Abstract
Coronavirus 2019 (COVID‐19) is a global concern for public health. Thus, early and accurate diagnosis is a critical step in management of this infectious disease. Currently, RT‐PCR is routine diagnosis test for COVID‐19, but it has some limitations and false negative results. enzyme‐linked immunosorbent assay (ELISA) against SARS‐CoV‐2 antigens seems to be an appropriate approach for serodiagnosis of COVID‐19. In the current study, an ELISA system, using a recombinant nucleocapsid (N) protein, was developed for the detection of IgM and IgG antibodies to SARS‐CoV‐2. The related protein was expressed, purified, and used in an ELISA system. Sera samples (67) for COVID‐19 patients, as well as sera samples from healthy volunteers (112), along with sera samples from non‐COVID‐19 patients were examined by the ELISA system. The expression and purity of the recombinant N protein were approved by SDS‐PAGE and Western blotting. The sensitivity of ELISA system was 91.04 and 92.53% for the detection of IgG and IgM antibodies, respectively. Moreover, the specificity of the developed ELISA system for IgG and IgM were 98.21 and 97.32%, respectively. Our developed ELISA system showed satisfactory sensitivity and specificity for the detection of antiSARS‐CoV‐2 IgM and IgG antibodies and could be used as a complementary approach for proper diagnosis of COVID‐19.
Keywords: antibodies, COVID‐19, ELISA, nucleocapsid, recombinant, SARS‐CoV‐2
Abbreviations
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- LKR
linker region
- 1FN
type‐1 interferon
- ELISA
enzyme‐linked immunosorbent assay
- HRP
horseradish peroxidase
- DAB
3, 3′‐diaminobenzidine tetrahydrochloride
- TMB
3, 3′, 5, 5′‐Tetramethylbenzidine.
1. INTRODUCTION
At the end of 2019, several cases of unknown respiratory disease with symptoms of bronchitis were reported in Wuhan, China. 1 , 2 A few weeks later, the cause of this disease identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 3 The disease spread to 122 countries by March 14, 2020. The recent coronavirus 2019 (COVID‐19) outbreak has been classified as Public Health Emergency of International Concern. 4 SARS‐CoV‐2 is an enveloped, positive‐stranded RNA virus and it infects the human respiratory system. 5 SARS‐CoV‐2 is structurally similar to SARS‐CoV. SARS‐CoV‐2 contains four basic proteins comprising spike (S), membrane (M), envelope (E), and nucleocapsid (N). 6 Each of these proteins has a unique role. M and E proteins are essential for virus assembly, 7 whereas the S protein is required for virus binding to the ACE2 receptor on host cell. 8 Protein N is one of the important structural proteins of the virus and is involved in the transcription and translation of RNA and its packing in the virus envelope. 9
N protein has 422 amino acids, and based on bioinformatics studies, it is divided into three domains: the two independent structural domains, the N‐terminus and the C‐terminus, are separated by the disordered central region. The N‐terminal and C‐terminal domains are believed to play a key role in the association of this protein with other proteins. 10 A number of recent studies indicate that the C‐terminal of this protein in SARS‐CoV is involved in protein oligomerization. Structural studies show that the RNA‐binding domain comprises between amino acids 45 and 181, which is near the N‐terminal end. 11 The N‐terminal domain of this protein interacts with the 3′ side of the virus RNA, which in this interaction is probably electrostatic. The N‐terminal and C‐terminal interface region is also called the linker region. 12 , 13
In virus‐infected cells, the N protein in SARS‐CoV is predominantly in the cytoplasm. Functionally, although N protein is not essential for envelope formation, it plays a key role in the formation of complete virions. 14 Moreover, there are many studies showing that N protein is essential for optimal coronavirus replication. 15 The involvement of N protein in the early stages of virion synthesis involves two steps: first, the N protein in SARS‐CoV is located at the site of the replicase enzyme; and second, the induction of infection depends on N protein translocation. 16 On the one hand, this protein also plays a role in the pathogenesis of the virus as it inhibits type‐1 interferon (1IFN) synthesis in SARS‐CoV and on the other hand, the C‐terminal domain is known as the 1IFN induction antagonist. Thus, inhibition of the IFN response seems to be involved in the pathogenesis of SARS‐CoV. 17
At present, one of the main challenges in the diagnosis of COVID‐19 is the early diagnosis of the disease, as timely diagnosis leads to timely treatment and prevention of further transmission of the disease. 18 Currently, several different methods are available for the diagnosis of COVID‐19. One of the most important methods is real‐time PCR in which samples are taken from the lower part of the respiratory tract. Low sensitivity and difficulty of sample preparation are the main drawbacks of this method. 19 In this regard, finding a suitable, cheap, and fast method is necessary. One of these serological methods is enzyme‐linked immunosorbent assay (ELISA), which is mainly based on the detection of antibodies produced against SARS‐CoV‐2 in the patient's serum. 20
Previous studies showed that specific IgM and IgG antibodies against N and S proteins of SARS‐CoV‐2 virus are produced in patient's after symptom onset. 21 IgM and IgG antibodies against N and S proteins are detectable in 75% of non‐ICU patients during the first week of symptom onset and reaches 94.7 and 100% during the second and third weeks after. 22 N protein is appropriate for early detection of COVID‐19 due to its high immunogenicity and aggregation in the cells before packaging in which specific antibodies against N protein are detectable in the serum after 4 days of symptom onset. 21
The current study aimed to develop an ELISA system, using a recombinant N protein, for the detection of IgM and IgG antibodies to SARS‐CoV‐2.
2. MATERIALS AND METHODS
2.1. Serum samples
Serum samples were collected from 67 RT‐PCR confirmed SARS‐COV‐19‐infected patients admitted in University Affiliated Hospital in Shiraz, Fars Province, Iran. All of the patients had symptoms of COVID‐19 with positive chest CT scan. The patients were divided into four groups, based on the day's post symptoms onset, day 0–7, day 8–14, day 15–21, and >22 day. Moreover, 112 negative serum samples were obtained from healthy volunteer serving as controls. Also, 30 serum samples from patients with parasitic or viral diseases, other than SARS‐CoV‐2, were included to a assess no possibly cross‐reactivity of antibodies. These samples included EBV acute (3n), Influenza (2n), Rubella acute (3n), Lupus (3n), CMV acute (3n), Toxocara (3n), Toxoplasma gondii (3n), Fasciola hepatica (3n), fever of unknown origin (FUO) (1n), Plasmodium falciparum (3n), and HCV acute (3n). All sera samples were heated at 65°C for 30 min to inactivate any potential live viruses and were then stored at −70°C until use.
2.2. Vector designing and construction
In the present study, the coding sequence of one of the SARS‐CoV‐2 proteins, N protein, was cloned into the NcoI and XhoI sites of the pET28a expression vector, using the following procedure. At first, the amino acid sequence of N protein (code: P0DTC9) was obtained from Uniprot specific database for COVID‐19 (https://covid‐19.uniprot.org/). Then, the sequence was submitted into GenScript for gene optimization to improve the efficiency of gene expression in Escherichia coli and was then synthesized in pET28a expression cloning vector (Biomatik, Canada). Chemically competent E. coli DH5α cells were then transformed with the recombinant plasmids. Finally, the transformed bacteria were selected and after plasmid extraction, the recombinant plasmids were verified by colony PCR, restriction enzyme digestion, and nucleic acid sequencing.
2.3. Protein expression and purification
The confirmed recombinant N plasmids were extracted and transformed into E. coli BL21 (DE3) cells. Bacteria harboring the recombinant pET28a (+) were grown in Luria Bertani broth supplemented with kanamycin, and agitated (180 rpm) at 37°C for an additional 6 h. The protein expression was then induced by adding 1 mM isopropyl‐β‐d‐thiogalactopyranoside when the OD600 reached 0.6. Cells were then harvested at 5000×g, at 4°C for 15 min.
The harvested bacteria were lysed under the denaturing (8 M urea, 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole) conditions. The pellets were then sonicated (five cycles of sonication at 55% amplitude for 15 s and intervals of 15 s) on ice to disrupt the bacteria and the resulting lysates were centrifuged at 10,000×g, at 4°C for 30 min. The supernatants were collected and applied into a Ni‐NTA column for purification of His‐tagged proteins according to the manufacturer's instructions. To renature the protein, the recombinant eluted protein was transferred into a dialysis tubes (Sigma) and dialyzed using urea supplemented refolding buffer (Tris/phosphate) at 4°C for 24 h. Refolding process was performed using refolding buffer (10 mM Tris, 100 mM sodium phosphate, and 150 mM NaCl, pH 8.0) that its concentration of urea was decreased gradually in every step from 8 to 6 and to 4 and then to 2 and 0 M.
2.4. SDS‐PAGE and Western blotting analysis
The purified recombinant proteins were analyzed by SDS‐PAGE and Western blotting to evaluate their purity and identity. For SDS‐PAGE analysis, the protein fractions were mixed with the loading buffer (0.5 M Tris–HCL [pH 6.8], SDS 10%, glycerol, bromophenol blue, DTT), heated for 10 min at 95°C, and electrophoresed on a 12.5% poly acrylamide gel (180 min, 95 V). The band staining was performed, using Coomassie Brilliant Blue dye (BioRad, UK). In direct and indirect Western blot analysis, 65 ng of each purified recombinant protein was electrophoresed on a 12.5% SDS‐PAGE and the bands were transferred onto a nitrocellulose membrane (BioRad, UK) (at 50 V, overnight). The membranes were blocked in a blocking buffer (5% skimmed milk dissolved in Tris‐buffered saline with 0.05% Tween 20) for 12 h at 4°C. After three rounds of washing with TBST, in indirect Western blot analysis, the membrane was cut into two strips and were separately incubated with inactivated pool sera of patients with confirmed SARS‐CoV‐2 (positive control) and pool sera of healthy volunteers (negative controls) at a dilution of 1:100. Following 60 min of incubation at 37°C, horseradish peroxidase‐conjugated goat anti‐human IgG (Abcam; ab6858) and IgM (Abcam; ab97205) were added to the membrane and the bands were then visualized by adding 3, 3'‐diaminobenzidine tetrahydrochloride (DAB). On the other hand, in direct Western blot analysis, after blocking and washing, the membrane was incubated at 4°C with conjugated anti‐poly His antibody for 90 min, under shaking condition. DAB solution was used for visualizing the specific bands on the nitrocellulose membrane.
2.5. ELISA
Ninety‐six well ELISA microplates were coated (coating buffer: 15 mM Na2CO3, 34 mM NaHCO3 pH 9.6) with 100 μl (5 μg/ml) of each purified recombinant protein at 4°C, overnight. The coating buffer was washed out by adding 300 μl of PBST (PBS containing 0.0.5% Tween‐20), and the wells were then blocked by adding 100 μl of 5% nonfat skimmed milk diluted in PBST, overnight at room temperature (RT). In this section, serum dilution was optimized. For optimization, the negative and positive sera were diluted at 1:50, 1:100, 1:200, and 1:400. Then, 100 μl (1:100 dilutions) of positive and negative sera were added to each well and incubated for 60 min at RT. Following the washing steps, 100 μl of anti‐human IgG and IgM secondary antibodies (1: 20,000 and 1:10,000 dilutions, respectively) were added to each well, and incubated for 1 h at RT. 3,3',5,5'‐Tetramethylbenzidine solution (100 μl) was added to the wells and kept for 15 min at RT in the dark and the reaction was then stopped by adding 1 M H2SO4. Finally, the optical density values were measured at 630 and 430 nm by microplate reader (BioTek, USA).
3. RESULTS
3.1. Construction, expression, and purification of the recombinant antigens
The coding sequence of antigenic protein of SARS‐CoV‐2, protein N (1248 bp) was successfully synthesized and the pET 28a (+) expression vector was obtained. The results of the colony PCR and restriction enzyme digestion showed that the gene fragment was correctly inserted into the expression vector. Moreover, nucleic acid sequencing confirmed the accuracy of the synthesis and cloning. The majority of recombinant proteins with (His) 6‐tagged were in the insoluble form or inclusion bodies. Inclusion bodies converted into solubilized form using 8 M urea. Proteins expressed by pET28 a (+) were purified by Ni‐NTA column chromatography with the yield of 20 mg/ml. The molecular size of the purified recombinant N protein was estimated to be 42 kDa when expressed by pET 28a (+), (Figure 1(A)). Moreover, the Western blot analysis confirmed the identity of N protein, as well as revealing the fact that the produced recombinant protein can bind to the anti‐SARS‐CoV‐2 antibodies present in the sera of infected individuals (Figures 1(B) and 1(C)).
FIGURE 1.
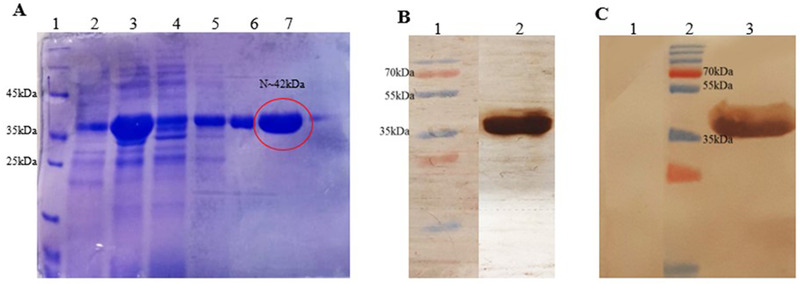
(A) SDS‐PAGE analysis of the recombinant N protein expression and purification. Lane 1: molecular weight marker; Lanes 2, 3, 4, 5, 6, and 7 noninduced, induced, prewash, wash 1, wash 2, and elution with His‐tag, respectively. (B) The membrane was incubated with anti‐His antibody to verify the identity of the N recombinant protein. Lane 1: size maker, Lane 2: protein incubated with anti‐His tag. (C) The membrane was cut into two strips and was separately incubated with sera of confirmed SARS‐CoV‐2 patients (Lane 3) and sera of healthy volunteers (Lane 1)
3.2. ELISA development
The optimal serum dilution for this ELISA was 1:100 that showed best result against different concentration of conjugates and coating antigens. Based on the determined cut off (Cutoff(IgG, IgM) = 0.8), our developed ELISA system average sensitivity for the detection of anti‐SARS‐CoV‐2 IgG and IgM antibodies was 91.79%. Accordingly, the specificity of the recombinant N antigen‐based ELISA for the detection of IgG and IgM antibodies were 98.21 and 97.32%, respectively. ELISA demonstrated high sensitivity at the first week of symptoms onset (day 0–7) and reached the highest (100%) sensitivity after 22 days of symptoms onset. No cross‐reactivity was detected with sera sample of non‐COVID‐19 patients. Accurate results of our developed ELISA system are shown in Table 1.
TABLE 1.
Sensitivity of the rN‐ELISA system for the detection of anti‐SARS‐CoV‐2 IgG and IgM antibodies
| Type of serum | Days post symptom onset | No. of samples | IgG | IgM | IgG and IgM | |||
|---|---|---|---|---|---|---|---|---|
| Positives | Positive percent | Positives | Positive percent | Positives | Positive percent | |||
| COVID‐19 patients | 0–7 | 3 | 3 | 100 | 3 | 100 | 6 | 100 |
| 8–14 | 25 | 21 | 84 | 24 | 96 | 45 | 90 | |
| 15–21 | 22 | 20 | 90.90 | 22 | 100 | 42 | 95.45 | |
| <22 | 17 | 17 | 100 | 13 | 76.47 | 30 | 88.23 | |
| Total | – | 67 | 61 | 91.04 | 62 | 92.53 | 123 | 91.79 |
4. DISCUSSION
These days people all around the world are struggling with the COVID‐19 pandemic. Despite the availability of several valuable vaccine, due to the low speed of vaccination and the emergence of new variants, a large number of people are being infecting every day. Early diagnosis is essential to prevent disease transmission and for appropriate treatment Currently, qRT‐PCR is the common technique to diagnose SARS‐CoV‐2 infection. However, due to the unavailability of sufficient equipment and the high rate of false negativity, which is usually linked to improper sampling, application of this method in practice faced several challenges. Because of these limitations and also the importance of virus‐specific IgM and IgG levels for diagnosis of the infection, researchers are working on serological techniques to find specific antibodies against SARS‐CoV‐2 and diagnose the disease. 23 The recombinant technology accelerates production of the proteins in large scales by inexpensive approach and homogeneous quality. 24 , 25 , 26 , 27 , 28 , 29 , 30 One of the efficient serological test approaches is the ELISA based on recombinant antigens to detect‐specific antibodies. 31 , 32 , 33 , 34 , 35 , 36
In this regard, in the present study, we designed and evaluated a recombinant nucleocapsid (rN) protein‐based ELISA system for the detection of specific IgM and IgG antibodies against SARS‐CoV‐2. Evaluation of 67 samples of COVID‐19 patients along with 112 negative samples, which were collected before the COVID‐19 pandemic, showed that the specificity and sensitivity of our rN‐based ELISA system for the detection of IgG antibodies against SARS‐CoV‐2 are 98.21 and 91.04%, respectively. Also, the specificity and sensitivity of this ELISA system for the detection of IgM antibodies against SARS‐CoV‐2 are 97.32 and 92.53%, respectively. This level of sensitivity and specificity for an ELISA system for the detection of anti‐SARS‐CoV‐2 antibodies is satisfactory compared with the previous studies. It is worth mentioning that no significant cross‐reactivity was detected through the investigation of 30 samples of patients who were suffering from parasitic or viral diseases. In line with our study, Liu et al. 37 reported a sensitivity of 97.02 and 95.24%, for the detection of IgG and IgM antibodies against SARS‐CoV‐2. Specificity of their method for detecting both antibodies was reported to be 100%.
Another study that was conducted by Liu et al., on the rN‐ and rS‐based ELISAs for the detection of the specific IgM and IgG antibodies against SARS‐CoV‐2, reported a sensitivity of 70.1 and 68.2% for IgG and IgM, respectively, which is lower than those reported in our study. The study indicated that the sensitivity of rS‐based ELISA is higher than rN‐based ELISA to detect IgM, which indicated the importance of evaluation of more than one antigen for diagnosis of this disease. Their designed ELISA based on N protein in early days 0–6 after symptom onset detected 31.8, 31.8, and 40.9% of positive cases for IgM, IgG, and combination of both antibodies. 23 In comparison with their results, our N‐based ELISA detected 100% of positive cases for IgM, IgG, and their combination in 0–7 days after symptom onset that is very critical for early diagnosis of COVID‐19 patients.
In another study, Roy et al. 38 developed an ELISA test that can detect and quantify IgA, IgM, and IgG antibodies against the SARS‐CoV‐2 receptor binding domain (RBD). According to their study, the specificity of IgG, IgA, and IgM was 99.56, 96.55, and 96.98%, respectively. Also, the reported sensitivity for these antibodies was 82.35, 94.12, and 77.94% in order. In addition, this study showed that the sensitivity of all these antibodies for more than 21 days from symptoms onset was 100%, which is satisfactory. 38
Indenbaum and her colleagues 39 who were working on an in‐house ELISA to detect the IgG, IgA, and IgM antibodies against RBD were reporting a close rate of specificity to Roy et al.’s study. According to their result, the specificity of IgG, IgA, and IgM was 98, 98, and 100%, and the sensitivity was 88, 80, and 47%, respectively. 39
In conclusion, the present study illustrated the importance of the rN‐based ELISA technique as a screening method for the diagnosis of COVID‐19. It should be noted that by reviewing previous studies, it seems that testing more than one SARS‐CoV‐2 antigenic protein could lead to more favorable results in diagnosis of COVID‐19. Accordingly, investigating other SARS‐CoV‐2 antigenic proteins to find the best combination of antigens for development of a high‐performance ELISA system for diagnosis of COVID‐19 can be suggested.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
5. ACKNOWLEDGMENT
This study was financially supported by the office of vice‐chancellor for research of Shiraz University of Medical Sciences (Grant No. 22116_106_01_98).
Ranjbar M, Asadi M, Nourigorji M, Sarkari B, Mostafavi‐Pour Z, Zomorodian K, et al. Development of a Recombinant Nucleocapsid Protein‐Based ELISA for Detection of IgM and IgG Antibodies to SARS‐CoV‐2. Biotechnol Appl Biochem. 2022;00 1–7. 10.1002/bab.2308
Contributor Information
Mohsen Moghadami, Email: moghadami@sums.ac.ir.
Amir Savardashtaki, Email: dashtaki63@gmail.com.
REFERENCES
- 1. Taheri‐Anganeh M, Khatami SH, Jamali Z, Savardashtaki A, Ghasemi Y, Mostafavi‐Pour Z. In silico analysis of suitable signal peptides for secretion of a recombinant alcohol dehydrogenase with a key role in atorvastatin enzymatic synthesis. Mol Biol Res Commun. 2019;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet North Am Ed. 2020;395(10224):565‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, et al. First known person‐to‐person transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in the USA. Lancet.. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gkiouras K, Nigdelis MP, Grammatikopoulou MG, Goulis DG. Tracing open data in emergencies: The case of the COVID‐19 pandemic. Eur J Clin Invest. 2020;50(9):e13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou L, Zhang M, Wang J, Gao J. Sars‐Cov‐2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;101642 (10.1016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaimes JA, André NM, Chappie JS, Millet JK, Whittaker GR. Phylogenetic analysis and structural modeling of SARS‐CoV‐2 spike protein reveals an evolutionary distinct and proteolytically‐sensitive activation loop. J Mol Biol. 2020;432(10):3309‐3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta MK, Vemula S, Donde R, Gouda G, Behera L, Vadde R. In‐silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J Biomol Struct Dyn. 2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karathanou K, Lazaratos M, Bertalan É, Siemers M, Buzar K, Schertler GF, et al. A graph‐based approach identifies dynamic H‐bond communication networks in spike protein S of SARS‐CoV‐2. J Struct Biol. 2020;212(2):107617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, Chow A, et al. SARS‐CoV‐2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell. 2020;183(5):1325‐1339.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang C‐K, Sue S‐C, Yu T‐H, Hsieh C‐M, Tsai C‐K, Chiang Y‐C, et al. Modular organization of SARS coronavirus nucleocapsid protein. J Biomed Sci. 2006;13(1):59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang Q, Yu L, Petros AM, Gunasekera A, Liu Z, Xu N, et al. Structure of the N‐terminal RNA‐binding domain of the SARS CoV nucleocapsid protein. Biochemistry. 2004;43(20):6059‐63. [DOI] [PubMed] [Google Scholar]
- 12. Chang C‐k Hou M‐H, Chang C‐F, Hsiao C‐D, Huang T‐H The SARS coronavirus nucleocapsid protein–forms and functions. Antiviral Res. 2014;103:39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang F‐Y, Lin L‐C, Ying T‐H, Yao C‐W, Tang T‐K, Chen Y‐W, et al. Immunoreactivity characterisation of the three structural regions of the human coronavirus OC43 nucleocapsid protein by Western blot: Implications for the diagnosis of coronavirus infection. J Virol Methods. 2013;187(2):413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurst KR, Koetzner CA, Masters PS. Identification of in vivo‐interacting domains of the murine coronavirus nucleocapsid protein. J Virol. 2009;83(14):7221‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wurm T, Chen H, Hodgson T, Britton P, Brooks G, Hiscox JA. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J Virol. 2001;75(19):9345‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grossoehme NE, Li L, Keane SC, Liu P, Dann CE III, Leibowitz JL, et al. Coronavirus N protein N‐terminal domain (NTD) specifically binds the transcriptional regulatory sequence (TRS) and melts TRS‐cTRS RNA duplexes. J Mol Biol. 2009;394(3):544‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu X, Ja P, Tao J, Guo D. SARS‐CoV nucleocapsid protein antagonizes IFN‐β response by targeting initial step of IFN‐β induction pathway, and its C‐terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai L, Yang D, Wang X, Tong L, Zhu X, Zhong N, et al. Chinese experts’ consensus on the Internet of Things‐aided diagnosis and treatment of coronavirus disease 2019 (COVID‐19). Clin eHealth. 2020;3:7‐15. [Google Scholar]
- 19. Chan JF‐W, Yip CC‐Y, To KK‐W, Tang TH‐C, Wong SC‐Y, Leung K‐H, et al. Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific COVID‐19‐RdRp/Hel real‐time reverse transcription‐PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grzelak L, Temmam S, Planchais C, Demeret C, Tondeur L, Huon C, et al. A comparison of four serological assays for detecting anti–SARS‐CoV‐2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12(559). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tantuoyir MM, Rezaei N. Serological tests for COVID‐19: potential opportunities. Cell Biol Int. 2021;45(4):740‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu S, Lu S. Antibody responses in COVID‐19 patients. J Biomed Res. 2020;34(6):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, et al. Evaluation of nucleocapsid and spike protein‐based enzyme‐linked immunosorbent assays for detecting antibodies against SARS‐CoV‐2. J Clin Microbiol. 2020;58(6):e00461‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asadi M, Taghizadeh S, Kaviani E, Vakili O, Taheri‐Anganeh M, Tahamtan M, et al. Caspase‐3: structure, function, and biotechnological aspects. Biotechnol Appl Biochem. 2021. [DOI] [PubMed] [Google Scholar]
- 25. Khatami SH, Vakili O, Ahmadi N, Soltani Fard E, Mousavi P, Khalvati B, et al. Glucose oxidase: applications, sources, and recombinant production. Biotechnol Appl Biochem. 2021. [DOI] [PubMed] [Google Scholar]
- 26. Movahedpour A, Ahmadi N, Ghalamfarsa F, Ghesmati Z, Khalifeh M, Maleksabet A, et al. β‐Galactosidase: from its source and applications to its recombinant form. Biotechnol Appl Biochem. 2021. [DOI] [PubMed] [Google Scholar]
- 27. Movahedpour A, Asadi M, Khatami SH, Taheri‐Anganeh M, Adelipour M, Shabaninejad Z, et al. A brief overview on the application and sources of α‐amylase and expression hosts properties in order to production of recombinant α‐amylase. Biotechnol Appl Biochem. 2021. [DOI] [PubMed] [Google Scholar]
- 28. Tehrani SS, Goodarzi G, Naghizadeh M, Khatami SH, Movahedpour A, Abbasi A, et al. Suitable signal peptides for secretory production of recombinant granulocyte colony stimulating factor in Escherichia coli . Recent Pat Biotechnol. 2020;14(4):269‐82. [DOI] [PubMed] [Google Scholar]
- 29. Khatami SH, Taheri‐Anganeh M, Arianfar F, Savardashtaki A, Sarkari B, Ghasemi Y, et al. Analyzing signal peptides for secretory production of recombinant diagnostic antigen B8/1 from Echinococcus granulosus: an in silico approach. Mol Biol Res Commun. 2020;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taheri‐Anganeh M, Khatami SH, Jamali Z, Movahedpour A, Ghasemi Y, Savardashtaki A, et al. LytU‐SH3b fusion protein as a novel and efficient enzybiotic against methicillin‐resistant Staphylococcus aureus. Mol Biol Res Commun. 2019;8(4):151. [PMC free article] [PubMed] [Google Scholar]
- 31. Savardashtaki A, Mostafavi‐Pour Z, Arianfar F, Sarkari B. Comparison of the utility of recombinant B8/2 subunit of the antigen B, native antigen, and a commercial ELISA Kit in the diagnosis of human cystic Echinococcosis. Iran Biomed J. 2019;23(4):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Savardashtaki A, Sarkari B, Arianfar F, Mostafavi‐Pour Z. Immunodiagnostic value of Echinococcus granulosus recombinant B8/1 subunit of antigen B. Iran J Immunol. 2017;14(2):111‐22. [PubMed] [Google Scholar]
- 33. Savardashtaki A, Sharifi Z, Hamzehlou S, Farajollahi MM. Analysis of immumoreactivity of heterologously expressed non‐structural protein 4B (NS4B) from hepatitis C virus (HCV) genotype 1a. Iran J Biotechnol. 2015;13(4):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khatami SH, Taheri‐Anganeh M, Movahedpour A, Savardashtaki A, Ramezani A, Sarkari B, et al. Serodiagnosis of human cystic echinococcosis based on recombinant antigens B8/1 and B8/2 of Echinococcus granulosus. J Immunoassay Immunochem. 2020;41(6):1010‐20. [DOI] [PubMed] [Google Scholar]
- 35. Mehrpour K, Mirzaei SA, Savardashtaki A, Nezafat N, Ghasemi Y. Designing an HCV diagnostic kit for common genotypes of the virus in Iran based on conserved regions of core, NS3‐protease, NS4A/B, and NS5A/B antigens: an in silico approach. Biologia (Bratisl). 2021;76(1):281‐96. [Google Scholar]
- 36. Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, et al. COVID‐19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther. 2021;19(3):345‐57. [DOI] [PubMed] [Google Scholar]
- 37. Liu P‐P, Zong Y, Jiang S‐P, Jiao Y‐J, Yu X‐J Development of a nucleocapsid protein‐based ELISA for detection of human IgM and IgG antibodies to SARS‐CoV‐2. ACS Omega. 2021;6(14):9667‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roy V, Fischinger S, Atyeo C, Slein M, Loos C, Balazs A, et al. SARS‐CoV‐2‐specific ELISA development. J Immunol Methods. 2020;484‐485:112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Zelm MC, Indenbaum V, Koren R, Katz‐Likvornik S, Yitzchaki M, Halpern O, et al. Testing IgG antibodies against the RBD of SARS‐CoV‐2 is sufficient and necessary for COVID‐19 diagnosis. PLoS One. 2020;15(11):e0241164. [DOI] [PMC free article] [PubMed] [Google Scholar]


