Abstract
Background
Serum procalcitonin (PCT) has become an emerging prognostic biomarker of disease progression in patients with COVID-19. This study aims to determine the optimal cut-off value of PCT with regards to important clinical outcomes, especially for mechanical ventilation and all-cause mortality among moderate to severe COVID-19 patients in Malaysia.
Methods
A total of 319 moderate to severe COVID-19 patients hospitalized at the National Referral Hospital in December 2020 were included in the study retrospectively. Demographics, comorbidities, the severity of COVID-19 infection, laboratory and imaging findings, and treatment given were collected from the hospital information system for analysis. The optimal cut-point values for PCT were estimated in two levels. The first level involved 276 patients who had their PCT measured within 5 days following their admission. The second level involved 237 patients who had their PCT measured within 3 days following their admission. Further, a propensity score matching analysis was performed to determine the adjusted relative risk of patients with regards to various clinical outcomes according to the selected cut-point among 237 patients who had their PCT measured within 3 days.
Results
The results showed that a PCT level of 0.2 ng/mL was the optimal cut-point for prognosis especially for mortality outcome and the need for mechanical ventilation. Before matching, patients with PCT ≥ 0.2 ng/mL were associated with significantly higher odds in all investigated outcomes. After matching, patients with PCT > 0.2 ng/mL were associated with higher odds in all-cause mortality (OR: 4.629, 95% CI 1.387–15.449, p = 0.0127) and non-invasive ventilation (OR: 2.667, 95% CI 1.039–6.847, p = 0.0415). Furthermore, patients with higher PCT were associated with significantly longer days of mechanical ventilation (p = 0.0213). There was however no association between higher PCT level and the need for mechanical ventilation (OR: 2.010, 95% CI 0.828–4.878, p = 0.1229).
Conclusion
Our study indicates that a rise in PCT above 0.2 ng/mL is associated with an elevated risk in all-cause mortality, the need for non-invasive ventilation, and a longer duration of mechanical ventilation. The study offers concrete evidence for PCT to be used as a prognostication marker among moderate to severe COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Risk factor, Procalcitonin, Mechanical ventilation, Mortality
Background
Since the outbreak of coronavirus disease 2019 (COVID-19) in December 2019 in Wuhan, China, the COVID-19 has rapidly spread across the world and was declared as a “Public Health Emergency of International Concern” on 30th January 2020. Then, it emerged as an unanticipated threat to global health and led the World Health Organisation (WHO) to further declare COVID-19 as a pandemic on 11th March 2020 [1]. As of 18th September 2021, there are more than 226 million confirmed COVID-19 cases with 4.7 million deaths reported globally. Malaysia has reported 2,049,750 cases with 22,355 deaths on the same day [2].
The clinical spectrum of COVID-19 ranges from asymptomatic to symptomatic severe disease with multiorgan involvement and failure requiring intensive care (ICU) admission and mechanical ventilation [3]. The broad clinical spectrum and highly variable clinical course among different COVID-19 patients have introduced great challenges in predicting the disease progression and outcome [4]. Laboratory biomarkers have always been very useful in day-to-day clinical practice to guide disease management and treatment decisions, especially in the management of infectious diseases like the COVID-19. Hence, ever since the start of the outbreak, the use of laboratory biomarkers including C-reactive protein (CRP), d-dimer, lactate dehydrogenase (LDH), and procalcitonin (PCT) to predict disease progression and severity have been explored and investigated extensively [1, 5].
Procalcitonin (PCT) is a 116-amino-acid peptide that shares a common molecular structure with the prohormone of calcitonin. It was first discovered in humans in 1981 by Allison et al. but its clinical use was never really explored until 1993 when Assicot et al. first suggested a positive association between elevated serum PCT and bacterial infection and sepsis [6, 7]. In today’s clinical practice, serum PCT has been increasingly instrumental in antibiotic stewardship and the diagnosis and management of sepsis due to bacterial infection [8].
Since the beginning of the COVID-19 pandemic, multiple observational studies and meta-analyses have been done to look into the utility of serum PCT level as a biomarker of clinical deterioration among COVID-19 patients and have shown encouraging results with various optimal PCT cut-points suggested [9–11]. A single-center retrospective study done in Wuhan, China showed that a serum PCT level of more than 0.2 ng/mL was found in severe and critical COVID-19 patients [12]. Another retrospective study of 1099 patients reported that a PCT of more than 0.5 ng/dL was associated with increased severity of COVID-19 infection [13]. Theoretically, PCT, an acute phase peptide, is released in response to proinflammatory cytokines like IL-1β, IL-6, and TNF-α typically induced by bacterial infection and are not seen in viral infections [14]. However, it was found that these pro-inflammatory cytokines are also raised in COVID-19 infection, especially among severe diseases, which makes serum PCT a promising biomarker of clinical deterioration among COVID-19 patients [15].
This study aims to investigate the optimal cut-point of PCT and its relationship with regards to various clinical outcomes, especially in all-cause mortality and the need for mechanical ventilation among moderate to severe COVID-19 patients requiring hospitalization and treatment.
Methods
Study design and setting
A total of 319 patients who were diagnosed with moderate to severe COVID-19 pneumonia and admitted to Sungai Buloh Hospital in December 2020 were included and studied retrospectively. Demographic information, including age, gender, race, concomitant medical illnesses, the severity of COVID-19 infection, laboratory and imaging findings, and treatment given were retrieved from the hospital information system for analysis. The study included category 4 and 5 adult patients above 18 years old with a diagnosis of COVID-19 confirmed via polymerase chain reaction (PCR) test (Table 1). Patients were excluded if no reported PCT values.
Table 1.
Classification of COVID-19 infection severity
| Clinical stage | Severity | Description |
|---|---|---|
| 1 | Mild |
Asymptomatic - Only RT-PCR test positive |
| 2 |
Symptomatic, but no pneumonia - Upper respiratory tract (URT) symptoms (e.g., pharyngeal congestion, sore throat, cough, or fever) - Other symptoms, like vomiting, diarrhea, abdominal pain, myalgia, loss of smell/taste |
|
| 3 | Moderate | Symptomatic, pneumonia, but no hypoxemia |
| 4 | Severe |
Symptomatic, pneumonia, requiring supplemental oxygen OR New requirement of supplemental oxygen or increased requirement from baseline without need for non-invasive or invasive ventilation |
| 5 | Critical |
Critically Ill with multiorgan involvement OR New or increased need for non-invasive or invasive ventilation, including high flow nasal cannula |
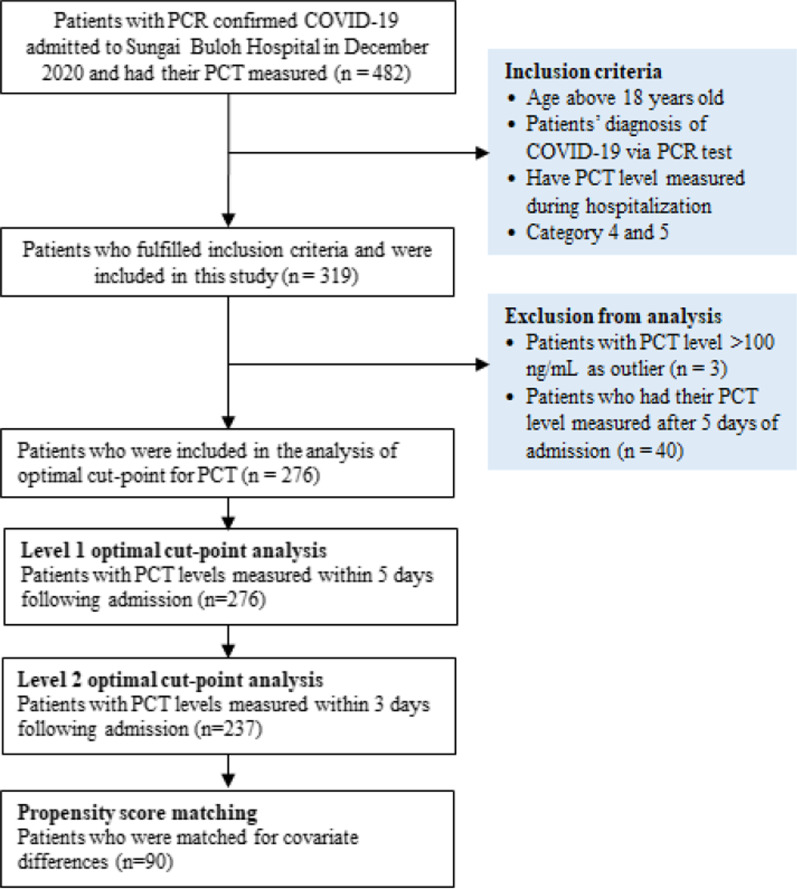
The majority of patients (87.1%) had their PCT levels measured within 5 days following their admission. As PCT levels may reduce over time after receiving treatment, we included patients with their PCT levels measured within 5 days and 3 days for the analysis of the optimal cut-point. Hence, the analysis of the optimal cut-point for PCT was performed in two levels. The first level involved 276 patients with their PCT levels measured within 5 days following admission and the second level involved 237 patients with their PCT levels measured within 3 days following admission. Besides, the study excluded patients with extremely high PCT levels (> 100 ng/mL) to avoid overly skewed findings. Clinical outcomes included in the analysis for the optimal PCT cut-point were all-cause mortality, mechanical ventilation, the occurrence of thrombotic events, ICU admission, and bacterial infection. Figure 1 summarizes the patient recruitment process.
Fig. 1.
Flowchart of patient recruitment
In the study, COVID-19 infection was classified according to the Malaysian Clinical Practice Guideline for Management of COVID-19 [3]. The guideline classifies COVID-19 severity into five clinical stages as shown in Table 1. Stage 1 and 2 consist of patients with asymptomatic infection or symptomatic infection with no pneumonia. Patients in these two categories are considered to have mild disease. Patients with stage 3 disease have lung involvement or pneumonia, but have not experienced hypoxemia, while stage 4 and 5 consist of critically ill patients requiring oxygen support and often intensive care.
Analysis of PCT
In Sungai Buloh Hospital, serum PCT was measured by SIEMENS ATELLICA IMMUNOASSAY (IM). Atellica IM BRAHMS PCT assay is a 2-site sandwich immunoassay using direct chemiluminescent technology that uses 3 mouse monoclonal antibodies specific for PCT. The first antibody, in the Lite Reagent, is a mouse monoclonal anti‑PCT antibody labeled with acridinium ester. The second and third antibodies, in the Ancillary Reagent, are mouse monoclonal anti‑PCT antibodies labeled with fluorescein. The immunocomplex formed with PCT is captured with mouse monoclonal anti-fluorescein antibody coupled to paramagnetic particles in the Solid Phase. This 18-min sandwich immunoassay with a measuring range of 0.03 to 50.00 ng/mL, is aligned to the B·R·A·H·M·S PCT sensitive KRYPTOR® assay [16].
Statistical analysis
Demographics and clinical characteristics were summarized in Table 2 for overall 319 patients, 276 and 237 patients who had their PCT measured within 5 days and 3 days following their admission. The optimal PCT cut-points were estimated using the receiver operative characteristic (ROC) method in two levels. In the first level, we included 276 patients who had their PCT levels measured within 5 days following their admission. In the second level, we included 237 patients who had their PCT levels measured within 3 days following their admission. The optimal cut-point was determined with priority on all-cause mortality and the need for mechanical ventilation. In further analysis, patients with their PCT levels measured within 3 days following admission were divided into two groups according to the selected cut-point. Patients with PCT levels above the cut-point were assumed to have a higher risk for inferior clinical outcomes, and vice versa. We compared and tested the differences of demographics, clinical characteristics, baseline laboratory findings, and co-morbidity profile between the two groups to identify covariates for adjustment in the propensity score matching analysis. The odds ratio (OR) was calculated and reported for various clinical outcomes with regards to the selected cut-point for PCT before and after the matching analysis. Independent t-test and Mann–Whitney U test were used to test differences of continuous variables between two groups, while Chi-squared test and Fisher’s exact test were used to assess categorical variables. All statistical tests were performed at two-sided 5% significance level.
Table 2.
Demographics and clinical characteristics of the study cohort
| Characteristics | Overall n (%) |
First level n (%) |
Second level n (%) |
|---|---|---|---|
| Total | 319 (100.0) | 276 (100.0) | 237 (100.0) |
| Age, years: Mean (SD) | 56 (13.6) | 54.8 (13.7) | 55.0 (13.7) |
| Gender: Female | 111 (34.8) | 102 (37.0) | 84 (35.4) |
| Race | |||
| Malay | 168 (52.7) | 147 (53.3) | 122 (51.5) |
| Chinese | 68 (21.3) | 54 (19.6) | 46 (19.4) |
| Indian | 52 (16.3) | 48 (17.4) | 44 (18.6) |
| Others | 31 (9.7) | 27 (9.8) | 25 (10.5) |
| Comorbidities profile | |||
| Hypertension: Yes | 179 (56.1) | 158 (57.2) | 135 (57.0) |
| Chronic cardiac disease: Yes | 64 (20.1) | 54 (19.6) | 46 (19.4) |
| Chronic pulmonary disease: Yes | 10 (3.1) | 8 (2.9) | 7 (3.0) |
| Asthma: Yes | 14 (4.4) | 13 (4.7) | 10 (4.2) |
| Diabetes mellitus: Yes | 147 (46.1) | 128 (46.4) | 110 (46.4) |
| Pre-existing renal disease: Yes | 73 (22.9) | 56 (20.3) | 46 (19.4) |
| Chronic liver disease: Yes | 3 (0.9) | 2 (0.7) | 2 (0.8) |
| Dementia: Yes | 3 (0.9) | 3 (1.1) | 2 (0.8) |
| Chronic neurological conditions: Yes | 16 (5.0) | 13 (4.7) | 10 (4.2) |
| Connective tissue disease: Yes | 7 (2.2) | 7 (2.5) | 5 (2.1) |
| HIV/AIDS: Yes | 2 (0.6) | 1 (0.4) | 1 (0.4) |
| Malignancy: Yes | 11 (3.4) | 9 (3.3) | 8 (3.4) |
| Current smoking: Yes | 9 (2.8) | 5 (1.8) | 4 (1.7) |
| Obesity: Yes | 17 (5.3) | 17 (6.2) | 16 (6.8) |
| Others: Yes | 81 (25.4) | 67 (24.3) | 51 (21.5) |
| COVID-19 severity when procalcitonin first sent | |||
| Category 4 | 254 (79.6) | 217 (78.6) | 181 (76.4) |
| Category 5 | 65 (20.4) | 59 (21.4) | 56 (23.6) |
| 4C mortality score | |||
| 0–3 (low-risk in-hospital mortality) | 16 (5.0) | 15 (5.4) | 13 (5.5) |
| 4–8 (intermediate-risk in-hospital mortality) | 117 (36.7) | 105 (38.0) | 87 (36.7) |
| 9–14 (high-risk in-hospital mortality) | 145 (45.5) | 124 (44.9) | 107 (45.1) |
| 15–21 (very high-risk in-hospital mortality) | 41 (12.8) | 32 (11.6) | 30 (12.7) |
| Steroid use: Yes | 297 (93.1) | 257 (93.1) | 219 (92.4) |
| Bloodstream infection: Yes | 35 (11.0) | 28 (13.0) | 25 (13.4) |
| Clinical outcomes | |||
| ICU admission: Yes | 143 (44.8) | 129 (46.7) | 120 (50.6) |
| NIV use: Yes | 65 (20.4) | 60 (21.7) | 57 (24.1) |
| Duration of NIV use, days, median (IQR) | 3 (1.8, 5.3) | 3 (1.8, 5.3) | 3 (1, 5) |
| Mechanical ventilation: Yes | 101 (31.7) | 89 (32.2) | 85 (35.9) |
| Duration of mechanical ventilation use, days, median (IQR) | 6 (4, 12) | 7 (4, 12) | 7 (4, 12) |
| Organizing pneumonia | |||
| Yes | 135 (42.3) | 118 (42.8) | 102 (43.0) |
| No | 26 (8.2) | 21 (7.6) | 19 (8.0) |
| CT scan not done | 158 (49.5) | 137 (49.6) | 116 (48.9) |
| Thrombotic event: Yes | 83 (26.0) | 69 (25.0) | 62 (26.2) |
| All-cause mortality: Yes | 71 (22.3) | 59 (21.4) | 55 (23.2) |
| Due to severe COVID-19 pneumonia | 48 (67.6) | 43 (72.9) | 39 (70.9) |
| Due to thrombotic event | 8 (11.3) | 7 (16.3) | 7 (17.9) |
| Due to comorbid | 7 (9.8) | 4 (9.3) | 4 (10.3) |
| Due to bacterial infections | 8 (11.3) | 5 (11.6) | 5 (12.8) |
| Bacteremia | 3 (4.2) | 1 (2.3) | 1 (2.6) |
| Intra-abdominal infection | 1 (1.4) | 0 (0) | 0 (0) |
| Necrotizing fasciitis | 1 (1.4) | 1 (2.3) | 1 (2.6) |
| Non-specified site | 1 (1.4) | 0 (0) | 0 (0) |
| Non-bacteremia | 5 (7.0) | 4 (9.3) | 4 (10.3) |
| Pulmonary infection | 2 (2.8) | 1 (2.3) | 1 (2.6) |
| Intra-abdominal infection | 1 (1.4) | 1 (2.3) | 1 (2.6) |
| Non-specified site | 2 (2.8) | 2 (4.7) | 2 (5.1) |
Column percentages are reported in parenthesis for categorical variables; mean and standard deviation are reported for continuous variable age; median and interquartile range are reported for continuous variables duration of non-invasive ventilation and mechanical ventilation
CT computed tomography, HIV/AIDS human immunodeficiency virus/acquired immunodeficiency syndrome, ICU intensive care unit, NIV non-invasive ventilation
In propensity score matching analysis, patients were matched for covariates that were statistically different. Baseline laboratory parameters and vital signs were not included in the matching analysis as they were not inherited but clinical manifestations of COVID-19 infection or complications. Propensity score matching was done based on the nearest-neighbor method within a caliper width equal to 0.25 times the SD of the logit of the calculated propensity score. All analyses were performed in IBM SPSS version 26.0 for Windows and R version 4.1.0 with packages such as “cutpointr” and “MatchIt” [17–19].
Results
Demographics, clinical characteristics, and clinical outcomes of patients were summarized in Table 2 according to the overall cohort, first and second level cohorts. Even with the exclusion of patients due to delayed measurement of PCT, first and second level cohorts were still similar to the overall cohort in terms of clinical characteristics, comorbidities profile, and clinical outcomes.
Determination of the optimal cut-point
In the determination of optimal cut-points for PCT, we included clinical outcomes, such as all-cause mortality, mechanical ventilation, thrombotic events, ICU admissions, and bacterial infection among moderate to severe COVID-19 patients. Tables 3 and 4 present various performance indexes and optimal cut-points for PCT with regards to the aforementioned clinical outcomes based on PCT levels measured within 5 days and 3 days following admission.
Table 3.
Optimal cut-off PCT values (within 5 days from admission)
| Cut-point and indexes | All-cause mortality | Mechanical ventilation | Thrombotic events | ICU admission | Bacterial infection culture |
|---|---|---|---|---|---|
| Number of patients | 276 | 276 | 276 | 276 | 216 |
| Positive cases | 59 | 89 | 69 | 129 | 28 |
| Negative cases | 217 | 187 | 207 | 147 | 188 |
| AUC | 0.7741 | 0.7773 | 0.6666 | 0.7223 | 0.6444 |
| Optimal cut-point (ng/mL) | 0.20 | 0.33 | 1.21 | 0.09 | 1.2 |
| Accuracy | 0.6703 | 0.7391 | 0.7536 | 0.6848 | 0.7454 |
| Sensitivity | 0.8136 | 0.6742 | 0.4203 | 0.7442 | 0.5357 |
| Specificity | 0.6313 | 0.7701 | 0.8647 | 0.6327 | 0.7766 |
| Precision | 0.3750 | 0.5825 | 0.5088 | 0.6400 | 0.2632 |
| TP | 48 | 60 | 29 | 96 | 15 |
| FN | 11 | 29 | 40 | 33 | 13 |
| FP | 54 | 43 | 28 | 43 | 42 |
| TN | 93 | 144 | 179 | 144 | 146 |
TP true positive, FN false negative, FP false positive, TN true negative, AUC area under curve
Table 4.
Optimal cut-off PCT values (within 3 days from admission)
| Cut-point and Indexes | All-cause mortality | Mechanical ventilation | Thrombotic events | ICU admission | Bacterial infection culture |
|---|---|---|---|---|---|
| Number of patients | 237 | 237 | 237 | 237 | 186 |
| Positive cases | 55 | 85 | 62 | 120 | 25 |
| Negative cases | 182 | 152 | 175 | 117 | 161 |
| AUC | 0.7812 | 0.7676 | 0.6711 | 0.7365 | 0.6465 |
| Optimal cut-point (ng/mL) | 0.20 | 0.21 | 1.21 | 0.07 | 1.25 |
| Accuracy | 0.6667 | 0.7046 | 0.7468 | 0.7046 | 0.7419 |
| Sensitivity | 0.8364 | 0.7528 | 0.4355 | 0.8333 | 0.5600 |
| Specificity | 0.6154 | 0.6776 | 0.8571 | 0.5726 | 0.7702 |
| Precision | 0.3966 | 0.5663 | 0.5192 | 0.6667 | 0.2745 |
| TP | 46 | 64 | 27 | 100 | 14 |
| FN | 9 | 21 | 35 | 20 | 11 |
| FP | 70 | 49 | 25 | 50 | 37 |
| TN | 112 | 103 | 150 | 67 | 124 |
TP true positive, FN false negative, FP false positive, TN true negative, AUC area under curve
For PCT levels measured within 5 days following admission, the optimal cut-points for PCT were found to be 0.20 ng/mL for all-cause mortality, 0.33 ng/mL for mechanical ventilation, 1.21 ng/mL for thrombotic events, 0.09 ng/mL for ICU admission dan 1.2 ng/mL for bacterial infection (Table 3). Highest sensitivity was found in all-cause mortality (0.8136) for PCT cut-point of 0.20 ng/mL. For PCT levels measured within 3 days following admission, the optimal cut-points for PCT were found to be 0.20 ng/mL for all-cause mortality, 0.21 ng/mL for mechanical ventilation, 1.21 ng/mL for thrombotic events, 0.07 ng/mL for ICU admission dan 1.25 ng/mL for bacterial infection (Table 4). Highest sensitivity was also found in all-cause mortality (0.8364) for PCT cut-point of 0.20 ng/mL.
As 0.2 ng/mL appeared to be the optimal cut-point in all-cause mortality for PCT levels measured within 5 days and 3 days following admission, and in mechanical ventilation for PCT levels measured within 3 days following admission, therefore, 0.2 ng/mL was selected as the final PCT cut-point in the matching analysis using propensity scores. The PCT of 0.2 ng/mL represented the optimal cut-point for the two clinically important outcomes, all-cause mortality and mechanical ventilation, especially for moderate to severe COVID-19 patients with PCT measured within 3 days following admission.
Propensity scores matching analysis
To assess the prognostic value of the optimal cut-point, the analysis focused on those who had their PCT levels measured within 3 days following admission. Moreover, the selected cut-point of 0.2 ng/mL was optimal for all-cause mortality and mechanical ventilation among those with PCT levels measured within 3 days following admission. Table 5 summarizes the covariate differences according to the selected optimal cut-point for PCT among 237 patients. Significant differences were identified for age (p = 0.0122), COVID-19 disease stage (p < 0.0001), days of illness before admission (p = 0.0036), SPO2 under room temperature (RA) (p = 0.0005), Glasgow Coma Scale (GCS) (p < 0.0001), urea (p < 0.0001), C-reactive protein (CRP) (p < 0.0001), white blood cells count (WBC) (p = 0.0003), absolute neutrophil count (ANC) (p < 0.0001), number of comorbidities (p < 0.0001), presence of hypertension (p = 0.0007), chronic cardiac diseases (p = 0.0018), diabetes mellitus (DM) (p = 0.0036), pre-existing renal disease (p < 0.0001) and malignancy (p = 0.0327).
Table 5.
Demographics, clinical characteristics and baseline laboratory findings, and comorbidity profile of COVID-19 patients according to procalcitonin level
| Variables | Category | PCT < 0.2 (ng/mL) (n = 121) |
PCT ≥ 0.2 (ng/mL) (n = 116) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Gender | Female | 42 (34.7) | 42 (36.2) | 0.8097 |
| Age (years) | Mean (SD) | 52.8 (12.6) | 57.3 (14.4) | 0.0122 |
| Age groups (years) | < 50 | 45 (37.2) | 33 (28.4) | 0.0945 |
| 50–59 | 38 (31.4) | 28 (24.1) | ||
| 60–60 | 23 (19.0) | 34 (29.3) | ||
| ≥ 70 | 15 (12.4) | 21 (18.1) | ||
| Ethnicity | Malay | 60 (62.3) | 62 (53.4) | 0.0675 |
| Chinese | 22 (18.2) | 24 (20.7) | ||
| Indian | 20 (16.5) | 24 (20.7) | ||
| Others | 19 (15.7) | 6 (5.2) | ||
| Clinical characteristics and baseline laboratory | ||||
| Severity of disease | Category 4 | 111 (91.7) | 70 (60.3) | < 0.0001 |
| Category 5 | 10 (8.3) | 46 (39.7) | ||
| Days of illness | Mean (SD) | 6.5 (3.0) | 5.3 (3.1) | 0.0036 |
| SPO2 under RA (%) | < 92% | 98 (81.0) | 111 (95.7) | 0.0005 |
| ≥ 92% | 23 (19.0) | 5 (4.3) | ||
| RR, bpm | < 20 | 13 (10.7) | 14 (12.1) | 0.2520 |
| 20–29 | 93 (76.9) | 79 (68.1) | ||
| ≥ 30 | 15 (12.4) | 23 (19.8) | ||
| GCS | < 15 | 11 (9.1) | 50 (43.1) | < 0.0001 |
| 15 | 110 (90.9) | 66 (56.9) | ||
| Urea, mmol/dL | < 7 | 87 (71.9) | 31 (26.7) | < 0.0001 |
| 7–14 | 29 (24.0) | 31 (26.7) | ||
| > 14 | 5 (4.1) | 54 (46.6) | ||
| CRP, mg/dL | < 5 | 49 (40.5) | 8 (6.9) | < 0.0001 |
| 5–9.9 | 31 (25.6) | 69 (59.5) | ||
| ≥ 10 | 41 (33.9) | 39 (33.6) | ||
| WBC (× 1012/L) | Mean (SD) | 8.56 (3.88) | 10.90 (5.80) | 0.0003 |
| ANC (× 109/L) | Mean (SD) | 6.75 (3.81) | 9.26 (5.53) | < 0.0001 |
| Comorbidity profile | ||||
| No. of comorbidity | 0 | 67 (55.4) | 33 (28.4) | < 0.0001 |
| 1 | 42 (34.7) | 33 (28.4) | ||
| ≥ 2 | 12 (9.9) | 50 (43.1) | ||
| Hypertension | Yes | 56 (46.3) | 79 (68.1) | 0.0007 |
| Chronic cardiac disease (excluding HPT) | Yes | 14 (11.6) | 32 (27.6) | 0.0018 |
| Asthma | Yes | 6 (5.0) | 4 (3.4) | 0.7491 |
| Chronic pulmonary disease (excluding asthma) | Yes | 3 (2.5) | 4 (3.4) | 0.7174 |
| Diabetes Mellitus | Yes | 45 (37.2) | 65 (56.0) | 0.0036 |
| Pre-existing renal disease | Yes | 5 (4.1) | 41 (35.3) | < 0.0001 |
| Stage 2 | 2 (40.0) | 0 | ||
| Stage 3 | 0 | 6(14.6) | ||
| Stage 4 | 2 (40.0) | 8 (19.5) | ||
| Stage 5 | 1 (20.0) | 27 (65.9) | ||
| HIV/AIDS | Yes | 1 | 0 | n.a |
| Malignancy | Yes | 1 (0.8) | 7 (6.0) | 0.0327 |
| Smoking | Yes, current | 1 (0.8) | 3 (2.6) | n.a |
| Obesity (> 30 kg/m2) | Yes | 7 (5.8) | 9 (7.8) | 0.5449 |
| Chronic liver disease | Yes | 0 | 2 | n.a |
| Dementia | Yes | 0 | 2 | n.a |
| Chronic neurological disease | Yes | 2 (1.7) | 8 (6.9) | 0.0555 |
| Connective tissue disease | Yes | 2 (1.7) | 3 (2.6) | 0.6783 |
ANC absolute neutrophil count, CRP C-reactive protein, GCS Glasgow coma scale, HIV/AIDS human immunodeficiency virus/acquired immunodeficiency syndrome, PCT procalcitonin, RR respiratory rate, RA room air, SD standard deviation, WBC white blood cell count
Table 6 presents the odds ratio (OR) with regards to various clinical outcomes based on the optimal cut-point of 0.2 ng/mL for PCT. Prior to matching, our analysis found that patients with PCT level above 0.2 ng/mL were associated with significantly higher risk in all-cause mortality (OR: 8.178, 95% CI 3.770–17.738, p < 0.0001), mechanical ventilation (OR: 5.861, 95% CI 3.229–10.637, p < 0.0001), non-invasive ventilation (OR: 2.898, 95% CI 1.541–5.541, p = 0.0010), ICU admission (OR: 4.166, 95% CI 2.422–7.166, p < 0.0001) and thrombotic events (OR: 2.158, 95% CI 1.190–3.915, p = 0.0013). Besides, patients with PCT levels above 0.2 ng/mL also experienced significantly longer days of mechanical ventilation (p < 0.0106) and length of hospital stay (p < 0.0001). Table 7 shows the mortality and causes of death before propensity score matching.
Table 6.
Clinical outcomes before propensity score matching
| Clinical outcomes | Category | PCT level (ng/mL) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| PCT < 0.2 (n = 121) | PCT ≥ 0.2 (n = 116) | ||||
| Procalcitonin level | Mean (SD) | 0.0674 (0.0331) | 6.002 (11.556) | n.a | < 0.0001 |
| All-cause mortality | Yes | 9 (7.4) | 46 (39.7) | 8.178 (3.770–17.738) | < 0.0001 |
| No | 112 (92.6) | 70 (60.3) | |||
| Mechanical ventilation | Yes | 21 (17.4) | 64 (55.2) | 5.861 (3.229–10.637) | < 0.0001 |
| No | 100 (82.6) | 52 (44.8) | |||
| Non-invasive ventilation | Yes | 18 (14.9) | 39 (33.6) | 2.898 (1.541–5.541) | 0.0010 |
| No | 103 (85.1) | 77 (66.4) | |||
| ICU admission | Yes | 41 (33.9) | 79 (68.1) | 4.166 (2.422–7.166) | < 0.0001 |
| No | 80 (66.1) | 37 (31.9) | |||
| Thrombotic events | Yes | 23 (19.0) | 39 (33.6) | 2.158 (1.190–3.915) | 0.0113 |
| No | 98 (81.0) | 77 (66.4) | |||
| Positive culture | Yes | 68 (89.5) | 93 (84.5) | 0.643 (0.263–1.578) | 0.3354 |
| No | 8 (10.5) | 17 (15.5) | |||
| Days of mechanical ventilation | Median (IQR) | 5 (4–6) | 9 (5–12.3) | n.a | 0.0106 |
| Days of non-invasive ventilation | Median (IQR) | 3 (2–4) | 3 (1–6) | 0.8071 | |
| Length of hospital stay, days | Median (IQR) | 11 (9–14) | 16 (11.3–20.8) | n.a | < 0.0001 |
CI confidence interval, ICU intensive care unit, IQR interquartile range, MV mechanical ventilation, NIV non-invasive ventilation, OR odds ratio, PCT procalcitonin
Table 7.
Mortality and causes of death before propensity score matching
| Mortality outcomes | Cases (%) |
|---|---|
| Total number of deaths | 55 |
| Cause of death | |
| Severe COVID-19 pneumonia | 39 (70.9) |
| Bacterial infection | 5 (9.1) |
| Bacteremia | 1 |
| Non-bacteremia | 4 |
| Thrombotic event | 7 (12.7) |
| Comorbidity | 4 (7.3) |
As the relative risk of patients categorized based on the optimal cut-point could be confounded by covariates that were significantly different, a propensity score matching was performed with the aforementioned covariates to derive two comparable arms for further analysis. The propensity score matching eventually identified 90 patients with balanced demographics, clinical characteristics, comorbidities profile, and laboratory findings except for urea (p = 0.0139) and CRP (p < 0.0001) as shown in Table 8. Subsequent analyses showed that patients with PCT above 0.2 ng/mL were associated with a significantly higher risk in all-cause mortality (OR: 4.629, 95% CI 1.387–15.449, p = 0.0127), and marginally higher risk for non-invasive ventilation (OR: 2.667, 95% CI 1.039–6.847, p = 0.0415). Besides, patients with PCT above 0.2 ng/mL tended to experience longer days of mechanical ventilation (p = 0.0213) as summarized in Table 9. There was no significant association between PCT level and risk of mechanical ventilation (OR; 2.010, 95% CI 0.828–4.878, p = 0.1229) after being matched for covariate differences.
Table 8.
Demographics, clinical characteristics and baseline laboratory findings and comorbidity profile of COVID-19 patients according to procalcitonin level after propensity score matching
| Variables | Category | PCT < 0.2 (n = 45) |
PCT ≥ 0.2 (n = 45) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Gender | Female | 17 (37.8) | 17 (37.8) | 1.0000 |
| Age | Mean (SD) | 55.1 (11.2) | 53.4 (13.1) | 0.5070 |
| Age groups | < 50 | 13 (28.9) | 16 (35.6) | 0.7838 |
| 50–59 | 14 (31.1) | 15 (33.3) | ||
| 60–60 | 13 (28.9) | 9 (20.0) | ||
| ≥ 70 | 5 (11.1) | 5 (11.1) | ||
| Ethnicity | Malay | 22 (48.9) | 27 (60.0) | 0.6236 |
| Chinese | 9 (20.0) | 7 (15.6) | ||
| Indian | 8 (17.8) | 8 (17.8) | ||
| Others | 6 (13.3) | 3 (6.7) | ||
| Clinical characteristics and baseline laboratory | ||||
| Severity of disease | Category 4 | 35 (77.8) | 37 (82.2) | 0.5982 |
| Category 5 | 10 (22.2) | 8 (17.8) | ||
| Days of illness | Mean (SD) | 5.7 (2.8) | 5.6 (3.0) | 0.8852 |
| SPO2 under RA (%) | < 92% | 38 (84.4) | 42 (93.3) | 0.1797 |
| ≥ 92% | 7 (15.6) | 3 (6.7) | ||
| RR, bpm | < 20 | 5 (11.1) | 4 (8.9) | |
| 20–29 | 33 (73.3) | 31 (68.9) | ||
| ≥ 30 | 7 (15.6) | 10 (22.2) | ||
| GCS | < 15 | 9 (20.0) | 11 (24.4) | 0.6121 |
| 15 | 36 (80.0) | 34 (75.6) | ||
| Urea, mmol/dL | < 7 | 24 (53.3) | 20 (44.4) | 0.0139 |
| 7–14 | 17 (37.8) | 10 (22.2) | ||
| > 14 | 4 (8.9) | 15 (33.3) | ||
| CRP, mg/dL | < 5 | 17 (37.8) | 1 (2.2) | < 0.0001 |
| 5–9.9 | 13 (28.9) | 15 (33.3) | ||
| ≥ 10 | 15 (33.3) | 29 (64.4) | ||
| WBC (× 1012/L) | Mean (SD) | 9.79 (4.26) | 10.69 (6.09) | 0.4148 |
| ANC (× 109/L) | Mean (SD) | 7.89 (4.32) | 8.74 (5.82) | 0.4328 |
| Comorbidity profile | ||||
| No. of comorbidity | 0 | 15 (33.3) | 15 (33.3) | 0.9654 |
| 1 | 19 (42.2) | 18 (40.0) | ||
| ≥ 2 | 11 (24.4) | 12 (26.7) | ||
| Hypertension | Yes | 24 (53.3) | 27(60.0) | 0.5234 |
| Chronic cardiac disease (excluding HPT) | Yes | 12 (26.7) | 12 (26.7) | 1.0000 |
| Asthma | Yes | 1 (2.2) | 0 | n.a |
| Chronic pulmonary disease (excluding asthma) | Yes | 1 (2.2) | 2 (4.4) | n.a |
| Diabetes mellitus | Yes | 24 (53.3) | 21 (46.7) | 0.5271 |
| Pre-existing renal disease | Yes | 5 (11.1) | 9 (20.0) | 0.2447 |
| Stage 2 | 2 | 0 | ||
| Stage 3 | 0 | 1 | ||
| Stage 4 | 2 | 0 | ||
| Stage 5 | 1 | 8 | ||
| HIV/AIDS | Yes | 0 | 0 | n.a |
| Malignancy | Yes | 1 (2.2) | 0 | n.a |
| Smoking | Yes, current | 0 | 2 (4.4) | n.a |
| Obesity (> 30 kg/m2) | Yes | 2 (4.4) | 4 (8.9) | 0.6766 |
| Chronic liver disease | Yes | 0 | 1 (2.2) | |
| Dementia | Yes | 0 | 0 | |
| Chronic neurological disease | Yes | 2 (4.4) | 1 (2.2) | |
| Connective tissue disease | Yes | 2 (4.4) | 2 (4.4) | |
ANC absolute neutrophil count, CRP C-reactive protein, GCS Glasgow Coma Scale, HIV/AIDS human immunodeficiency virus/acquired immunodeficiency syndrome, PCT procalcitonin, RR respiratory rate, RA room air, SD standard deviation, WBC white blood cell count
Table 9.
Clinical outcomes after propensity score matching
| Clinical outcomes | Category | PCT level | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| PCT < 0.2 (n = 45) | PCT ≥ 0.2 (n = 45) | ||||
| Procalcitonin level | Mean (SD) | 0.068 (0.033) | 4.849 (11.624) | n.a | 0.0084 |
| All-cause mortality | Yes | 4 (8.9) | 14 (31.1) | 4.629 (1.387–15.449) | 0.0127 |
| No | 41 (91.1) | 31 (68.9) | |||
| Mechanical ventilation | Yes | 12 (26.7) | 19 (42.2) | 2.010 (0.828–4.878) | 0.1229 |
| No | 33 (73.3) | 26 (57.8) | |||
| Non-invasive ventilation | Yes | 9 (20.0) | 18 (40.0) | 2.667 (1.039–6.847) | 0.0415 |
| No | 36 (80.0) | 27 (60.0) | |||
| ICU admission | Yes | 23 (51.1) | 28 (62.2) | 1.575 (0.681–3.648) | 0.2886 |
| No | 22 (48.9) | 17 (37.8) | |||
| Thrombotic events | Yes | 10 (22.2) | 15 (33.3) | 1.750 (0.686–4.467) | 0.2418 |
| No | 35 (77.8) | 30 (66.7) | |||
| Positive culture | Yes | 3 (10.0) | 4 (9.5) | 0.947 (0.196–4.582) | 0.9464 |
| No | 27 (90.0) | 38 (90.5) | |||
| Days of mechanical ventilation | Median (IQR) | 5 (4–6) | 8 (5–12) | n.a | 0.0213 |
| Days of non-invasive ventilation | Median (IQR) | 3 (2–4) | 3.5 (1–6) | n.a | 0.7928 |
| Length of hospital stay, days | Median (IQR) | 13.5 (9.8–17) | 15 (10–19.5) | n.a | 0.1304 |
CI confidence interval, ICU intensive care unit, IQR interquartile range, MV mechanical ventilation, NIV non-invasive ventilation, OR odds ratio, PCT procalcitonin
Discussion
The identification of patients with moderate to severe COVID-19 diseases who are at risk of deterioration and death is very important to guide the administration of appropriate treatment promptly to improve prognosis. Our study provided a comprehensive analysis of the prognostic value of serum PCT in predicting various clinical outcomes, especially for mortality and the requirement of mechanical ventilation among patients with category 4 and 5 COVID-19 diseases.
Our study results confirmed that the optimal PCT cut-point of 0.2 ng/mL is a useful biomarker to predict the risk of deterioration and death. Procalcitonin is a precursor of calcitonin which is synthesized and released by thyroid parafollicular C cells and usually remain undetectable in physiological condition, however, in the presence of inflammatory cytokines and endotoxins, it can be secreted by extrathyroidal tissues in high amounts [20].
Traditionally, an elevated PCT level is more suggestive of a bacterial infection and has long been used to guide decisions of antibiotic initiation [21]. Some studies suggested that an elevated PCT level in association with clinical deterioration of patients with COVID-19 was attributed to the secondary bacterial co-infection, which further exacerbated the primary COVID-19 infection [1, 11, 21–23]. However, the ability of PCT to accurately differentiate between bacterial and viral infection remains controversial [24]. Our study results did not identify a clear significant association between elevated PCT levels and bacterial co-infection as evidenced by the lack of positive culture yield in the majority of our patients.
The primary mechanism behind the clinical deterioration observed amongst COVID-19 patients is a supraphysiological response known as cytokine release storm. In a study by Guo et al., hypercytokinemia, especially pro-inflammatory cytokines like IL-1β, IL-6, IP-10, G-CSF, IL-8, IL-17, TNF-alpha, and IFN-gamma were seen in patients with COVID-19 and were positively associated with disease severity, multi-organ failure, and death [15, 25, 26]. The IL-6, in particular, is a useful non-specific inflammatory marker to predict disease severity [27, 28]. An increase in IL-6 and other cytokines can also trigger the increase in PCT, especially in the presence of a hyperinflammatory state, which possibly explains the positive association between PCT levels and disease severity and clinical deterioration [11, 22, 27].
Being consistent with previous studies, our study shows that a PCT level above 0.2 ng/mL is significantly associated with a higher risk of mortality, especially among severe and critically ill COVID-19 patients [1, 5, 23]. Furthermore, a meta-analysis by Huang et al. showed that an elevated PCT level was associated with increased mortality, which is in agreement with our results [29]. While our study did not find a relationship between PCT level of 0.2 ng/mL and risk of mechanical ventilation, we observed a significantly higher risk of non-invasive ventilation amongst severe and critically ill COVID-19 patients with elevated PCT levels above 0.2 ng/mL. One plausible explanation for this observation is the strategy of initiation of non-invasive ventilation, in particular high flow nasal cannula practiced in our center to reduce the rate of invasive mechanical ventilation. Several studies have found a significant reduction in invasive mechanical ventilation rates with high flow nasal cannula, which supports our strategy [30–32].
Another notable finding of our study is a longer duration of mechanical ventilation seen amongst severe and critically ill COVID-19 patients with an elevated PCT level of 0.2 ng/mL or more. This finding is very relevant, especially at the peak of this pandemic which sees a scarcity of resources like mechanical ventilators at COVID-19 treatment centers which have contributed to an increase in mortality rate [33, 34]. Furthermore, a longer duration of mechanical ventilation brings about a host of complications like ventilator-induced lung injury, major functional disabilities due to ICU acquired weakness, and cognitive impairment, which can further contribute to increased morbidity and mortality [35]. Thus, prognostication using the PCT level is important to guide rationing of medical resources and implementing relevant management strategies to minimize complications related to mechanical ventilation to minimize mortality.
Conclusion
The results of our study clearly illustrate that an elevated serum PCT of 0.2 ng/mL or more is associated with a higher risk of mortality, NIV, and a longer duration of mechanical ventilation. Our study however did not find an association between serum PCT and risk of mechanical ventilation. On this basis, serum PCT could be effectively used as a prognostic biomarker to predict mortality, the requirement of NIV, and duration of mechanical ventilation in severe and critical COVID-19 patients. However, to better understand the implications of these results, further large adequately powered studies could be done to look into the efficacy of various COVID-19 treatment strategies by looking at its ability to reduce serum PCT, which could signify a reduction in risk of mortality, requirement of NIV and a shorter duration of mechanical ventilation.
Acknowledgements
The authors would like to thank the Director-General of Health for the approval to publish the research findings.
Abbreviations
- COVID-19
Coronavirus disease 2019
- PCT
Procalcitonin
- OR
Odds ratio
- CI
Confidence interval
- p
P-value
- WHO
World Health Organisation
- ICU
Intensive care unit
- CRP
C-reactive protein
- LDH
Lactate dehydrogenase
- PCR
Polymerase chain reaction
- URT
Upper respiratory tract
- IM
Immunoassay
- MREC
Medical Research Ethics Committee
- ROC
Receiver operative characteristic
- IBM
International Business Machines Corporation
- SPSS
Statistical Package for the Social Sciences
- SD
Standard deviation
- HIV
Human immunodeficiency virus
- AIDS
Acquired immunodeficiency syndrome
- NIV
Non-invasive ventilation
- IQR
Interquartile range
- CT scan
Computed tomography
- AUC
Area under curve
- TP
True positive
- FN
False negative
- FP
False positive
- TN
True negative
- SPO2
Oxygen saturation
- GCS
Glasgow Coma Scale
- WBC
White blood cells
- ANC
Absolute neutrophil count
- DM
Diabetes mellitus
- RR
Respiratory rate
- MV
Mechanical ventilation
- HPT
Hypertension
- RA
Room air
- IL
Interleukin
- IP-10
Interferon gamma-induced protein 10
- G-CSF
Granulocyte colony-stimulating factor
- TNF-alpha
Tumour Necrosis Factor alpha
- IFN-gamma
Interferon gamma
Author contributions
CWT, DKYK, NSBAN, SS, and KCL conceived and designed the study. CWT, DKYK, NSBAN and SS collected the data. KBL and KCL carried out the analysis and interpretation of the results. SKA contributed to the funding acquisition. CWT, DKYK, KBL, NSBAN, SS, KCL, SKA, SNABSB, and SKC contributed to writing, review, and/or revision of the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the GitHub repository, https://github.com/william81/procalcitonin
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Research Ethics Committee (MREC), Ministry of Health, Malaysia, on April 6, 2021 (NMRR-21-458-59017) and (KKM/NIH/P21-564(4)). All methods were performed in accordance with the relevant guidelines and regulations. Consent to participate was waived as the study analyzed data retrospectively.
Consent for publication
Not applicable, as there is no identifying participants’ data in the manuscript.
Competing interests
The authors declared that no competing interest exists.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu J, et al. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci Rep. 2020;10:15058. doi: 10.1038/s41598-020-72164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int/.
- 3.Ministry of Health Malaysia. Clinical management of confirmed COVID-19 case in adult. 2020. https://covid-19.moh.gov.my/garis-panduan/gp-umum-covid19.
- 4.Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients With Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahtasakal CA, et al. Could we predict the prognosis of the COVID-19 disease? J Med Virol. 2021;93:2420–2430. doi: 10.1002/jmv.26751. [DOI] [PubMed] [Google Scholar]
- 6.Allison J, Hall L, MacIntyre I, Craig RK. The construction and partial characterization of plasmids containing complementary DNA sequences to human calcitonin precursor polyprotein. Biochem J. 1981;199:725–731. doi: 10.1042/bj1990725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assicot M, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 9.Cen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 2020;26:1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2021;49:82–89. doi: 10.1016/j.ajic.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J, et al. Serum procalcitonin levels on admission predict death in severe and critical COVID-19 patients in Wuhan, China. Cardiovasc Innov Appl. 2020;5:37–44. [Google Scholar]
- 13.Guan W-J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slaats J, ten Oever J, van de Veerdonk FL, Netea MG. IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLOS Pathog. 2016;12:e1005973. doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, et al. Cytokine signature associated with disease severity in COVID-19. Front Immunol. 2021;12:3276. doi: 10.3389/fimmu.2021.681516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manufacturer Insert Procalcitonin (PCT) on Atellica IM 11202687_EN Rev. 03, 2019-07.
- 17.R Core Team . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 18.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Soft. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 19.Thiele C, Hirschfeld G. cutpointr: improved estimation and validation of optimal cutpoints in R. J Stat Soft. 2021;98:1–27. doi: 10.18637/jss.v098.i11. [DOI] [Google Scholar]
- 20.Vanhomwegen C, et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir J Med Sci. 2021 doi: 10.1007/s11845-020-02485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J, et al. Serum IL-6 and procalcitonin are two promising novel biomarkers for evaluating the severity of COVID-19 patients. Medicine. 2021;100:e26131. doi: 10.1097/MD.0000000000026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56:106051–106051. doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An P-J, Zhu YZ, Yang L-P. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol Res. 2020;159:104946–104946. doi: 10.1016/j.phrs.2020.104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2020;70:538–542. doi: 10.1093/cid/ciz545. [DOI] [PubMed] [Google Scholar]
- 25.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 26.Que Y, et al. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int Rev Immunol. 2021 doi: 10.1080/08830185.2021.1884248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji P, et al. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore) 2020;99:e23315. doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370–104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal A, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67:1217–1248. doi: 10.1007/s12630-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demoule A, et al. High-flow nasal cannula in critically iii patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. doi: 10.1136/bmj.n436. [DOI] [PubMed] [Google Scholar]
- 33.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuel EJ, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 35.Pham T, Brochard LJ, Slutsky AS. Mechanical ventilation: state of the art. Mayo Clin Proc. 2017;92:1382–1400. doi: 10.1016/j.mayocp.2017.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the GitHub repository, https://github.com/william81/procalcitonin