Abstract
Purpose of review
This article reviews tau PET imaging with an emphasis on first-generation and second-generation tau radiotracers and their application in neurodegenerative disorders, including Alzheimer’s disease and non-Alzheimer’s disease tauopathies.
Recent findings
Tau is a critical protein, abundant in neurons within the central nervous system, which plays an important role in maintaining microtubules by binding to tubulin in axons. In its abnormal hyperphosphorylated form, accumulation of tau has been linked to a variety of neurodegenerative disorders, collectively referred to as tauopathies, which include Alzheimer’s disease and non-Alzheimer’s disease tauopathies [e.g., corticobasal degeneration (CBD), argyrophilic grain disease, progressive supranuclear palsy (PSP), and Pick’s disease]. A number of first-generation and second-generation tau PET radiotracers have been developed, including the first FDA-approved agent [18F]-flortaucipir, which allow for in-vivo molecular imaging of underlying histopathology antemortem, ultimately guiding disease staging and development of disease-modifying therapeutics.
Summary
Tau PET is an emerging imaging modality in the diagnosis and staging of tauopathies.
Keywords: hybrid imaging, PET, PET/MRI, tau
INTRODUCTION
Molecular imaging of tau pathology is a novel modality which has risen to prominence over the last year since the FDA approval of the first-generation tau PET radiopharmaceutical, [18F]-flortaucipir, in May 2020 [1]. In this review, we provide an overview of recent advancements in the field by highlighting the clinically relevant biochemistry of tau, the development of first-generation and second-generation tau radiotracers, and the use of tau PET in Alzheimer’s disease and non-Alzheimer’s disease tauopathies. We also discuss potential future applications, including the role of tau PET in drug development and clinical treatment trials.
TAU STRUCTURE
Tau belongs to the family of microtubule-binding proteins, and it is especially common in neurons, where it binds to the microtubule cytoskeleton and stabilizes it to enable functions such as retrograde and anterograde axonal transport [2]. Tau is encoded by the microtubule associated protein tau (MAPT) gene on chromosome 17, with alternative splicing yielding six different isoforms of the protein [2,3]. Binding to tubulin, the monomers of microtubules, is made possible by up to four tubulin-binding regions [2]. The number of these repeats, however, differs depending on the specific form of tau: three isoforms only have three tubulin-binding regions [3-repeats (3R) isoforms] while the rest of the transcripts have four [4-repeats (4R) isoforms]. Importantly, the proportion of 3R to 4R isoforms is different in health and disease. Physiologically, there is an equal proportion of 3R and 4R isoforms. This also applies to the composition of aggregates in certain tauopathies, such as Alzheimer’s disease, primary age-related tauopathy (PART), and chronic traumatic encephalopathy (CTE). In contrast, 3R isoforms comprise the inclusions found in Pick’s disease, while 4R isoforms predominate in tauopathies such as progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and argyrophilic grain disease [4■]. Therefore, tauopathies can be subdivided into different categories according to the predominant primary structure of tau. However, research has shown that tau fibrils also differ in their geometric arrangements depending on the disease subtype. Abnormally hyperphosphorylated tau typically aggregates in fibrous structures, which can be distinguished by ultrastructural methods, such as cryogenic electron microscopy (Cryo-EM). These fibrils [e.g., paired helical filaments (PHF) in Alzheimer’s disease] assemble to form aggregates visible on light microscopy, including the classical neurofibrillary tangles (NFT) in Alzheimer’s disease and Pick bodies in Pick’s disease [5,6]. In recent years, ultrastructural analysis of pathologic fibrils has shown that the folding of tau is constant in patients with the same disease, but varies among those with different neurodegenerative tauopathies [4■,5,7,8]. Considering these findings, a structure-based sub-classification of tauopathies has been proposed, further highlighting the heterogeneity of disease subtypes [4■]. With respect to tau PET imaging, awareness of these differences is relevant given that while all radiotracers target pathological tau, affinities and patterns of binding will differ amongst tauopathies.
RADIOTRACERS
Over the last decade, several compounds have been investigated as potential PET radiopharmaceuticals targeting pathological tau aggregates. Ideally, such molecules should exhibit advantageous pharmacokinetic properties, demonstrate high affinity for pathological tau aggregates, and be selective over physiological tau and other proteins such as amyloid-β (Aβ), α-synuclein, TAR DNA-binding protein 43 (TDP-43) and monoamine oxidase (MAO). To that end, several first-generation and second-generation tau radiotracers have been developed.
FIRST-GENERATION TRACERS
First-generation tau PET radiotracers include arylquinoline derivatives ([18F]THK5317 and [18F]THK5351), a pyridoindole derivative ([18F]AV-1451 also known as [18F]-flortaucipir), and a phenyl/pyridinyl-butadienyl-benzothiazone/benzothiazolium (PBB) derivative ([11C]PBB3). Of these, the prototypical molecule of the first-generation tracers is [18F]-flortaucipir, which in May 2020 became the first molecule of its kind to be approved for clinical use by the FDA (Fig. 1). The compound was initially found and characterized as having high affinity for tau aggregates in Alzheimer’s disease-brain derived PHF, with very low to no binding to β-amyloid [9]. Similar results were independently reported by another group that observed no binding in a brain specimen exhibiting a very severe case of cerebral amyloid angiopathy [10]. Furthermore, flortaucipir was found to be lipophilic enough to show good permeability across the blood–brain barrier, as well as demonstrated a desirable profile of metabolization [9]. In the first human in-vivo tau-PET experiment, flortaucipir not only exhibited higher uptake in Alzheimer’s disease patients compared with healthy controls, but also a topographical binding pattern similar to the expected NFT distribution in the disease, based on histopathologic Braak staging [11-13]. In 1991, Braak and Braak published their seminal study describing six distinct stages of NFT pathology in Alzheimer’s disease patients, in which abnormal tau deposition can be detected initially in the trans-entorhinal region before progressing to the hippocampus (stages I/II = entorhinal stage), limbic structures (stages III/IV = limbic stage) and finally involving the association cortices (stages V/VI = isocortical stage) [12]. Visual reads of antemortem flortaucipir PET scans have been shown to have high sensitivity, as well as moderate-to-high specificity in predicting both postmortem Braak stage V or VI pathological tau accumulation and the presence of amyloid-β plaques sufficient to meet the criteria for high levels of Alzheimer’s disease neuropathological change [14■■]. Overall, these findings demonstrate flortaucipir PET to be a reproducible and reliable method to noninvasively diagnose pathological changes in Alzheimer’s disease.
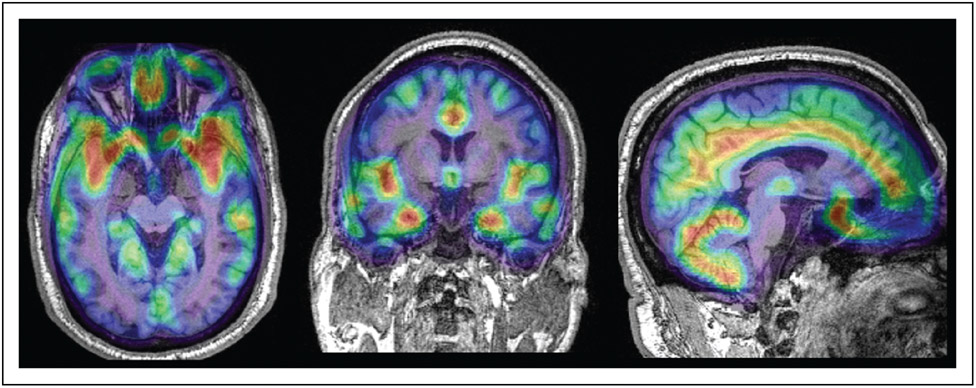
FIGURE 1.
Fifty-year-old man with amnestic mild cognitive impairment. Montreal Cognitive Assessment (MoCA) score was 20 of 30. 18F-flortacupir PET/MRI in the axial, coronal and sagittal plane demonstrates increased tracer uptake with standardized uptake value ratio more than 1.22 relative to cerebellar gray matter in a combined region including the entorhinal cortex, amygdala, inferior temporal gyrus and lateral occipital cortex. This corresponds to in vivo tau-PET Stage I/II, with Z-score in the entorhinal cortex more than 2.5 compared with cognitively normal controls, as defined by Cho et al. [40].
While flortaucipir has been validated as a surrogate biomarker for tau NFT in the setting of Alzheimer’s disease, its performance in non-Alzheimer’s disease tauopathies has been modest. In-vitro experiments analyzing the binding of the tracer to non-Alzheimer’s disease tauopathy brain slices have found either no [10] or low-to-moderate binding [15,16]. Significantly, while binding was faint in these cases, it did colocalize with abnormal tau aggregates, indicating that the specificity of flortaucipir for tau aggregates is conserved but that the target affinity is reduced [15]. In line with these studies, flortaucipir PET imaging studies in PSP patients have shown increased uptake in some, but not all, disease-associated regions [17]. Overall, flortaucipir has been shown to have limited utility in the diagnosis of non-Alzheimer’s disease tauopathies, due to its low affinity for non-Alzheimer’s disease fibrils. As for other neurodegenerative proteinopathies, the compound has not shown binding to aggregates of α-synuclein or TDP-43 [10,15].
Several studies have found clinically significant off-target binding of flortaucipir to pigment-containing structures, including neuromelanin in the substantia nigra, lipofuscin-containing neurons in the lateral geniculate nucleus, and calcified structures such as the choroid plexus, possibly confounding the ability to visualize binding in key mesial temporal structures [10,14■■] and cerebellum, which may cause bias for standardized uptake value ratio (SUVR) measures using cerebellum as reference [18]. Case reports of flortaucipir binding to meningiomas, chronic infarcts and cavernous malformations further support the notion that flortaucipir binding is affected by pigmentation and mineralization [19]. In addition, flortaucipir has been found to bind to MAO A/B, compromising the tracer’s specificity in the brain where these enzymes are common [20].
NEXT-GENERATION TRACERS
While flortaucipir has been proven to be advantageous in the diagnosis of Alzheimer’s disease, in view of its limitations including off-target binding in clinically important structures and low affinity for tau fibrils in non-Alzheimer’s disease tauopathies, there is an unmet need for alternative clinically applicable radiopharmaceuticals. A number of potential second-generation agents have been investigated, including [18F]-RO-948, [18F]-MK-6240, [18F]-PI-2620, [18F]-JNJ-311, and [18F]-GTP1 [21■]. Initial in vitro experiments with second-generation radiotracers demonstrated not only high-selective binding to Alzheimer’s disease-type NFT and 3R-rich and 4R-rich brain homogenates derived from Pick’s disease and PSP patients, respectively, but also low off-target binding to β-amyloid and MAO [22■■,23,24,25■]. With respect to Alzheimer’s disease, several studies have shown increased uptake in disease-relevant brain regions, low uptake in healthy controls, and a positive correlation between binding and cognitive decline, suggesting that these tracers are highly suitable for the diagnosis and monitoring of Alzheimer’s disease (Figs. 2 and 3) [25■,26■■,27■■,28].
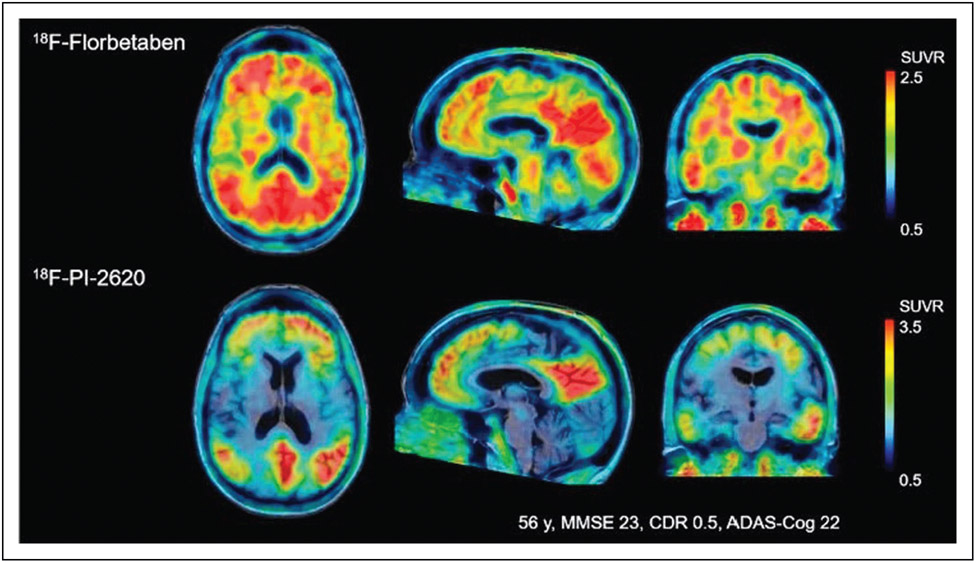
FIGURE 2.
Comparison of 18F-florbetaben amyloid-PET and [18F] PI-2620 tau PET obtained in a 56-year-old subject with Mini-Mental State Examination (MMSE) score of 23. Images were normalized to the cerebellar gray matter and co-registered to the subject’s MRI. Please note increased cortical amyloid burden (A+) with associated tau-PET binding in disease relevant-areas (T+), with lack of off-target binding in the choroid plexus and subcortical structures typically seen with first-generation tau radiotracers. Reproduced with permission [27■■].
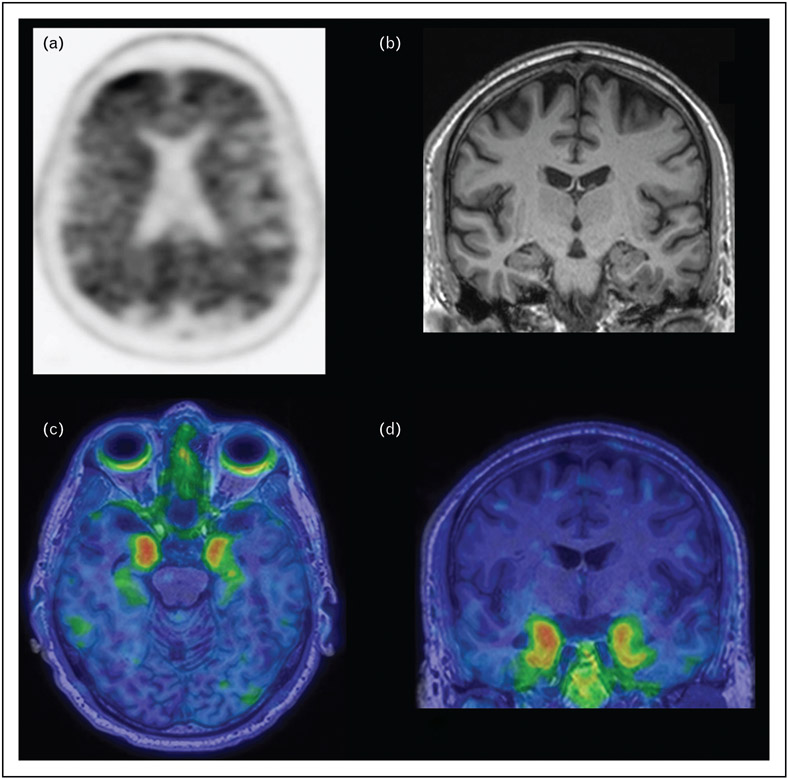
FIGURE 3.
Sixty-year-old man presenting with memory loss. Amyloid PET scan with Pittsburgh Compound B demonstrates diffuse cortical amyloid deposition (a). Coronal T1-weighted MRI demonstrates no significant hippocampal or lobar-specific atrophy (b). Axial (c) and coronal (d) tau PET images utilizing the 2nd generation tracer 18F-MK6240 fused to T1-weighted MRI show increased tau deposition in the bilateral mesial temporal lobes.
There have been mixed reports regarding the in-vivo performance of next-generation radiotracers as viable biomarkers for 4R tauopathies. A PI-2620 PET study imaged a cohort of PSP, CBD, Alzheimer’s disease patients and healthy controls utilizing static imaging at 60–90 min post injection (p.i) and found increased uptake in the globus pallidus of 4R tauopathy patients, but not in other subcortical areas where pathology would be expected. Furthermore, the cohort of healthy volunteers also demonstrated some increased uptake in the globus pallidus, limiting diagnostic performance [28]. On the other hand, a different study with a larger cohort posed the same question but acquired the images 0–60 min p.i with dynamic technique [22■■]. This group found increased PI-2620 uptake in the globus pallidus and other expected subcortical regions, as well as a moderate-to-high-discriminative performance between PSP and controls (Fig. 4) [22■■]. Importantly, these studies did not find any correlation between PI-2620 uptake and disease severity, and no significant in-vivo binding in areas such as the dorsal midbrain and the prefrontal cortex, which are known to be affected in PSP [22■■,28]. Another study compared PI-2620 uptake in patients with corticobasal syndrome (CBS), either with or without evidence of Aβ positivity, representing either Alzheimer’s disease-CBS or 4R-CBS. Comparing these groups, in-vivo uptake was higher in the Aβ + group, group, but the Aβ − group showed increased uptake in frontal cortical regions typically affected in CBS [29■■]. Overall, study results suggest that second-generation tau radiotracers show great promise as Alzheimer’s disease biomarkers with less off-target binding than flortaucipir, as well as some potential as diagnostic biomarkers in patients with suspected 4R tauopathy, particularly if utilizing dynamic acquisition protocols [25■].
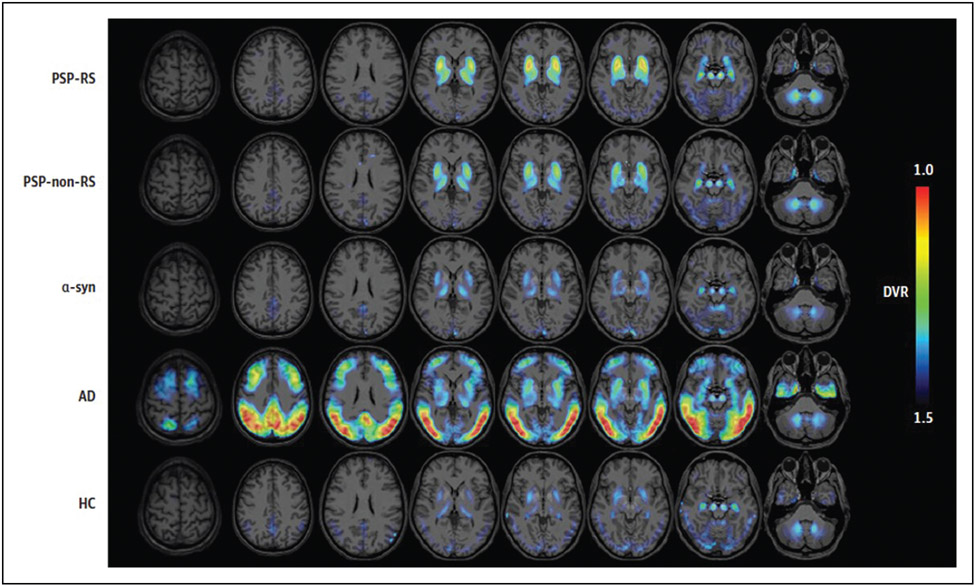
FIGURE 4.
Average [18F]-PI-2620 distribution volume ratio binding maps presented as axial overlays on a standard MRI template for patients with progressive supranuclear palsy-Richardson’s syndrome, progressive supranuclear palsy-non-Richardson’s syndrome, α-synucleinopathy, Alzheimer’s disease and healthy controls. Please note disease-specific uptake in the basal ganglia, subthalamic nucleus, substantia nigra and dentate nuclei in progressive supranuclear palsy patients. Reproduced with permission [22■■].
TAU PET IN ALZHEIMER’S DISEASE
Tau PET has been a field of intense research in the last decade. In the next section, we highlight the role of tau PET in Alzheimer’s disease, its use in defining and staging the disease, and its role in atypical Alzheimer’s disease variants.
Tau PET in Alzheimer’s disease diagnosis
Alzheimer’s disease has long been considered a clinico-pathological entity that comprises both a typical amnestic syndrome and the presence of three hallmark pathological features in postmortem brain: Aβ plaques, NFT, and neurodegeneration [30]. However, it has since been recognized that a proportion of patients presenting with the typical clinical syndrome will not have amyloidopathy and/or tauopathy on neuropathology [31,32]. Furthermore, patients with Alzheimer’s disease-associated neuropathological changes postmortem can present clinically with nonamnestic syndromes. Therefore, a biological definition of Alzheimer’s disease has been advocated by different scientific communities, including the National Institute of Aging (NIA) and the Alzheimer’s Association. This collaboration has jointly released a research framework definition for Alzheimer’s disease, where the disease is defined as a function of the presence of biomarkers of amyloidopathy, tauopathy, and neurodegeneration/neuronal injury (A/T/N) [30], as well as the clinical syndrome.
Due to studies that have successfully correlated antemortem tau tracer uptake with the presence of NFT on pathology, tau PET is now recognized as a valid imaging biomarker to assess the tauopathy, ‘T component’, of Alzheimer’s disease [14■■]. Cerebrospinal fluid (CSF) concentration of hyperphosphorylated tau (p-tau) is another tool used to determine tau pathology, but it provides different information than PET; while tracer binding in PET can monitor tau accumulation over time, CSF measurements typically provide only a snapshot of the current pathological state and are less sensitive to changes over time [30]. Considering this key difference, one may hypothesize that the more advanced the disease, the higher the relevance of tau PET. Mattsson and colleagues compared the diagnostic ability of CSF p-tau and flortaucipir PET in differentiating cognitively healthy controls from Alzheimer’s disease patients. They found that while the diagnostic performance did not differ in patients with prodromal Alzheimer’s disease, tau PET was superior in the dementia stage, with close to perfect diagnostic performance for mild to moderate Alzheimer’s disease [33]; thereby showing that even though both methods can identify tauopathy in Alzheimer’s disease, tau PET outperforms CSF parameters particularly in advanced disease.
Apart from assessing the degree of tauopathy, tau PET also provides information on neuronal injury, a pathological hallmark traditionally defined by regional hypometabolism on FDG-PET, increased total CSF tau, and cortical atrophy on structural imaging [30]. It has been shown that early-phase (0–5 min p.i.) tau PET, which reflects cerebral blood perfusion, correlates well with FDG-PET hypometabolism in Alzheimer’s disease patients [34■,35,36], raising the possibility of optimizing one-stop shop protocols to enable noninvasive assessment of both tauopathy and neuronal injury in a single PET study. Finally, combining tau PET with novel serum-based plasma phosphorylated tau (p-tau) may achieve earlier diagnosis and optimal accuracy in following progression of Alzheimer’s disease [37].
Tau PET in staging of Alzheimer’s disease
Tau PET has great potential in contributing to the accurate diagnosis of Alzheimer’s disease, adding to our ability to stage disease beyond amyloid PET. Moreover, postmortem NFT burden in cortical areas is significantly correlated with the severity of clinical symptoms, which is not the case for β-amyloid [38,39]. These studies also demonstrated that patients with early stages of NFT pathology (Braak stages I/II) may not have apparent cognitive deficits, establishing that tau pathology is present even during the preclinical stages of the disease [12,39]. Overall, these findings raise the possibility that tau PET can be used to stage patients and identify those in the preclinical stages of disease. Schöll et al. [40] examined the possibility of replicating Braak staging in vivo with tau PET. They imaged a cohort composed of healthy young adults, cognitively healthy older adults, and patients with probable Alzheimer’s disease. By delineating regions-of-interest that approximated the entorhinal, limbic, and isocortical stages, they measured tracer uptake and computed thresholds for assigning a stage to each participant. They identified uptake patterns as expected neuropathologically, as well as concordance of the distribution of PET-derived stages in healthy older adults with the distribution of neuropathologically-derived Braak stages found in cognitively healthy older adults [41,42]. Furthermore, increased tau PET stage was associated with declining cognitive status [40]. In another study avoiding a priori parcellation of Braak regions, Cho and colleagues attempted to use tau-PET to stage participants in different stages of the Alzheimer’s disease continuum [43]. They delineated 25 cortical regions and calculated the Z-scores of regional SUVR) values. By assuming that areas with more frequent and higher flortaucipir uptake were affected first, they sorted regions by Z-score values and established a spreading pattern. Strikingly, the resulting spreading pattern closely resembled the histopathological Braak stages [43]. Due to this observation and the stepwise nature of the spreading pattern, the group was able to define a tau PET-based staging system (Fig. 1) [43]. Demonstrating that in-vivo tau PET can closely reproduce the Braak spreading pattern of NFT pathology without a priori knowledge of the regions highlights the potential of tau-PET in Alzheimer’s disease staging. Nonetheless, both the Schöll and Cho studies are limited by the lack of postmortem neuropathological validation. Meanwhile, Fleisher and colleagues’ PET-to-autopsy validation study demonstrated that prediction of postmortem high neuropathological Braak stage is possible with high sensitivity and specificity by antemortem visual assessment of flortaucipir PET scans [14■■]. These findings further highlight the relevance of in-vivo tau PET imaging as a reliable method for Alzheimer’s disease staging.
The potential of tau PET to identify individuals in preclinical stages of Alzheimer’s disease has been researched by different groups. Ossenkoppele and colleagues examined the ability of tau PET to predict cognitive decline in large discovery and validation cohorts of participants across the Alzheimer’s disease clinical continuum. They found that tracer uptake in the temporal cortex by both flortaucipir and the second-generation tracer [18F]RO-948 predicted cognitive decline in all patient groups including Aβ-positive cognitively normal participants, a group likely representing the early preclinical stages of Alzheimer’s disease. In addition, the predictive performance in this cohort was superior to volumetric MRI and Aβ PET [44■■]. Another group found brain regions of increased uptake of flortaucipir helpful in detecting patients with preclinical Alzheimer’s disease [45]. Through computational models, they arrived at the conclusion that the entorhinal cortex, inferior temporal cortex, lateral occipital cortex, and amygdala were the most informative regions. They also averaged the SUVR in these regions to come up with a summary measure of flortaucipir uptake and defined a threshold above which a cognitively normal patient may be classified as having high tau-PET burden. Patients belonging to the cognitively normal high-tau group had positive amyloid PET and worse cognitive performance, indicating that such a summary measure of tau tracer binding can in fact be used for identifying Alzheimer’s disease patients prior to onset of clinical symptoms [45]. Overall, multiple studies have demonstrated that not only is tau-PET comparable with amyloid-PET in establishing the presence of Alzheimer’s disease-type pathology, but it has the added benefit of enabling antemortem biological staging of disease.
Tau PET in atypical Alzheimer’s disease
As discussed earlier, a limitation in the diagnosis of Alzheimer’s disease is the heterogeneous clinical presentation of patients, particularly those with atypical Alzheimer’s disease syndromes, entities that are characterized neuropathologically by tau and amyloid aggregates, but often in a different spatial distribution, leading to distinct clinical syndromes: posterior cortical atrophy (PCA), logopenic variant primary progressive aphasia (lvPPA) and frontal (behavioral/dysexecutive) variant Alzheimer’s disease (fvAD). Tau-PET has also been used to characterize these diseases in vivo, and appears to mirror patterns described by neuropathology. In PCA and lvPPA, two independent groups demonstrated that flortaucipir binding was most prominent in the occipital regions and in the language-dominant left hemisphere, respectively [46,47]. Furthermore, flortaucipir binding was found to be inversely correlated to FDG metabolism [46] and positively correlated to cortical atrophy [47]. In addition, amyloid tracer binding showed extensive but diffuse binding in these Alzheimer’s disease subtypes, not corresponding to areas of regional atrophy. The flortaucipir binding patterns corresponded spatially to regions of cortical atrophy [48,49], strongly suggesting that tau accumulation, rather than β-amyloid, drives neurodegeneration and is responsible for the cognitive deterioration in both syndromes. As for fvAD, most studies have found neurodegeneration in temporoparietal regions, but some studies have noted pronounced frontal involvement [50-52]. Using antemortem tau-PET imaging, one group found heterogeneous frontal binding patterns in a cohort of patients with fvAD [52]. Since frontal lobe associated behavioral symptoms are predominant in fvAD, these findings suggest that the clinical symptomatology might not be driven solely by tau deposition.
Overall, tau-PET not only shows the ability to reliably reproduce tau pathology patterns in atypical Alzheimer’s disease syndromes, but also sheds light onto the pathophysiology of these Alzheimer’s disease subtypes. Furthermore, tau imaging promises to serve as a reliable tool in differentiating atypical Alzheimer’s disease from nontau differential diagnoses such as dementia with Lewy bodies, other variants of primary progressive aphasia, and behavioral variant frontotemporal dementia (bvFTD).
TAU PET IN NON-ALZHEIMER’S DISEASE TAUOPATHIES
Tau PET can also be useful in non-Alzheimer’s disease tauopathies, most notably frontotemporal lobar degeneration (FTLD), which encompasses a group of heterogeneous neurodegenerative disorders typically demonstrating frontal and/or temporal cortical atrophy. Several pathologic FTLD subtypes are currently recognized (e.g., FTLD-tau, FTLD-TDP, and FTLD-FUS [fused in sarcoma]). Within FTLD-tau, three pathological types exist based on location and type of tau pathology: CBD, PSP, and Pick’s disease. Similar to the concordance seen between tau PET uptake and expected neuropathologic distribution of tau deposition in Alzheimer’s disease, studies have demonstrated tracer uptake at the expected sites of neurodegeneration in these disorders (Fig. 4) [53-56]. However, results have been mixed depending on the specific PET radiopharmaceutical used, as detailed in NEXT-GENERATION TRACERS section. In addition, postmortem studies have demonstrated discordance between tau pathology and in-vivo PET imaging, suggesting that off-target binding in the midbrain and basal ganglia related to MAO-B and/or mineralization may be confounding interpretation in some cases, as previously described.
In addition to pathologic heterogeneity, FTLD also consists of distinct clinical syndromes, including semantic variant primary progressive aphasia (svPPA), nonfluent variant PPA (nfvPPA) and bvFTD, which are associated with differing proportions of pathological tau subtypes. While svPPA is most commonly associated with TAR DNA-binding protein 43 (TDP-43) type C pathology, increased flortaucipir uptake has been identified at the site of asymmetric atrophy in some patients, suggesting possible off-target binding of this tracer to nontau molecules associated with neurodegeneration [57]. Tau tracer uptake has also been demonstrated in some cases of bvFTD involving the basal ganglia, anterior cingulate, and insular white matter, corresponding to expected regions of neurodegeneration [58].
Increased tau tracer uptake has been identified in patients with CTE (CTE), in which abnormal tau deposition results from the sequela of repetitive traumatic brain injury [59]. In patients with Lewy body pathology (α-synucleinopathies), including Parkinson’s disease, tau pathology is uncommon and reported antemortem binding on flortaucipir PET likely reflects off-target binding [60,61].
Further research investigating the utility of first-generation and second-generation tau tracers in non-Alzheimer’s disease tauopathies and other neurodegenerative disorders is warranted.
TAU PET IN DEMENTIA THERAPEUTICS
As with other molecular neuroimaging biomarkers, such as amyloid PET, there is considerable hope that the development of tau radiopharmaceuticals will not only aid in the diagnosis of tauopathies, but also facilitate the development of tau-targeting dementia therapeutics. A recently published study surveyed the drug development pipeline for Alzheimer’s disease by interrogating the Clinical-Trials.gov registry [62■■]. They found that among 126 agents currently being evaluated, 11 candidates are classified as disease-modifying treatments targeting tau neurobiology. The group also surveyed diagnostic biomarkers used in the drug development pipeline. Importantly, while far less frequently used than CSF tau measurements, tau-PET is incorporated in seven clinical trials as an outcome measure and in two as an entry criterion [61]. These trends highlight that this novel molecular imaging modality is rapidly gaining acceptance in the research community and emphasize the relevance of tau PET not only as a tool for clinical diagnosis, but also for the development of new dementia pharmaceuticals as they emerge in the research setting and hopefully transition into future clinical practice.
CONCLUSION
Currently, tau PET is an emerging imaging tool for the diagnostic assessment of patients with cognitive impairment, which has vast potential in identifying tau pathology in vivo and biologically staging neurodegenerative disorders. Furthermore, it facilitates identification of atypical Alzheimer’s disease-variants and helps distinguish Alzheimer’s disease from other dementia subtypes with overlapping clinical phenotypes. In FTLD, tau PET has demonstrated a role in diagnosing 4R tauopathies including PSP and CBS, and there is hope that further advancements and new tracers will allow for better characterization of this heterogeneous disease group. Finally, tau PET has a major role in aiding current and future tau-targeting and other disease modifying drug development in Alzheimer’s disease, by serving as a molecular imaging biomarker which reliably depicts spatiotemporal disease progression in vivo and correlates with clinical symptoms and disease severity.
KEY POINTS.
The development of tau-specific PET radiopharmaceuticals allows for detection of tau deposition in vivo, which was previously detectable only postmortem.
Tau PET can be used in the evaluation of both Alzheimer’s disease and non-Alzheimer’s disease tauopathies, including FTLD-tau (e.g., CBD, PSP and Pick’s disease).
First-generation, and more recently, second-generation tau radiotracers have been developed, each with important strengths and limitations.
Financial support and sponsorship
The current work was supported in part by the NIH/NIA R01AG068398 (G.C.), NIH/NIA R01AG057848 (Y.L.), and Foundation of the ASNR Boerger grant (A.F.).
Footnotes
Conflicts of interest
Dr Gordon has received research support without direct compensation from Eisai, AbbVie, Janssen, and Novo Nordisk, and has been a consultant for METiS Pharmaceuticals. Dr Franceschi has received research support without direct compensation from Life Molecular Imaging GmbH, and has served as a consultant for Biogen Inc and Life Molecular Imaging GmbH. Dr Chiang has served as a consultant for Biogen Inc. and Life Molecular Imaging GmbH.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Food and Drug Administration. URL: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease. [Accessed date 1st Oct 2021]
- 2.Avila J, Jimenez JS, Sayas CL, et al. Tau structures. Front Aging Neurosci 2016; 8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neve RL, Harris P, Kosik KS, et al. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 1986; 387:271–280. [DOI] [PubMed] [Google Scholar]
- 4.■. Shi Y, Zhang W, Yang Y, et al. Structure-based classification of tauopathies. Nature 2021; 598:359–363. Study outlines a hierarchical classification of tauopathies on the basis of their filament folds, which complements clinical diagnosis and neuropathology and also allows the identification of new entities.
- 5.Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017; 547:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci 2016; 17:5–21. [DOI] [PubMed] [Google Scholar]
- 7.Falcon B, Zhang W, Murzin AG, et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 2018; 561:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Tarutani A, Newell KL, et al. Novel tau filament fold in corticobasal degeneration. Nature 2020; 580:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia CF, Arteaga J, Chen G, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 2013; 9:666–676. [DOI] [PubMed] [Google Scholar]
- 10.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015; 78:787–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis 2013; 34:457–468. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–259. [DOI] [PubMed] [Google Scholar]
- 13.Marquié M, Siao Tick Chong M, Antón-Fernández A, et al. [F-18]-AV-1451 binding correlates with postmortem neurofibrillary tangle Braak staging. Acta Neuropathol 2017; 134:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.■■. Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol 2020; 77:829–839. This study’s findings suggest that PET imaging with flortaucipir can be used to identify the density and distribution of Alzheimer’s disease-type tau pathology and the presence of high levels of Alzheimer’s disease neuropathological change, supporting a neuropathological diagnosis of Alzheimer’s disease.
- 15.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 2016; 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [(18)F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement 2016; 12:1116–1124. [DOI] [PubMed] [Google Scholar]
- 17.Schonhaut DR, McMillan CT, Spina S, et al. (18) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann Neurol 2017; 82:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Li Y, Pirraglia E, et al. Alzheimer’s Disease Neuroimaging Initiative. Quantitative evaluation of tau PET tracers 18F-THK5351 and 18F-AV-1451 in Alzheimer’s disease with standardized uptake value peak-alignment (SUVP) normalization. Eur J Nucl Med Mol Imaging 2018; 45:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockhart SN, Ayakta N, Winer JR, et al. Elevated (18)F-AV-1451 PET tracer uptake detected in incidental imaging findings. Neurology 2017; 88:1095–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeiren C, Motte P, Viot D, et al. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov Disord 2018; 33:273–281. [DOI] [PubMed] [Google Scholar]
- 21.■. Bischof GN, Dodich A, Boccardi M, et al. Clinical validity of second-generation tau PET tracers as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur J Nucl Med Mol Imaging 2021; 48:2110–2120. Study reviews current literature which provides preliminary evidence on the establishment of the second-generation tau PET tracers into the clinical context, thereby successfully addressing some methodological issues from the tau PET tracer of the first generation.
- 22.■■. Brendel M, Barthel H, van Eimeren T, et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol 2020; 77:1408–1419. This multicenter evaluation indicates a value of 18F-PI-2620 to differentiate suspected patients with PSP, potentially facilitating more reliable diagnosis of PSP (4R tauopathy).
- 23.Kroth H, Oden F, Molette J, et al. Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging 2019; 46:2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murugan NA, Chiotis K, Rodriguez-Vieitez E, et al. Cross-interaction of tau PET tracers with monoamine oxidase B: evidence from in silico modelling and in vivo imaging. Eur J Nucl Med Mol Imaging 2019; 46:1369–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.■. Yap SY, Frias B, Wren MC, et al. Discriminatory ability of next-generation tau PET tracers for Alzheimer’s disease. Brain 2021; 144:2284–2290. Study provides head-to-head comparison of next generation tau PET tracers PI2620, RO948, MK6240 and JNJ067 in human postmortem brain tissue from a cohort of 25 dementia cases and age-matched controls using quantitative phosphorimaging with tritium-labeled radiotracers in conjunction with phosphotau specific immunohistochemistry.
- 26.■■. Mormino EC, Toueg TN, Azevedo C, et al. Tau PET imaging with (18)F-PI-2620 in aging and neurodegenerative diseases. Eur J Nucl Med Mol Imaging 2021;48:2233–2244. Preliminary results suggest strong differences in the medial temporal lobe and cortical regions known to be impacted in Alzheimer’s disease using 18F-PI-2620 in patients along the Alzheimer’s disease trajectory.
- 27.■■. Mueller A, Bullich S, Barret O, et al. Tau PET imaging with (18)F-PI-2620 in patients with Alzheimer disease and healthy controls: a first-in-humans study.J Nucl Med 2020; 61:911–919. Study presents initial clinical data obtained in Alzheimer’s disease and HC subjects demonstrating high-image quality and excellent signal-to-noise ratio of 18F-PI-2620 for imaging tau deposition in Alzheimer’s disease.
- 28.Tezuka T, Takahata K, Seki M, et al. Evaluation of [(18)F]PI-2620, a second-generation selective tau tracer, for assessing four-repeat tauopathies. Brain Commun 2021; 3:fcab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.■■. Palleis C, Brendel M, Finze A, et al. Cortical [(18) F]PI-2620 binding differentiates corticobasal syndrome subtypes. Mov Disord 2021; 36:2104–2115. Study data indicates value of [18F]PI-2620 for evaluating corticobasal syndrome, providing quantitatively and regionally distinct signals in β-amyloid-positive and negative corticobasal syndrome.
- 30.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not ‘brain aging’: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011; 121:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrano-Pozo A, Qian J, Monsell SE, et al. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol 2014; 75:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattsson N, Smith R, Strandberg O, et al. Comparing (18)F-AV-1451 with CSF t-tau and p-tau for diagnosis of Alzheimer disease. Neurology 2018; 90:e388–e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.■. Beyer L, Nitschmann A, Barthel H, et al. Early-phase [(18)F]PI-2620 tau-PET imaging as a surrogate marker of neuronal injury. Eur J Nucl Med Mol Imaging 2020; 47:2911–2922. This study suggests that early-phase imaging of [18F]PI-2620 can serve as a surrogate biomarker for neuronal injury. Dynamic imaging or a dual time-point protocol for tau-PET imaging may supersede additional [18F]FDG-PET imaging by indexing both the distribution of tau and the extent of neuronal injury.
- 35.Brendel M, Wagner L, Levin J, et al. Perfusion-phase [(18)F]THK5351 tau-PET imaging as a surrogate marker for neurodegeneration. J Alzheimers Dis Rep 2017; 1:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Vieitez E, Leuzy A, Chiotis K, et al. Comparability of [(18)F]THK5317 and [(11)C]PIB blood flow proxy images with [(18)F]FDG positron emission tomography in Alzheimer’s disease. J Cereb Blood Flow Metab 2017; 37:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leuzy A, Smith R, Cullen NC, et al. Biomarker-based prediction of longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol 2021. doi: 10.1001/jamaneurol.2021.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 1992; 42:1681–1688. [DOI] [PubMed] [Google Scholar]
- 39.Bierer LM, Hof PR, Purohit DP, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 1995; 52:81–88. [DOI] [PubMed] [Google Scholar]
- 40.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016; 89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 42.Braak H Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997; 18:351–357. [DOI] [PubMed] [Google Scholar]
- 43.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 2016; 80:247–258. [DOI] [PubMed] [Google Scholar]
- 44.■■. Ossenkoppele R, Smith R, Mattsson-Carlgren N, et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol 2021; 78:961–971. The findings of this prognostic study suggest that tau PET is a promising tool for predicting cognitive change that is superior to amyloid PET and MRI and may support the prognostic process in preclinical and prodromal stages of Alzheimer’s disease.
- 45.Mishra S, Gordon BA, Su Y, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 2017; 161:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016; 139:1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia C, Makaretz SJ, Caso C, et al. Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol 2017; 74:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crutch SJ, Lehmann M, Schott JM, et al. Posterior cortical atrophy. Lancet Neurol 2012; 11:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R. Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: a review. Front Neurol 2018; 9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blennerhassett R, Lillo P, Halliday GM, et al. Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis 2014; 39:63–70. [DOI] [PubMed] [Google Scholar]
- 51.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 2015; 138:2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singleton E, Hansson O, Pijnenburg YAL, et al. Heterogeneous distribution of tau pathology in the behavioural variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2021; 92:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Utianski RL, Schwarz CG, Murray ME, et al. In vivo imaging and autoradiography in a case of autopsy-confirmed pick disease. Neurol Clin Pract 2021; 11:e11–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho H, Choi JY, Hwang MS, et al. Subcortical (18) F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord 2017; 32:134–140. [DOI] [PubMed] [Google Scholar]
- 55.Schroter N, Blazhenets G, Frings L, et al. Tau imaging in the 4-repeat-tauopathies progressive supranuclear palsy and corticobasal syndrome: a 11C-pyridinyl-butadienyl-benzothiazole 3 PET pilot study. Clin Nucl Med 2020; 45:283–287. [DOI] [PubMed] [Google Scholar]
- 56.Smith R, Scholl M, Widner H, et al. In vivo retention of (18)F-AV-1451 in corticobasal syndrome. Neurology 2017; 89:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makaretz SJ, Quimby M, Collins J, et al. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry 2018; 89:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho H, Seo SW, Choi JY, et al. Predominant subcortical accumulation of (18)F-flortaucipir binding in behavioral variant frontotemporal dementia. Neurobiol Aging 2018; 66:112–121. [DOI] [PubMed] [Google Scholar]
- 59.Lesman-Segev OH, La Joie R, Stephens ML, et al. Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. Neuroimage Clin 2019; 24:102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marquie M, Verwer EE, Meltzer AC, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol Commun 2017; 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen AK, Damholdt MF, Fedorova TD, et al. In Vivo cortical tau in Parkinson’s disease using 18F-AV-1451 positron emission tomography. Mov Disord 2017; 32:922–927. [DOI] [PubMed] [Google Scholar]
- 62.■■. Cummings J, Lee G, Zhong K, et al. Alzheimer’s disease drug development pipeline. Alzheimers Dement (N Y) 2021; 7:e12179. This pipeline analysis shows that target biological processes are more diversified, biomarkers are more regularly used, and repurposed agents are being explored to determine their utility for the treatment of Alzheimer’s disease.