Abstract
Linseed oil is rich in unsaturated fatty acids, and its increased consumption could aid in health-promoting nutrition. However, rapid oxidation of linseed oil and concomitant development of bitterness impair consumers’ acceptance. Previous research revealed that cyclolinopeptides, a group of cyclic peptides inherent to linseed oil, dominantly contribute to the observed bitterness. In the present study, fresh and stored linseed oil and flaxseed were analyzed for the presence of cyclolinopeptides using preparative high-performance liquid chromatography combined with mass spectrometry- and nuclear magnetic resonance-based identification and quantification. The purified compounds were tested for the activation of all 25 human bitter taste receptors of which only two responded exclusively to methionine-oxidized cyclolinopeptides. Of those, the methionine sulfoxide-containing cyclolinopeptide-4 elicited responses at relevant concentrations. We conclude that this compound is the main determinant of linseed oil’s bitterness and propose strategies to reduce the development of bitterness.
Keywords: linseed oil, cyclolinopeptide, bitter taste, TAS2R
Introduction
The composition of macronutrients in the human diet plays a major role in the maintenance of a healthy lifestyle and is a matter of constant changes and discussions.1 Among the three macronutrients carbohydrate, protein, and fat, fat is the component with the highest energy density, and thus, the fat content of food items is linked to the total energy consumption.2 However, not only the total fat content in the diet is considered important for the well-being of humans, but also the composition of the lipids is a crucial determinant for its nutritional value.3 Linseed oil with its high content in poly-unsaturated α-linolenic acid could contribute to a well-balanced diet.4 Unfortunately, upon storage cold-pressed linseed oil rapidly develops a strong bitterness, which reduces its acceptance by consumers.5 Previous studies revealed that the bitterness of linseed oil is, next to the oxidative development of hydroxylated fatty acids, due to a group of cyclic peptides, called “cyclolinopeptides.”5
Cyclolinopeptides are cyclic octa- and nonapeptides present in flaxseed (for a review see ref (6)). Because of their hydrophobicity, cyclolinopeptides are found in the oil after processing of flaxseed. Most of the cyclolinopeptides contain one or two frequently oxidized methionine residues in their peptide sequence.7 It is suggested that cyclolinopeptides exert profound biological functions such as anti-inflammatory8 and hepatoprotective effects.9
The human sense of taste is able to assess the chemical composition of food prior to ingestion rapidly. To achieve this, the taste buds present in the oral cavity are equipped with taste receptors specific for the five basic taste qualities salty, sour, umami, sweet, and bitter.10 Because numerous bitter compounds present in nature represent toxins, bitter taste is considered to caution against the consumption of potentially poisonous food items, although not all bitter substances are harmful.10 Nevertheless, bitter taste is innately linked to aversive behavior, a fact that is phylogenetically conserved, for example, in rodents, where bitter-elicited rejection behavior has been shown to be hard-wired within the central nervous system.11 However, at later stages in life humans can learn to tolerate a moderate bitterness and may even start to enjoy it in the context of particular food items and beverages.12 The human genome harbors about 25 putatively functional taste 2 receptor (TAS2R) genes of which 21 have been associated with bitter agonists in the past.13,14 The functional TAS2Rs can be classified into four groups, TAS2Rs with numerous diverse agonists (“generalists”), TAS2Rs with few bitter agonists (“specialists”), intermediately tuned TAS2Rs, and TAS2Rs with pronounced selectivity for distinct chemical classes of bitter substances.13 Previous studies have revealed that some TAS2Rs are involved in the detection of bitter amino acids and peptides.15−17 These receptors, TAS2R1, −R4, −R14, −R39, and −R46, although not specifically tuned to this class of bitter substances, may also be responsible for the bitter taste of the cyclolinopeptides of linseed oil.
To identify the TAS2Rs responsible for the development of bitter taste of linseed oil, we screened all 25 human TAS2Rs for activation with a variety of cyclolinopeptides including derivatives in which the native methionine residues are partially or fully oxidized to methionine sulfoxides or sulfones. The identification of the TAS2R activation profile and the assessment of the bitter-inducing activities of individual cyclolinopeptides would be important for devising strategies to reduce the bitterness and consumer acceptance of linseed oil. As the current nomenclature of cyclolinopeptides is lacking a clear structure, we propose a new more systematic organization of the compound family names.
Materials and Methods
Purification of Cyclolinopeptides from Linseed Oil
Virgin, cold-pressed linseed oils from two market-leading producers were purchased and either used fresh (= fresh linseed oil) or stored for 8 months at room temperature with access to atmospheric oxygen (= aged linseed oil). Fresh and aged linseed oils (400 mL) were diluted with equal volumes of n-hexane in separating funnels and subjected to three consecutive extractions with 800 mL each of a methanol/water mixture (60/40, v/v). The combined aqueous phases were filtered using a folded filter prewetted with H2O and subsequently dried in a rotary evaporator. The resulting extracts were dissolved in methanol and stored at −18 °C. Aliquots of the extracts were separated on a semipreparative phenyl-hexyl-column (Luna 250 × 10 mm, 5 μm particle size, 100 Å pore size, Phenomenex, Aschaffenburg, Germany) by high-performance liquid chromatography-diode array detection (HPLC-DAD) (λ = 210 nm) at a flow rate of 5 mL/min with gradient elution (H2O = eluent A, MeCN = eluent B) and 500 μL injections. Gradient elution started with 60% B (3 min), increased to 75% B in 3 min, kept for 1 min, increased to 100% B in 3.5 min, kept for 3 min, and to 60% B within 4 min followed by re-equilibration. Manually collected fractions from multiple runs were evaporated, and the residues were freeze-dried. Purity of isolates was checked by analytical HPLC-DAD (Luna 250 × 4.6 mm, 5 μm particle size, 100 Å pore size, Phenomenex, Aschaffenburg, Germany) and found to be 95–99% based on the peak area at 210 nm. Isolated compounds were identified based on published5,18−21 and own experimental data (Tables S1 and S4) by exact mass and nuclear magnetic resonance (NMR) experiments.
Synthesis of the Internal Standard (IS) Substance 1-Abu-CLB (= 1-Abu-CL2)
The IS was synthesized as reported in ref (22). Briefly, 1-Met-CLB (= 1-Met-CL2, see the Results section) was purified from fresh linseed oil by HPLC-DAD (see the previous paragraph). The pure compound (20 mg) was dissolved in aqueous ethanol (H2O + EtOH, 1 + 1.5 mL) and mixed with Raney nickel (1 mL, activated nickel catalyst, Sigma-Aldrich, Steinheim, Germany). The mixture was heated in a closed reaction vessel (3 h, 95 °C), cooled to room temperature, and filtered. The solvent was evaporated in a stream of nitrogen, and the concentrated solution was subsequently purified by semipreparative HPLC using the same conditions (see the previous paragraph).
Ultraperformance Liquid Chromatography–High-Definition Mass Spectrometry (UPLC-HDMS)
The procedure was mainly performed as described before.23 Briefly, the chromatographic system was an Acquity UPLC (Waters, Milford, MA, USA) connected to a Waters Synapt G2S HDMS mass spectrometer (Waters, Manchester, UK). Ionization was electrospray (ESI±) with the following instrument settings: capillary voltage (±3 kV), sampling cone voltage (±30 V), source temperature (120 °C), cone gas flow (50 L/h), desolvation gas flow (850 L/h), and desolvation temperature (450 °C). MassLynx 4.1 SCN 851 was used for operating the system. Mass data were autocorrected by infusing (20 μL/mL) of a solution (1 ng/μL) of the pentapeptide leucine enkephaline (Tyr-Gly-Gly-Phe-Leu, m/z 556.2771, [M + H]+, m/z 554.2615, [M – H]−) in a mixture (1:1, v/v) of acetonitrile and aqueous formic acid (0.1% in water) into the ion source during data acquisition. Scan time for the lock mass was 0.3 s (15 s interval). Mass calibration of the Synapt G2S in the mass range of m/z 50–1200 was performed with sodium formate (5 mmol/L in 2-propanol/water, 9:1, v/v). Chromatographic separation was achieved on a BEH phenyl column (150 mm × 2 mm, 1.7 μm particle size, 130 Å pore size, Waters, Manchester, UK) with 1% aqueous formic acid (solvent A) and acetonitrile (containing 1% formic acid, solvent B) at a flow rate of 0.3 mL/min. After isocratic elution with 50% solvent A for 2 min, solvent B was increased to 95% within 6 min followed by isocratic elution (1 min) prior to reconditioning (0.5 min) and re-equilibration (3 min). Centroid MS data were acquired in the mass range of m/z 50–1200 using the MSe experiment (10 Hz).
Quantitation of Cyclolinopeptides in Linseed Oil and Flaxseed by HPLC-MS/MS
The system consisted of an HPLC (Agilent 1200) connected to a 3200 triple quad MS/MS spectrometer (Applied Biosystems Sciex, Darmstadt, Germany). Analyst 1.5.1 (Sciex) was used for instrument control. For analysis in multiple reaction monitoring (MRM, ESI+) mode, ionization and fragmentation parameters of the analytes and IS were automatically optimized with the tool “quantitative optimization.” Ion source parameters were as follows: curtain gas 25 psi, nebulizer gas 45 psi, heater gas 65 psi, heater temperature 500 °C, and ion spray voltage +5.5 kV. The injection volume was 5 μL. Gradient elution on a phenyl-hexyl column (Luna 50 × 2 mm, 3 μm particle size, 100 Å pore size, Phenomenex, Aschaffenburg, Germany) used eluent A (1% formic acid in MeCN) and B (1% formic acid in water). Elution (flow 0.3 mL/min) started at 50% A (2 min), and A was increased to 100% in 6 min followed by isocratic elution (1 min), re-establishing the starting conditions (within 0.5 min) and re-equilibration (3.5 min).
Individual stock solutions and dilutions of the IS and the analytes were prepared in ethanol (∼1 μmoL/mL). Calibration curves in neat solvent were established by analyzing mixtures of the analytes and the IS in different ratios (0.1–6.5) and plotting area ratios (A/IS) versus concentration ratios (A/IS) followed by linear regression. Quantitation was limited to the concentration range where standards had a precision ≤20% relative standard deviation (RSD) and back-calculated accuracy values between 85 and 118% (see Table S2). For analysis, the sample (1 g, oil or ground seeds) was transferred into a centrifugation glass vessel with a screw cap and mixed with n-hexane (1 mL), and the IS (6.4 μg in 50 μL ethanol) was added. The mixture was incubated (10 min) and extracted with aqueous methanol (water/methanol 40/60, v/v, 2 mL). After centrifugation, an aliquot (5 μL) of the aqueous layer was injected into the HPLC-MS/MS system.
NMR Spectroscopy
One-dimensional (1D)- and two-dimensional (2D)-NMR experiments were performed on a Bruker 500 MHz Avance III spectrometer (Bruker, Rheinstetten, Germany) equipped with a Z-gradient 5 mm cryo probe (TCI) using standard pulse programs from the Bruker library. DMSO-d6 (600 μL, 99.9 atom % D) was used as the solvent, and chemical shifts are reported in parts per million referenced to the residual 0.1% DMSO-d5 1H signal, of 2.50 ppm. Data processing was performed using the Topspin NMR software (version 2.1; Bruker, Rheinstetten, Germany).
Functional Screening of Cyclolinopeptides
The functional screening experiments were performed as before.24,25 Briefly, for the functional experiments HEK 293 T-Gα16gust44 cells were cultivated in poly-d-lysine-coated (10 μg/mL, Sigma-Aldrich, Steinheim, Germany) 96-well plates in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, 1% l-glutamine at 37 °C, 5% CO2, and saturated air humidity. The cells were transiently transfected with cDNA constructs of the 25 TAS2Rs using lipofectamine 2000 (Thermo Fisher Scientific, Darmstadt, Germany). For a negative control, empty vector (mock) was transfected. After ∼20 h post-transfection, cells were loaded with Fluo-4 am (Thermo Fisher Scientific, Darmstadt, Germany) in the presence of 2.5 mM probenecid (Sigma-Aldrich, Steinheim, Germany). Excess Fluo-4 am was removed by two washes with C1-buffer, spaced 30 min. apart. Directly after the final C1-buffer wash, the 96-well plates were placed in a fluorometric imaging plate reader (FLIPRtetra, Molecular Devices, San Jose, USA). For the assay, stock solutions of cyclolinopeptides were prepared in C1-buffer and applied to the cells at final concentrations of 10 and 100 μM. Because of the very low amounts of CLI/CLF (= CL5, see the Results section) peptides in linseed oil, this group was not available for the functional experiments in sufficient quantities. As positive controls for the screening, additional wells expressing the aristolochic acid-sensitive TAS2R1426 as well as the strychnine-sensitive receptors TAS2R1027 and TAS2R4628 were challenged with the corresponding agonists. During the measurement, changes in fluorescence (excitation wavelength = 488 nm; detection wavelength = 510 nm) were recorded. The cell viability was confirmed by a second application of somatostatin 14 (100 nM, Bachem, Bubendorf, Switzerland). Agonist-receptor pairs considered positive after the initial screening were subjected to experimental confirmation before monitoring dose–response relationships.
Recording and Calculation of Dose–Response Relationships
As described in the screening procedure, HEK 293 T-Gα16gust44 cells were transfected with cDNA of the previously identified responsive TAS2Rs along with empty vector (mock) controls. Cyclolinopeptides were applied in the final concentration range of 0.1–100 μM. Because of the limited solubility of cyclolinopeptides in the assay buffer, higher concentrations could not be tested. Data were mock-subtracted and baseline-corrected. For the calculations, three independent experiments were performed in duplicate. Plots were obtained using SigmaPlot 14.0 software.
Results
Proposed Nomenclature for Cyclolinopeptides
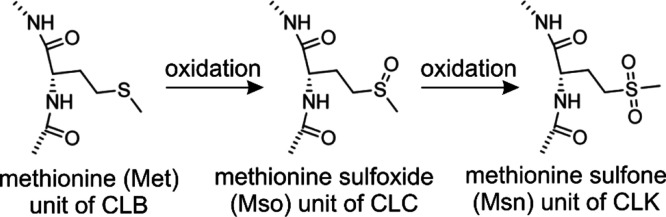
Cyclolinopeptides occurred in the literature already in the 1950s, and the first identified compound of this class was named cyclolinopeptide A (CLA).18,29 In the course of the discovery of further members of this compound class, the authors opted to name them alphabetically corresponding to the chronological sequence of their discovery as cyclolinopeptides B (CLB) to K (CLK).5,19−21,30 Despite their overall chemical stability, cyclolinopeptides B to K undergo oxidative processes because of the presence of one or two methionine residues within the peptide chains being modified to methionine sulfoxides or even sulfones. These oxidative changes were not taken into account for the historical nomenclature, and thus, the third discovered cyclolinopeptide C actually represents the methionine sulfoxide of cyclolinopeptide B.21,31 The two possible oxidation products of methionine which occur in linseed oil already at room temperature upon contact with air are depicted in Figure 1.
Figure 1.
Sequential oxidation of methionine-containing cyclolinopeptides CLB, CLC, and CLK (old nomenclature).
Later, it was discovered that the methionine sulfoxide-containing cyclolinopeptides CLD, CLE, CLH, and CLI also exist in nonoxidized form, a fact that was indicated by adding a prime to the original symbols (e.g., CLD′).20,31−33 To improve the already confusing nomenclature, Olivia et al. introduced a new more systematic naming of cyclolinopeptides calling CLB, CLC, and CLK now 1-Met-CLB, 1-Mso-CLB, and 1Msn-CLB, respectively.22 However, this systematic cannot be applied to all cyclolinopeptides without creating new problems especially for those receiving names in older publications (e.g., the proposed name for CLF is 1-Mso,3-Mso-CLF thus obscuring the fact that it is the oxidized form of CLI). Therefore, it is evident that another adjustment of the nomenclature (see Table 1) is inevitable.
Table 1. Historic and Proposed New Nomenclature of Cyclolinopeptidesa.
| group | amino acid sequence | new names | old names |
|---|---|---|---|
| CL1 | cyclo-(I-L-V-P-P-F-F-L-I) | CL1 | CLA |
| CL2 | cyclo-(R1-L-I-P-P-F-F-V-I) | ||
| R1 = Met | 1-Met-CL2 | CLB, 1-Met-CLB | |
| R1 = Mso | 1-Mso-CL2 | CLC, 1-Mso-CLB | |
| R1 = Msn | 1-Msn-CL2 | CLK, 1-Msn-CLB | |
| R1 = Abu(*) | 1-Abu-CL2 | 1-Abu-CLB | |
| CL3 | cyclo-(R1-L-L-P-F-F-W-I) | ||
| R1 = Met | 1-Met-CL3 | CLD′, 1-Met-CLD | |
| R1 = Mso | 1-Mso-CL3 | CLD, 1-Mso-CLD | |
| R1 = Msn | 1-Msn-CL3 | ||
| R1 = Abu | 1-Abu-CL3 | ||
| CL4 | cyclo-(R1-L-V-F-P-L-F-I) | ||
| R1 = Met | 1-Met-CL4 | CLE′ | |
| R1 = Mso | 1-Mso-CL4 | CLE, 1-Mso-CLE | |
| R1 = Msn | 1-Msn-CL4 | CLJ, 1-Msn-CLE | |
| R1 = Abu | 1-Abu-CL4 | ||
| CL5 | cyclo-(R1-L-R3-P-F-F-W-V) | ||
| R1 = Met, R3 = Met | 1-Met,3-Met-CL5 | CLI′ | |
| R1 = Met, R3 = Mso | 1-Met,3-Mso-CL5 | CLI | |
| R1 = Mso, R3 = Met | 1-Mso,3-Met-CL5 | 1-Mso,3-Met-CLF | |
| R1 = Mso, R3 = Mso | 1-Mso,3-Mso-CL5 | CLF, 1-Mso,3-Mso-CLF | |
| R1 = Mso, R3 = Msn | 1-Mso,3-Msn-CL5 | ||
| R1 = Msn, R3 = Mso | 1-Msn,3-Mso-CL5 | ||
| R1 = Msn, R3 = Msn | 1-Msn,3-Msn-CL5 | ||
| R1 = Abu, R3 = Abu | 1-Abu,3-Abu-CL5 | ||
| CL6 | cyclo-(R1-L-R3-P-F-F-W-I) | ||
| R1 = Met, R3 = Met | 1-Met,3-Met-CL6 | CLH′, 1-Met,3-Met-CLG | |
| R1 = Met, R3 = Mso | 1-Met,3-Mso-CL6 | CLP-N | |
| R1 = Mso, R3 = Met | 1-Mso,3-Met-CL6 | CLH, 1-Mso,3-Met-CLG | |
| R1 = Mso, R3 = Mso | 1-Mso,3-Mso-CL6 | CLG, 1-Mso,3-Mso-CLG | |
| R1 = Mso, R3 = Msn | 1-Mso,3-Msn-CL6 | ||
| R1 = Msn, R3 = Mso | 1-Msn,3-Mso-CL6 | ||
| R1 = Msn, R3 = Msn | 1-Msn,3-Msn-CL6 | ||
| R1 = Abu, R3 = Abu | 1-Abu,3-Abu-CL6 | ||
Met = methionine, Mso = methionine sulfoxide, Msn = methionine sulfone, and Abu(*) = aminobutyric acid (synthetic compound).
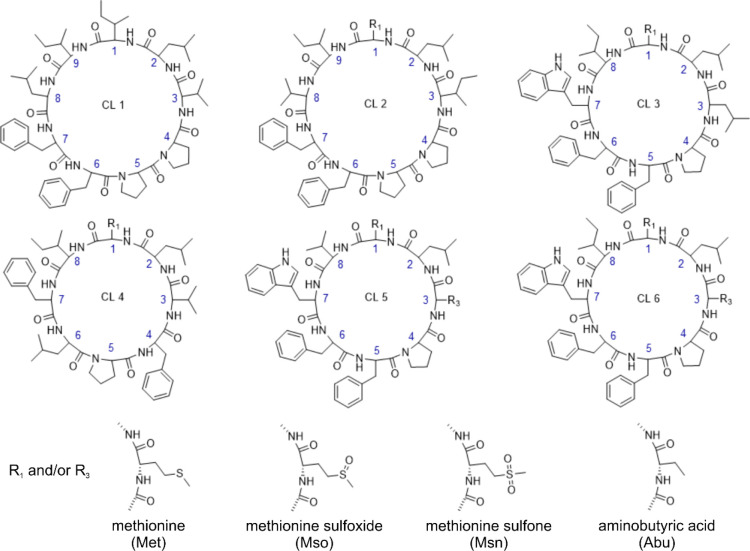
The proposed new nomenclature provides a number of improvements over older versions: (1) its systematic includes all known cyclolinopeptides, (2) it incorporates information about the oxidized forms, and (3) it allows easy incorporation of novel, so far unrecognized, oxidated cyclolinopeptides. The structures of the six classes of cyclolinopeptides are depicted in Figure 2.
Figure 2.
Structures of the six classes of cyclolinopeptides. The circular structures and amino acid sequences of the six classes of cyclolinopeptides are depicted in the top panel. The oxidized forms of methionines at positions 1 and 3 are shown at the bottom.
Purification and Identification of Cyclolinopeptides
Cyclolinopeptides are not commercially available; therefore, we needed to isolate the compounds. For the isolation of pure original and oxidized derivatives, we extracted the fraction of cyclolinopeptides from both fresh and correspondingly aged linseed oil with aqueous methanol. The volumes of the extracts were reduced and separated by HPLC on the phenyl-hexyl material (Figure 3). Single peaks were manually collected and freeze-dried, thus affording the substances in a purity ≥95%. We identified the compounds by NMR and UPLC-time-of-flight MS as sodiated and/or protonated pseudomolecular ions based on literature data (see Table S1). However, for one of the isolated CLPs, the 1-Met,3-Met-CL6, no NMR data were available in the literature. This cyclolinopeptide was identified by means of UPLC-HDMS and 1D- and 2D-NMR spectroscopy (cf. Tables S1 and S4). According to the elementary composition of C56H76N9S2 (determined by high-resolution mass spectrometry), all assignments of C, H, and N signals of the eight amino acids and their connections to each other could be achieved by 1D and 2D NMR experiments (see Table S4). In particular, the ε-methyl groups directly bound to the sulfur of the two methionine moieties could be identified by the typical chemical shifts of 2.00 or 2.02 ppm and the respective singlet with an integral of three protons.
Figure 3.
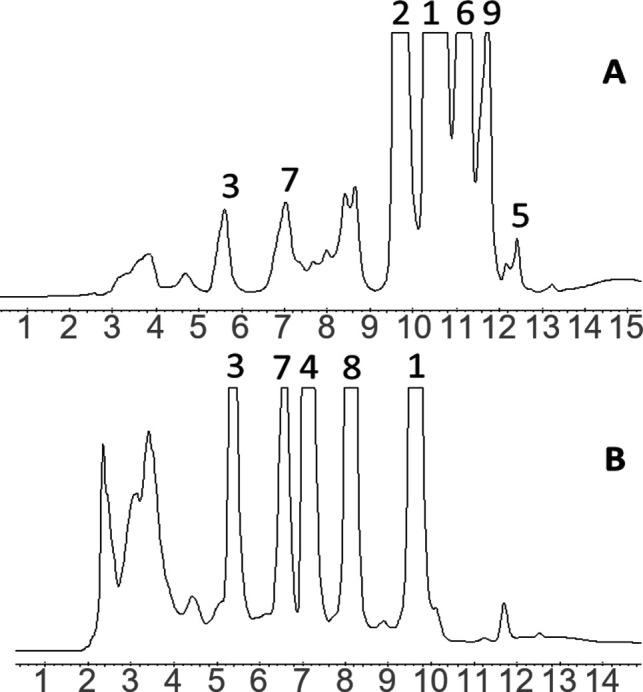
Semipreparative HPLC-DAD separation of H2O/methanol extracts from (A) fresh and (B) aged (8 months) linseed oils (1: CL1, 2: 1-Met-CL2, 3: 1-Mso-CL2, 4: 1-Msn-CL2, 5: 1-Met-CL3, 6: 1-Met-CL4, 7: 1-Mso-CL4, 8: 1-Msn-CL4, and 9: 1-Met,3-Met-CL6).
We used the compounds to develop a targeted MS/MS method, which enabled quantitative analysis of CLPs. The structurally related substance, 1-Abu-CL2, used as the IS was obtained by oxidative modification of isolated 1-Met-CL2. Subsequent semipreparative purification delivered the synthetic CLP derivative 1-Abu-CL222 in a yield of 12 mg (63%), ≥99% purity (HPLC-DAD). The sum formula calculated based on the exact mass (UPLC-HDMS-ESI+: measured m/z 1012.6234 [M + H]+, calculated m/z 1012.6236 for C55H82C9O9 [M + H]+ (Δ −0.2 mDa); m/z 1034.6052 [M + Na]+, calculated m/z 1034.6055 [M + Na]+ (Δ −0.3 mDa); MS/MS (Sciex API 3200 QQQ, ESI+, 50/50 MeOH/H2O, collision energy 30 V): 588(10), 489(20), 392(25), 342(32), 267(20), 245(55), 217(75), 120(85), 70(100)) was C55H82N9O9. Solutions of both the IS and analytes were individually infused into the MS/MS system for tuning, and optimized ion source and ion path parameters were compiled in an multiple reaction monitoring (MRM-) method (cf. Table S2). The chromatographic separation succeeded on the phenyl-hexyl material, and calibration curves were established from the isolated analytes and the synthetic IS for quantitative analysis. Sample preparation involved mixing the sample with hexane and the IS, subsequent extraction with aqueous methanol, and injection of an aliquot of the aqueous layer into the HPLC-MS/MS system (see Tables S2 and S3) for precision and accuracy of standard analysis and precision of sample analysis.
Recovery of the IS after sample workup (peak area relative to the standard solution in neat solvent) in white oil, sunflower oil, and linseed oil was 100, 100, and 115% (means of n = 3 each). Precision (n = 3) was 8.3, 7.2, and 8.5% RSD. Precision of the method was further evaluated by replicate analysis of authentic linseed oil. In fresh samples, precision of replicate sample workups was found to be ≤16.8% for the analytes with the exception of the minor compounds 1-Mso,3-Mso-CLF (= 1-Mso,3-Mso-CL5, see the Results section) (22.5%) and 1-Mso,3-Mso-CLG (= 1-Mso,3-Mso-CL6, see the Results section). In aged samples, precision was ≤8.5% for all analytes within the calibrated range (Table S3).
Quantitation of Cyclolinopeptides in Linseed Oil and Flaxseed
Using the developed method, we analyzed linseed oils for cyclolinopeptide content (see Table 2).
Table 2. Cyclolinopeptide Concentrations in Different Linseed Samples and Flaxseeda.
| concentration
in food (μmol/kg) |
||||||
|---|---|---|---|---|---|---|
| linseed oil | flaxseed | |||||
| compounds | 1 (fresh) | 2 (fresh) | 2 (stored) | 3 | 4 | 5 |
| Σ CL1 | 148 | 196 | 196 | 144 | 138 | 207 |
| CL1 | 148 ± 6 | 196 ± 12 | 196 ± 10 | 144 ± 4 | 138 ± 17 | 207 ± 3 |
| Σ CL2 | 150 | 175 | 135 | 125 | 101 | 177 |
| 1-Met- CL2 | 137.4 ± 0.6 | 154 ± 15 | n.d. | 121 ± 6 | 94.7 ± 0.5 | 173.8 ± 0.1 |
| 1-Mso-CL2 | 10.7 ± 1.0 | 20.6 ± 7.9 | 133 ± 2 | 4.1 ± 0.2 | 5.4 ± 0.4 | 3.2 ± 0.3 |
| 1-Msn-CL2 | 1.5 ± 0.1 | 0.33 ± 0.10 | 1.8 ± 0.1 | n.d. | 0.45 ± 0.06 | n.d. |
| Σ CL3 | 58 | 42 | 42 | 56 | 84 | 57 |
| 1-Met- CL3 | 55.0 ± 1.3 | 38.8 ± 1.7 | 23.0 ± 2.4 | 51.0 ± 0.2 | 79.2 ± 2.2 | 49.6 ± 2.1 |
| 1-Mso-CL3 | 2.9 ± 1.1 | 2.8 ± 1.0 | 19.4 ± 0.1 | 4.73 ± 0.05 | 4.9 ± 0.2 | 7.4 ± 0.3 |
| Σ CL4 | 189 | 236 | 232 | 231 | 200 | 322 |
| 1-Met- CL4 | 138 ± 5 | 208 ± 15 | 105 ± 9 | 221 ± 18 | 189 ± 4 | 314 ± 9 |
| 1-Mso-CL4 | 49.9 ± 1.0 | 27.7 ± 13.0 | 125 ± 3 | 9.8 ± 2.4 | 11.5 ± 0.9 | 7.7 ± 1.9 |
| 1-Msn-CL4 | 1.0 ± 0.1 | 0.7 ± 0.2 | 2.6 ± 0.3 | n.d. | n.d. | n.d. |
| Σ CL5 | 2.5 | 5.3 | 8.5 | 0.7 | 1.1 | 0.6 |
| 1-Met,3-Met-CL5 | 1.0 ± 0.1 | 3.6 ± 1.2 | 2.2 ± 0.2 | 0.7 ± 0.1 | 1.1 ± 0.1 | 0.63 ± 0.03 |
| 1-Met,3-Mso-CL5 | n.d. | 1.3 ± 0.2 | 1.8 ± 0.1 | n.d. | n.d. | n.d. |
| 1-Mso,3-Mso-CL5 | 1.5 ± 0.1 | 0.36 ± 0.43 | 4.56 ± 0.01 | n.d. | n.d. | n.d. |
| Σ CL6 | 127 | 193 | 235 | 187 | 246 | 176 |
| 1-Met,3-Met-CL6 | 91.8 ± 0.9 | 101 ± 15 | 38.3 ± 0.2 | 183 ± 2 | 238 ± 4 | 176 ± 2 |
| 1-Mso,3-Met-CL6 | 31.3 ± 0.6 | 78.9 ± 16.5 | 79.0 ± 1.2 | n.d. | n.d. | n.d. |
| 1-Mso,3-Mso-CL6 | 3.5 ± 0.2 | 12.6 ± 8.5 | 117 ± 2 | 4.25 ± 0.09 | 7.8 ± 0.3 | n.d. |
| Σ CL1 – CL6 | 675 | 847 | 849 | 744 | 770 | 940 |
n.d. = not detectable.
Determination of the Bitter Taste Receptor Activation Profiles of Cyclolinopeptides
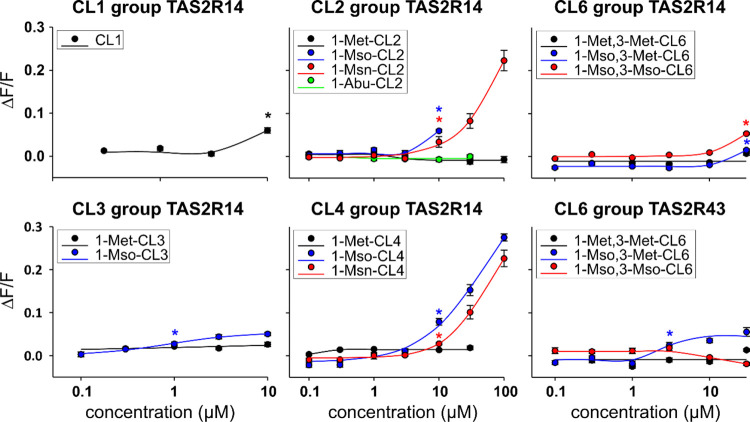
The purified cyclolinopeptides (CL1, 1-Met-CL2, 1-Mso-CL2, 1-Msn-CL2, 1-Abu-CL2, 1-Met-CL3, 1-Mso-CL3, 1-Met-CL4, 1-Mso-CL4, 1-Msn-CL4, 1-Met,3-Met-CL6, 1-Mso,3-Met-CL6, and 1-Mso,3-Mso-CL6) were used to screen the 25 human TAS2Rs, which were transiently expressed in HEK 293 T-Gα16gust44 cells. Because the solubility of cyclolinopeptides in the assay buffer was limited, we screened the cells with a maximum concentration of 100 μM and a tenfold dilution thereof. Of the 25 TAS2Rs, only two, the TAS2R14 and the TAS2R43, showed responses (Figure 4). Whereas only one cyclolinopeptide, namely 1-Mso,3-Met-CL6, elicited responses in TAS2R43-transfected cells, TAS2R14-expressing cells responded to CL1, 1-Mso-CL2, 1-Msn-CL2, 1-Mso-CL3, 1-Mso-CL4, 1-Msn-CL4, 1-Mso,3-Met-CL6, and 1-Mso,3-Mso-CL6. Hence, the TAS2R14 seems to be the dominant bitter taste receptor for the detection of cyclolinopeptides which is in agreement with previous findings where TAS2R14 was identified as one of the five receptors responding to amino acids and/or peptides.15 We noted that the stimulation of cells with 100 μM of some CLPs resulted in signals from cells transfected with empty vector (mock-controls), thus limiting the applicable concentration in our assay.
Figure 4.
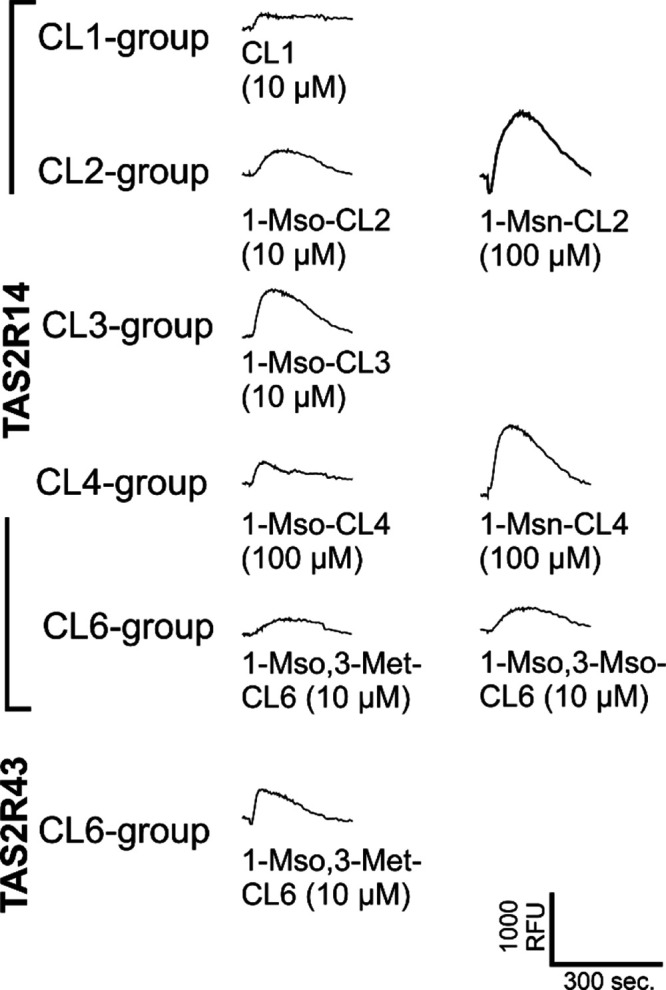
Cyclolinopeptides activate two human bitter taste receptors. HEK 293 T-Gα16gust44 cells transiently transfected with all 25 human TAS2Rs were screened with cyclolinopeptides at concentrations of 10 and 100 μM. Only the mock-subtracted raw fluorescence traces of responding cells at the indicated concentrations are shown.
Dose–Response Relationships of Cyclolinopeptides and Responding Bitter Taste Receptors
To identify the concentration ranges at which the different cyclolinopeptides activate TAS2R14 and TAS2R43, we monitored the dose–response relationships of CLPs (Figure 5). For the four cyclolinopeptides, CL1, 1-Mso-CL2, 1-Mso,3-Met-CL6, and 1-Mso,3-Mso-CL6, we observed that only the highest applicable concentration led to a significant activation of receptor-expressing cells. For other CLPs, more activating concentrations were observed. Determinations of EC50 concentrations were due to a lack of receptor signal saturation, only for 1-Mso,3-Met-CL6 with TAS2R43-expressing cells possible (EC50-concentration = 2.92 ± 1.35 μM). The highest signal amplitudes were found for TAS2R14-expressing cells stimulated with 1-Msn-CL2 and even more pronounced for 1-Mso-CL4 and 1-Msn-CL4. Our data clearly demonstrate that, except for CL1, which is lacking methionine residues, all cyclolinopeptides eliciting responses in TAS2R-expressing cells are active only if at least one methionine is present in oxidized form thus confirming the development of bitterness in the aged linseed oil samples.
Figure 5.
Dose–response relationships of cyclolinopeptides with TAS2R14- and TAS2R43-expressing HEK 293 T-Gα16gust44 cells. The graphs show the relative fluorescence changes (ΔF/F) upon agonist application. The applied agonist concentrations are provided at the logarithmically scaled x-axis. The threshold concentrations (defined as lowest compound concentrations resulting in statistically significant signals (Student’s t-test, p ≤ 0.05) from stimulated receptor-transfected cells compared to empty vector-transfected cells) are labeled by asterisks.
Discussion
Linseed oil is not widely consumed. The main reason for the lack of consumer acceptance is the rapidly developing bitter taste mediated by a variety of constituents such as hydroxylated fatty acids and, in particular, cyclolinopeptides.5 In the present work, cyclolinopeptides from different specimens of linseed oils and flaxseeds were purified and quantified, and it was observed that, while the initial levels of the six classes of cyclolinopeptides are not too different among the various specimens, storage causes a rapid shift toward methionine-oxidized cyclolinopeptide variants. Of those cyclolinopeptides that contain at least one methionine in their amino acid sequence, CL2 became fully methionine-oxidized upon storage, whereas CL3 (45% oxidized), CL4 (55%), CL5 (74%), and CL6 (84%) were partially oxidized (see Table 2).
Confirming previous observations5 suggesting that these oxidized cyclolinopeptides contribute substantially to the storage-induced bitterness of linseed oils, this study demonstrated that indeed preferentially methionine-oxidized variants of cyclolinopeptides elicited responses of functionally expressed human bitter taste receptors. These responses were limited to two of the 25 TAS2Rs, namely, TAS2R14 and TAS2R43, with the majority of responses seen for TAS2R14. The TAS2R43 solely responded to 1-Mso,3-Met-CL6, whereas CL1, 1-Mso-CL2, 1-Msn-CL2, 1-Mso-CL3, 1Mso-CL4, 1-Msn-CL4, 1-Mso,3-Met-CL6, and 1-Mso,3-Mso all activated TAS2R14-transfected cells (see Figures 4 and 5).
Although solubility issues and the occurrence of receptor-independent signals prevented monitoring of signal saturating concentrations of the tested cyclolinopeptides, except for 1-Mso,3-Met-CL6 with TAS2R43-expressing cells, our data allowed conclusions to be drawn about the contribution of cyclolinopeptide variants to the bitterness of stored/oxidized linseed oil. Except for CL1, which does not contain any methionine residue in its peptide chain, none of the cyclolinopeptide variants without oxidized methionines activate TAS2Rs and hence, should not taste bitter. While 1-Mso-CL3 with a threshold of 1 μM and 1-Mso,3-Met-CL6 exhibit the highest potencies for the activation of TAS2R14 and TAS2R43, respectively, the observed maximal signal amplitudes are low and therefore may not correlate with strong bitter perception (see Figure 5). The efficacy of 1-Msn-CL2, as indicated by the signal amplitude of ∼0.2 (ΔF/F) for the activation of TAS2R14-expressing cells, would suggest a more pronounced contribution for the overall bitterness of aged linseed oil. Because only a concentration of 1.8 μmol/kg of this cyclolinopeptide variant was determined in the stored sample (see Table 2), not even the activation threshold was reached (see Figure 5). Whether 1-Mso-CL2, which has been found at much higher concentrations in the stored sample (133 μmol/kg), has the capacity to elicit higher signal amplitudes in TAS2R14-transfected cells could not be tested because of unspecific signals at concentrations higher than 10 μM (see Figure 5). Therefore, we conclude that the cyclolinopeptide 1-Mso-CL4, which elicits high signal amplitudes in TAS2R14-expressing cells (see Figure 5) and occurs at high concentrations in aged linseed oil (see Table 2), together with the TAS2R14, represents a major determinant of its bitterness. This finding is in perfect agreement with the previous study by Brühl and colleagues who identified 1-Mso-CL4 (then termed cyclolinopeptide E) as the bitter principle in stored linseed oil.5 The human bitter taste receptor TAS2R14 is not only the most broadly tuned receptor of all human TAS2Rs;26,34−36 it has also been recognized as one of the five TAS2Rs responding to bitter peptides.15 Hence, it is not surprising that this receptor becomes activated by several cyclolinopeptides. Surprisingly, none of the other peptide-responsive TAS2Rs, TAS2R1, −R4, −R39, and −R46 responded to this class of cyclic peptides, suggesting that the peptidic nature of cyclolinopeptides may neither represent the only, nor the primary determinant for the observed bitterness. This is further supported by the fact that none of the cyclolinopeptides, except for CL1 at high concentrations, is bitter if the methionine is not oxidized, arguing against the peptide sequence as the main bitter determinant. However, it has been noticed that cyclolinopeptides, such as oxidized CL5 and CL6 forms, exhibit osteoclast inhibitory activities,7 CL1 and derivatives show antimalarial activities,37 and CL2 and CL4 forms exert immunosuppressive activities.21 Therefore, these profound pharmacological functions warrant that their ingestion is accompanied by an alerting bitter taste. Because bitter taste receptors are not only expressed in the gustatory system but in numerous extra-oral tissues including white blood cells38−41 it might even be possible that some TAS2Rs are directly involved in those physiological responses.
Our data suggest that the rapidly developing bitterness upon linseed oil storage could be improved by a reduction of the production of the CL4 class of cyclolinopeptides, for example, by breeding to reduce the activity of the gene cyclolinopeptide-5142 or the usage of cultivars low in CL4 class production such as, for example, “Flanders.”42 Moreover, also the blending of cultivars rich in 1-Mso-CL3 such as “Somme,”42 a potent but low efficient agonist of TAS2R14 with low CL4 class producing cultivars may allow a reduction of bitterness because of a competition at the ligand binding site of this critical receptor.
Acknowledgments
The authors are grateful to Andreas Daschner (Chair of Food Chemistry and Molecular Sensory Science, Technical University of Munich, Freising, Germany) and Eva Boden (Leibniz-Institute for Food Systems Biology at the Technical University of Munich, Freising, Germany) for excellent technical assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.2c00976.
Exact mass data for compound identification, sample quantitation and precision, and NMR data (PDF)
This research was supported in part by the Deutsche Forschungsgemeinschaft (DFG – German Research Foundation) (BE 2091/7-1 to MB).
The authors declare no competing financial interest.
Supplementary Material
References
- Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity. Circulation 2016, 133, 187–225. 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach-Mardas M.; Rodacki T.; Dobrowolska-Iwanek J.; Brzozowska A.; Walkowiak J.; Wojtanowska-Krosniak A.; Zagrodzki P.; Bechthold A.; Mardas M.; Boeing H. Link between food energy density and body weight changes in obese adults. Nutrients 2016, 8, 229. 10.3390/nu8040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenighan Y. M.; McNulty B. A.; Roche H. M. Dietary fat composition: replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proc. Nutr. Soc. 2019, 78, 234–245. 10.1017/S0029665118002793. [DOI] [PubMed] [Google Scholar]
- Kostik V.; Memeti S.; Bauer B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Brühl L.; Matthäus B.; Fehling E.; Wiege B.; Lehmann B.; Luftmann H.; Bergander K.; Quiroga K.; Scheipers A.; Frank O.; Hofmann T. Identification of bitter off-taste compounds in the stored cold pressed linseed oil. J. Agric. Food Chem. 2007, 55, 7864–7868. 10.1021/jf071136k. [DOI] [PubMed] [Google Scholar]
- Picur B.; Cebrat M.; Zabrocki J.; Siemion I. Z. Cyclopeptides of Linum usitatissimum. J. Pept. Sci. 2006, 12, 569–574. 10.1002/psc.779. [DOI] [PubMed] [Google Scholar]
- Kaneda T.; Yoshida H.; Nakajima Y.; Toishi M.; Nugroho A. E.; Morita H. Cyclolinopeptides, cyclic peptides from flaxseed with osteoclast differentiation inhibitory activity. Bioorg. Med. Chem. Lett. 2016, 26, 1760–1761. 10.1016/j.bmcl.2016.02.040. [DOI] [PubMed] [Google Scholar]
- Górski A.; Kasprzycka M.; Nowaczyk M.; Wieczoreck Z.; Siemion I. Z.; Szelejewski W.; Kutner A. Cyclolinopeptide: a novel immunosuppressive agent with potential anti-lipemic activity. Transplant. Proc. 2001, 33, 553. 10.1016/S0041-1345(00)02139-4. [DOI] [PubMed] [Google Scholar]
- Kessler H.; Klein M.; Müller A.; Wagner K.; Bats J. W.; Ziegler K.; Frimmer M. Conformational Prerequisites for the in vitro Inhibition of Cholate Uptake in Hepatocytes by Cyclic Analogues of Antamanide and Somatostatin. Angew. Chem., Int. Ed. 1986, 25, 997–999. 10.1002/anie.198609971. [DOI] [Google Scholar]
- Lindemann B. Taste reception. Physiol. Rev. 1996, 76, 719–766. 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- Wang L.; Gillis-Smith S.; Peng Y.; Zhang J.; Chen X.; Salzman C. D.; Ryba N. J. P.; Zuker C. S. The coding of valence and identity in the mammalian taste system. Nature 2018, 558, 127–131. 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A.; Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- Meyerhof W.; Batram C.; Kuhn C.; Brockhoff A.; Chudoba E.; Bufe B.; Appendino G.; Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Thalmann S.; Behrens M.; Meyerhof W. Major haplotypes of the human bitter taste receptor TAS2R41 encode functional receptors for chloramphenicol. Biochem. Biophys. Res. Commun. 2013, 435, 267–273. 10.1016/j.bbrc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- Kohl S.; Behrens M.; Dunkel A.; Hofmann T.; Meyerhof W. Amino acids and peptides activate at least five members of the human bitter taste receptor family. J. Agric. Food Chem. 2013, 61, 53–60. 10.1021/jf303146h. [DOI] [PubMed] [Google Scholar]
- Maehashi K.; Matano M.; Wang H.; Vo L. A.; Yamamoto Y.; Huang L. Bitter peptides activate hTAS2Rs, the human bitter receptors. Biochem. Biophys. Res. Commun. 2008, 365, 851–855. 10.1016/j.bbrc.2007.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya J.; Pydi S. P.; Singh N.; Aluko R. E.; Chelikani P. Bitter taste receptor T2R1 is activated by dipeptides and tripeptides. Biochem. Biophys. Res. Commun. 2010, 398, 331–335. 10.1016/j.bbrc.2010.06.097. [DOI] [PubMed] [Google Scholar]
- Brewster A. I.; Bovey F. A. Conformation of cyclolinopeptide a observed by nuclear magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 1971, 68, 1199–1202. 10.1073/pnas.68.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav P. D.; Okinyo-Owiti D. P.; Ahiahonu P. W.; Reaney M. J. Detection, isolation and characterisation of cyclolinopeptides J and K in ageing flax. Food Chem. 2013, 138, 1757–1763. 10.1016/j.foodchem.2012.10.126. [DOI] [PubMed] [Google Scholar]
- Matsumoto T.; Shishido A.; Morita H.; Itokawa H.; Takeya K. Cyclolinopeptides F-I, cyclic peptides from linseed. Phytochemistry 2001, 57, 251–260. 10.1016/S0031-9422(00)00442-8. [DOI] [PubMed] [Google Scholar]
- Morita H.; Shishido A.; Matsumoto T.; Itokawa H.; Takeya K. Cyclolinopeptides B - E, new cyclic peptides from Linum usitatissimum. Tetrahedron 1999, 55, 967–976. 10.1016/S0040-4020(98)01086-2. [DOI] [Google Scholar]
- Olivia C. M.; Burnett P. G.; Okinyo-Owiti D. P.; Shen J.; Reaney M. J. Rapid reversed-phase liquid chromatography separation of cyclolinopeptides with monolithic and microparticulate columns. J. Chromatogr. B 2012, 904, 128–134. 10.1016/j.jchromb.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Lang R.; Klade S.; Beusch A.; Dunkel A.; Hofmann T. Mozambioside Is an Arabica-Specific Bitter-Tasting Furokaurane Glucoside in Coffee Beans. J. Agric. Food Chem. 2015, 63, 10492–10499. 10.1021/acs.jafc.5b04847. [DOI] [PubMed] [Google Scholar]
- Lang T.; Lang R.; Di Pizio A.; Mittermeier V. K.; Schlagbauer V.; Hofmann T.; Behrens M. Numerous Compounds Orchestrate Coffee’s Bitterness. J. Agric. Food Chem. 2020, 68, 6692–6700. 10.1021/acs.jafc.0c01373. [DOI] [PubMed] [Google Scholar]
- Ziegler F.; Behrens M. Bitter taste receptors of the common vampire bat are functional and show conserved responses to metal ions in vitro. Proc. R. Soc. B 2021, 288, 20210418 10.1098/rspb.2021.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak S.; Di Pizio A.; Levit A.; Niv M. Y.; Meyerhof W.; Behrens M. Reengineering the ligand sensitivity of the broadly tuned human bitter taste receptor TAS2R14. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2162–2173. 10.1016/j.bbagen.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Born S.; Levit A.; Niv M. Y.; Meyerhof W.; Behrens M. The human bitter taste receptor TAS2R10 is tailored to accommodate numerous diverse ligands. J. Neurosci. 2013, 33, 201–213. 10.1523/JNEUROSCI.3248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhoff A.; Behrens M.; Massarotti A.; Appendino G.; Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food Chem. 2007, 55, 6236–6243. 10.1021/jf070503p. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. P.; Tobschirbel A. Über ein Oligopeptid aus Leinsamen. Chem. Ber. 1959, 92, 2805–2809. 10.1002/cber.19590921122. [DOI] [Google Scholar]
- Morita H.; Shishido A.; Matsumoto T.; Takeya K.; Itokawa H.; Hirano T.; Oka K. A new immunosuppressive cyclic nonapeptide, cyclolinopeptide B from Linum usitatissimum. Bioorg. Med. Chem. Lett. 1997, 7, 1269–1272. 10.1016/S0960-894X(97)00206-0. [DOI] [Google Scholar]
- Stefanowicz P. Detection and sequencing of new cyclic peptides from linseed by electrospray ionization mass spectrometry. Acta Biochim. Pol. 2001, 48, 1125–1129. 10.18388/abp.2001_3877. [DOI] [PubMed] [Google Scholar]
- Aladedunye F.; Sosinska E.; Przybylski R. Flaxseed Cyclolinopeptides: Analysis and Storage Stability. J. Am. Oil Chem. Soc. 2013, 90, 419–428. 10.1007/s11746-012-2173-0. [DOI] [Google Scholar]
- Marr J.; Tremblay P.; Picard P.; Burnett P.-G.; Okinyo-Owiti D. P.; Reaney M. J. T.. High-Throughput cyclolinopeptide and triacylglycerol profiling of Linum usitatissimum using LDTD-MS/MS. In 58th American Society for Mass Spectrometry (ASMS); Salt Lake City, UT, USA, 2010. [Google Scholar]
- Behrens M.; Brockhoff A.; Kuhn C.; Bufe B.; Winnig M.; Meyerhof W. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun. 2004, 319, 479–485. 10.1016/j.bbrc.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Karaman R.; Nowak S.; Di Pizio A.; Kitaneh H.; Abu-Jaish A.; Meyerhof W.; Niv M. Y.; Behrens M. Probing the Binding Pocket of the Broadly Tuned Human Bitter Taste Receptor TAS2R14 by Chemical Modification of Cognate Agonists. Chem. Biol. Drug Des. 2016, 88, 66–75. 10.1111/cbdd.12734. [DOI] [PubMed] [Google Scholar]
- Levit A.; Nowak S.; Peters M.; Wiener A.; Meyerhof W.; Behrens M.; Niv M. Y. The bitter pill: clinical drugs that activate the human bitter taste receptor TAS2R14. FASEB J. 2014, 28, 1181–1197. 10.1096/fj.13-242594. [DOI] [PubMed] [Google Scholar]
- Bell A.; McSteen P. M.; Cebrat M.; Picur B.; Siemion I. Z. Antimalarial activity of cyclolinopeptide A and its analogues. Acta Pol. Pharm. 2000, 57, 134–136. [PubMed] [Google Scholar]
- Malki A.; Fiedler J.; Fricke K.; Ballweg I.; Pfaffl M. W.; Krautwurst D. Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J. Leukocyte Biol. 2015, 97, 533–545. 10.1189/jlb.2A0714-331RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer S.; Wabnitz G. H.; Kahle N. A.; Stegmaier S.; Prior B.; Giese T.; Gaida M. M.; Samstag Y.; Hänsch G. M. Tasting Pseudomonas aeruginosa Biofilms: Human Neutrophils Express the Bitter Receptor T2R38 as Sensor for the Quorum Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine Lactone. Front. Immunol. 2015, 6, 369. 10.3389/fimmu.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H. T. T.; Herz C.; Ruf P.; Stetter R.; Lamy E. Human T2R38 Bitter Taste Receptor Expression in Resting and Activated Lymphocytes. Front. Immunol. 2018, 9, 2949. 10.3389/fimmu.2018.02949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H. T. T.; Stetter R.; Herz C.; Spöttel J.; Krell M.; Hanschen F. S.; Schreiner M.; Rohn S.; Behrens M.; Lamy E. Allyl Isothiocyanate: A TAS2R38 Receptor-Dependent Immune Modulator at the Interface Between Personalized Medicine and Nutrition. Front. Immunol. 2021, 12, 669005 10.3389/fimmu.2021.669005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui B.; Shim Y. Y.; Datla R. S. S.; Covello P. S.; Stone S. L.; Reaney M. J. T. Identification and Quantification of Cyclolinopeptides in Five Flaxseed Cultivars. J. Agric. Food Chem. 2012, 60, 8571–8579. 10.1021/jf301847u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.