = −2 [−20 to 16] μm; P = 0.84; Table 2, Fig 3]. CI-DME was present in 61 (90%) PBM eyes and 57 (86%) placebo eyes at 4 months (PBM vs. placebo adjusted odds ratio [95% CI] = 1.30 [0.44–3.83]; P = 0.63). Additional information on retinal thickness outcomes, including the change in retinal volume, is given in Table 2 and Figure S1 (available at www.ophthalmologyretina.org). None of the preplanned subgroup analyses with at least 20 observations in each subgroup (sex, baseline CST, baseline glycosylated hemoglobin) indicated a significant interaction (Table S1, available at www.opththalmologyretina.org). Three (4%) of the PBM eyes versus 1 (2%) of the placebo eyes received non-Protocol DME treatment (anti-VEGF) (Table S2, available at www.opththalmologyretina.org).
Table 2.
OCT CST and Retinal Volume*
| OCT Outcomes | Treatment Group |
|||
|---|---|---|---|---|
| PBM (n = 68) | Placebo (n = 66) | PBM vs. Placebo Adjusted Mean Difference or Odds Ratio (95% CI) † | PBM vs. Placebo Adjusted Risk Difference (95% CI) † | |
|
| ||||
| OCT CST at baseline, μm (Spectralis equivalent) | ||||
| Mean (SD) | 366 (42) | 359 (32) | ||
| OCT CST at 4 mos, μm (Spectralis equivalent) | ||||
| Mean (SD) | 379 (69) | 374 (66) | ||
| OCT CST change from baseline to 4 mos, ‡,§μm | ||||
| Mean (SD) | 13 (53) | 15 (57) | −2 (−20, 16) P = 0.84 |
|
| Center-involved DME at 4 mos | ||||
| n (%) | 61 (90%) | 57 (86%) | 1.30 (0.44, 3.83) P = 0.63 |
3% (−19%, 27%) |
| OCT retinal volume at baseline,|| mm3 (Stratus equivalent) | ||||
| Mean (SD) | 7.82 (0.86) | 7.73 (0.69) | ||
| OCT retinal volume at 4 mos,|| mm3 (Stratus equivalent) | ||||
| Mean (SD) | 7.91 (1.01) | 7.85 (0.90) | ||
| OCT retinal volume change from baseline to 4 mos, ‡,¶ mm3 | ||||
| Mean (SD) | 0.12 (0.45) | 0.10 (0.42) | 0.01 (−0.14, 0.15) P = 0.93 |
|
Abbreviations: CI = confidence interval; CST = central subfield thickness; DME = diabetic macular edema; SD = standard deviation; PBM = photobiomodulation.
Only eyes that completed the 4-month visit were included in the calculation of descriptive statistics of OCT data. For eyes that received alternate DME treatment before 4 months (n = 3 [PBM]; n = 1 [placebo]), the last OCT measurements before alternative DME treatment were used in the place of the 4- month measurements. All analyses followed the intent-to-treat principle.
PBM vs. placebo mean differences, odds ratios and risk differences were adjusted for baseline values of the outcome and the randomization stratification factor of recent or planned intravitreous treatment in the nonstudy eye.
Multiple imputation (m = 100) was used for the missing values of CST and retinal volume change, with imputation models that were stratified by treatment and included variables for treatment group, baseline values, and change from baseline at all monthly interim visits up to the primary outcome visit and the randomization stratification factor of recent or planned intravitreous treatment in the nonstudy eye. Multiple imputation was not performed for CI-DME, given the thresholds are gender and machine specific.
OCT CST change was truncated to the mean ± 3 SD (13 ± 3 × 58)
OCT retinal volume was missing for 4 PBM and 3 placebo eyes at baseline and 5 PBM and 4 placebo eyes at 4 months.
OCT retinal volume change was truncated to the mean ± 3 SD (0.09 ± 3 × 0.42) and missing for 8 PBM and 5 placebo eyes.
Figure 3.
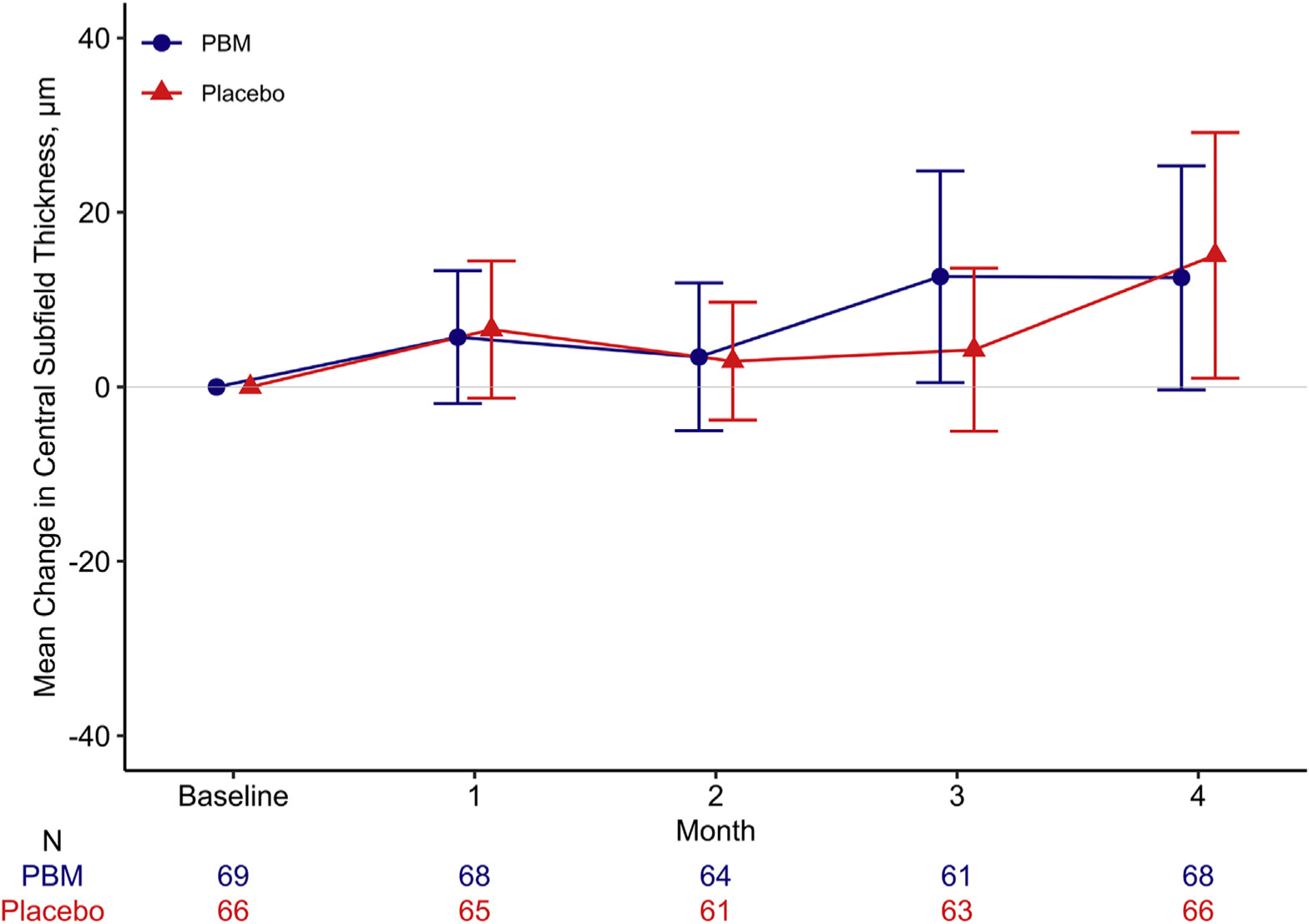
Mean change in OCT central subfield thickness (with 95% confidence limits) from baseline to 4 months among eyes in a pilot study evaluating PBM therapy for diabetic macular edema (Protocol AE). PBM = photobiomodulation.
VA decreased by at least 5 letters from baseline to 4 months in 12 (18%) of both PBM and placebo eyes (PBM vs. placebo adjusted odds ratio [95% CI] = 0.96 [0.39–2.35]; P = 0.93). VA decreased from baseline to 4 months by a mean (SD) of 0.2 (5.5) letters in PBM eyes and 0.6 (4.6) letters in placebo eyes (Table 3, Fig 4).
Table 3.
Visual Acuity
| Treatment Group |
|||
|---|---|---|---|
| Visual Acuity Outcomes | PBM (n = 68) | Placebo (n = 66) | PBM vs. Placebo Adjusted Mean Difference or Odds Ratio (95% CI) ‡ |
|
| |||
| VA at baseline, letters | |||
| Mean (SD) | 84.3 (4.0) | 84.9 (3.4) | |
| VA at 4 mos, letters | |||
| Mean (SD) | 84.1 (5.8) | 84.0 (6.6) | |
| VA change from baseline to 4 mos,*,† letters | |||
| Mean (SD) | −0.2 (5.5) | −0.6 (4.6) | 0.4 (−1.3, 2.0) P = 0.64 |
| VA decreased ≥5 letters from baseline to 4 mos† | |||
| n (%) | 12 (18%) | 12 (18%) | 0.96 (0.39, 2.35) P = 0.93 |
Abbreviations: CI = confidence interval; PBM = photobiomodulation; SD = standard deviation; VA = visual acuity.
Only eyes that completed the 4-month visit were included in the calculation of descriptive statistics of VA data. For eyes that received alternate DME treatment before 4 months (n = 3[PBM]; n = 1[placebo], the last VA measurement before alternative DME treatment were used in the place of the 4-month measurements.
VA change was truncated to the mean ± 3 SD (−0.3 ± 3 × 5.3).
Multiple imputation (m = 100) was used for the missing values of visual acuity change, with imputation models that were stratified by treatment and included variables for baseline visual acuity, change from baseline at all monthly interim visits up to the primary outcome visit, and the randomization stratification factor of recent or planned intravitreous treatment in the nonstudy eye.
PBM vs. placebo mean differences and odds ratios were adjusted for baseline VA and the randomization stratification factor of recent or planned intravitreous treatment in the nonstudy eye. All analyses followed the intent-to-treat principle.
Figure 4.
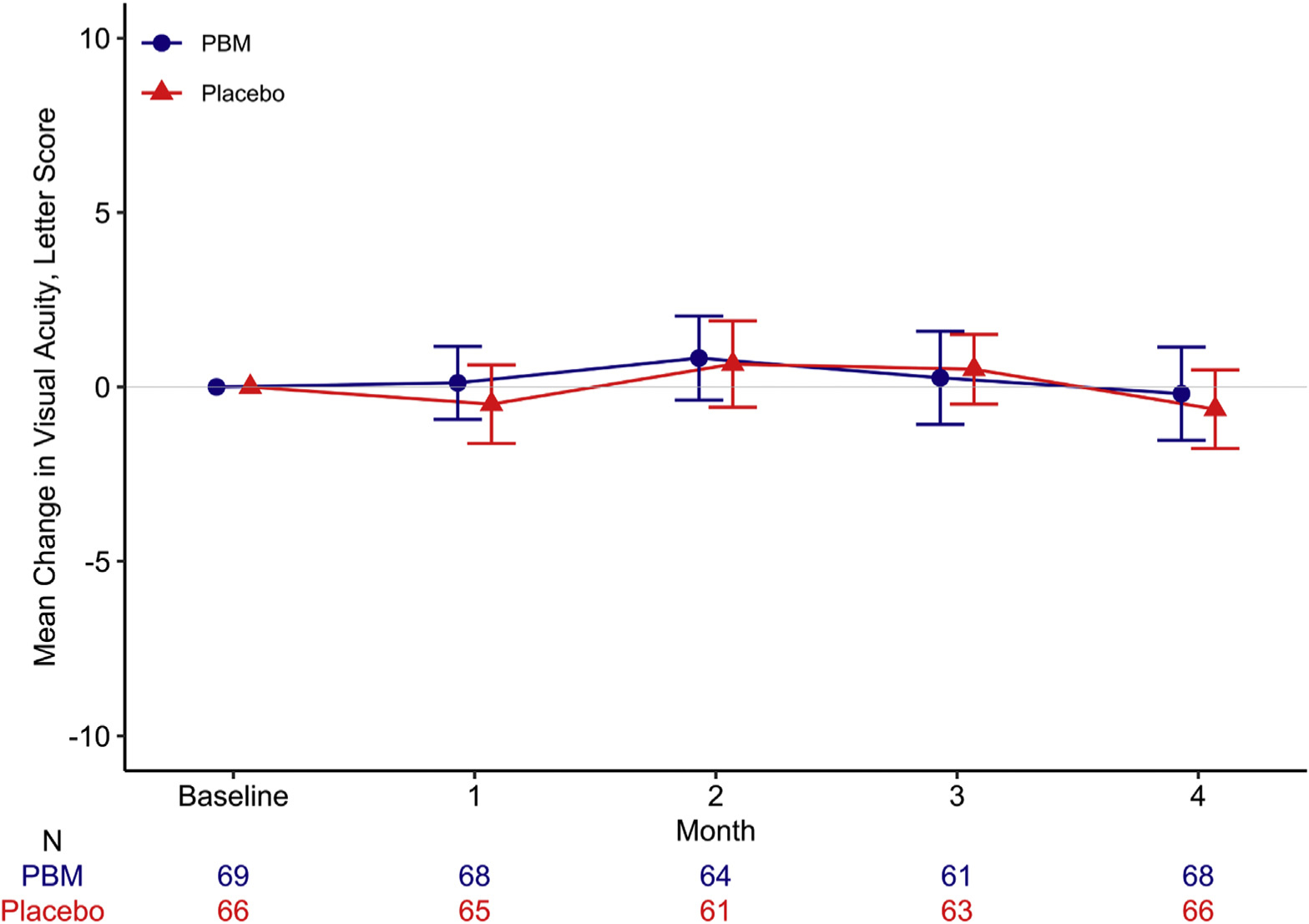
Mean change in visual acuity (with 95% confidence limits) from baseline to 4 months among eyes in a pilot study evaluating PBM therapy for diabetic macular edema (Protocol AE). PBM = photobiomodulation.
Phase 2
In eyes initially assigned to placebo that received PBM devices during phase 2 (n = 61), from 4 to 8 months OCT CST decreased by a mean (SD) of 1 (44) μm and VA decreased by a mean (SD) of 0. 9 (5.5) letters.
Treatment Compliance
Treatment compliance (defined as the proportion of device sessions completed divided by the total prescribed sessions during phase 1) was high for both groups, with a median (IQR) of 92% (82%–98%) in PBM eyes and 95% (86%–99%) in placebo eyes (Table S3, Figs S2, S3, available at www.opththalmologyretina.org). Of 13 430 treatment sessions in the PBM group, 13 000 (97%) were confirmed to be completed with the patch on the skin. There did not seem to be a significant relationship between treatment compliance and change in CST in either treatment group (Fig S4, available at www.opththalmologyretina.org).
The median treatment compliance was 95% in 40 participants who were randomly assigned to receive text reminders and 92% in the 41 participants not receiving text reminders (P = 0.44, Table S4, available at www.opththalmologyretina.org). Of the participants who received text message reminders, 60% reported that they were helpful.
Device Issues
Sixteen PBM and 22 sham devices had at least 1 issue reported, with a total of 22 PBM and 27 sham device issues. The inability to download data accounted for 29% of all reported issues. The device was replaced in 9 (41%) instances of PBM and 16 (59%) instances of sham issues. Only 1 device issue (intense brightness) was suspected to be associated with an adverse event (decreased vision up to 2–3 hours post use).
Safety
There were 8 adverse events possibly related to the PBM device, color vision changes (2), photophobia (2), eye ache (1), ocular discomfort (1), and decreased vision (2), and 2 adverse events possibly related to the placebo device, burning sensation in the face (1) and itching (1). No adverse events were serious (Table S5, available at www.opththalmologyretina.org).
Discussion
This phase 2 randomized controlled trial of PBM for eyes with CI-DME and good VA did not find beneficial anatomic or functional effects from PBM treatment as delivered in the study. Specifically, there were no significant differences identified between the PBM and placebo groups in terms of change in OCT CST or VA letter score from baseline to 4 months. PBM was safe and well-tolerated over 4 months of use, with no serious adverse events reported by study participants.
To the best of our knowledge, this is the largest randomized controlled trial to date of PBM for DME treatment. A previous nonrandomized, consecutive case series of patients with bilateral DME suggested that eyes treated with PBM for 160 seconds/day had greater reductions in retinal thickening than the untreated fellow eyes. However, this series contained only 4 patients who were followed over a wide range of 2–9 months.9 An unpublished randomized controlled trial in 10 eyes with treatment-resistant DME comparing anti-VEGF with or without PBM treatment for 4.5 J/cm2 per day, given 3 days per week over 8 weeks, was also suggestive of anatomic and functional benefit from PBM (Kim J, personal communication, 2021). Despite their promising results, both studies were inconclusive, given their small sample sizes.
In DRCR Protocol V, a similar group of eyes (n = 214) to those in the current study (CI-DME and VA 20/25 or better) that were assigned to initial observation had minimal change at 4 months in CST (mean [SD]: −11 [57] μm) and VA (mean [SD]: −0.5 [7.5] letters).12 The very small changes in Protocol AE eyes at 4 months in CST (mean [SD] PBM: 13 [53] μm; placebo: 15 [57] μm) and VA (mean [SD] PBM: −0.2 [5.5] letters; placebo: −0.6 [4.6] letters) are consistent with these results, suggesting that the primary results from this study are likely to be generalizable to larger, similar cohorts. There is no evidence to suggest that the lack of treatment effect is due to a beneficial effect of the placebo device, given the consistency with Protocol V. Also, the broad-spectrum, low-power white light that was chosen for placebo is below the thresholds used in previous preclinical and clinical studies to generate cellular or anatomic responses in the retina.
Additional data to support the lack of efficacy found for PBM in Protocol AE are found in the phase 2 results. Although the primary intent of the second phase in this study was to allow access to PBM for study participants and not to provide evidence of efficacy, results from phase 2 were consistent with phase 1 outcomes and did not identify anatomic or visual benefit from PBM.
Patient adherence to device use was excellent during phase 1 of this study, with median treatment compliance of 92% or more in both placebo and PBM groups. Compliance metrics included not only the frequency of use but also enabled analysis of whether devices were sitting on the skin when they were activated. These data suggested that the devices were used routinely and appropriately throughout the study. Patient compliance with study visits was also high with 100% of participants, excluding 1 death, completing the primary outcome visit. Furthermore, although the COVID-19 pandemic necessitated the discontinuation of phase 2 visits for some participants and stopped the switch to placebo treatment for participants assigned to PBM in phase 1, this had no impact on the study’s primary results.
Despite these strengths of the study, there are also limitations. It is possible that a different treatment algorithm using PBM at different frequencies, dosages, or wavelengths might lead to different results. In addition, this study only enrolled eyes with good vision. Despite the results from DRCR Protocol V, there may be some DME eyes with good VA that the investigators believed needed treatment and, therefore, did not enroll in the present DRCR AE study. Thus, a potential bias exists in enrolling eyes perceived to have a more favorable prognosis. It is unknown if a greater effect might be seen from PBM treatment of eyes with worse vision or greater CST than the participants in the study, although there is no known scientific rationale to suggest that an interaction with baseline vision is likely.
Although safe and well-tolerated, PBM, as given in this study, was not found to be effective for the treatment of CI-DME in eyes with good vision. Thus, the results from this trial do not support a future phase 3 study or clinical use of PBM at this dosing frequency for the treatment of DME. Additional efforts to develop safe and effective novel therapies for DME are needed to address this burgeoning global health issue.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award number UG1EY014231). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PhotoOptx provided the devices for the study. Supported by additional grants to the Jaeb Center for Health Research from the JDRF. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, all editorial content of presentations and publications related to the protocol, and the decision to submit for publication.
Abbreviations and Acronyms:
- CI
confidence interval
- CI-DME
center-involved diabetic macular edema
- CST
central subfield thickness
- DME
diabetic macular edema
- IQR
interquartile range
- PBM
photobiomodulation
- VA
visual acuity
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
HUMAN SUBJECTS: Human subjects were included in this study. Approved by the institutional review board of the Jaeb Center for Health Research. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
ANIMAL SUBJECTS: No animal subjects were included in this study.
Supplemental material available at www.ophthalmologyretina.org.
References
- 1.International Diabetes Federation. Diabetes facts and figures: IDF diabetes atlas ninth edition 2019. https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed June 23, 2021.
- 2.National Eye Institute. Facts about diabetic eye disease. https://nei.nih.gov/health/diabetic/retinopathy. Accessed June 23, 2021.
- 3.Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117: 1064–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. [DOI] [PubMed] [Google Scholar]
- 5.Korobelnik IF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. [DOI] [PubMed] [Google Scholar]
- 7.Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eells IT, Gopalakrishnan S, Valter K. Near-infrared photobiomodulation in retinal injury and disease. Adv Exp Med Biol. 2016;854:437–441. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Herda AA, Kern TS. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol. 2014;98: 1013–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Du Y, Lee CA, et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Invest Ophthalmol Vis Sci. 2013;54: 3681–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Du Y, Liu H, et al. Photobiomodulation inhibits long-term structural and functional lesions of diabetic retinopathy. Diabetes. 2018;67:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321:1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetic Retinopathy Clinical Research Network Writing Committee, Bressler SB, Edwards AR, et al. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132(9):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


