Abstract
Objective
The purpose of this study was to describe cerebrovascular, neuropathic, and autonomic features of post‐acute sequelae of coronavirus disease 2019 ((COVID‐19) PASC).
Methods
This retrospective study evaluated consecutive patients with chronic fatigue, brain fog, and orthostatic intolerance consistent with PASC. Controls included patients with postural tachycardia syndrome (POTS) and healthy participants. Analyzed data included surveys and autonomic (Valsalva maneuver, deep breathing, sudomotor, and tilt tests), cerebrovascular (cerebral blood flow velocity [CBFv] monitoring in middle cerebral artery), respiratory (capnography monitoring), and neuropathic (skin biopsies for assessment of small fiber neuropathy) testing and inflammatory/autoimmune markers.
Results
Nine patients with PASC were evaluated 0.8 ± 0.3 years after a mild COVID‐19 infection, and were treated as home observations. Autonomic, pain, brain fog, fatigue, and dyspnea surveys were abnormal in PASC and POTS (n = 10), compared with controls (n = 15). Tilt table test reproduced the majority of PASC symptoms. Orthostatic CBFv declined in PASC (−20.0 ± 13.4%) and POTS (−20.3 ± 15.1%), compared with controls (−3.0 ± 7.5%, p = 0.001) and was independent of end‐tidal carbon dioxide in PASC, but caused by hyperventilation in POTS. Reduced orthostatic CBFv in PASC included both subjects without (n = 6) and with (n = 3) orthostatic tachycardia. Dysautonomia was frequent (100% in both PASC and POTS) but was milder in PASC (p = 0.002). PASC and POTS cohorts diverged in frequency of small fiber neuropathy (89% vs 60%) but not in inflammatory markers (67% vs 70%). Supine and orthostatic hypocapnia was observed in PASC.
Interpretation
PASC following mild COVID‐19 infection is associated with multisystem involvement including: (1) cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction; (2) small fiber neuropathy and related dysautonomia; (3) respiratory dysregulation; and (4) chronic inflammation. ANN NEUROL 2022;91:367–379
Glossary
- BRAF‐NRS =
Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales, V2 Revised
- BFS =
Brain Fog Scale
- COVID‐19 =
coronavirus disease 19
- CVRi =
cerebrovascular resistance index
- ENFD =
epidermal nerve fiber density
- NTSS =
Neuropathy Total Symptom Score‐6, SAS
Survey of Autonomic Symptoms
- PASC =
post‐acute sequelae of COVID‐19
- POTS =
Postural Tachycardia Syndrome
- QASAT =
Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and small fibers from skin biopsies
- SARS‐CoV‐2 =
severe acute respiratory syndrome coronavirus 2
- SFN =
small fiber neuropathy
- SGNFD =
sweat gland nerve fiber density
Coronavirus disease 19 (COVID‐19) is a novel infectious disease caused by severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2), which incited a global pandemic. Post‐acute sequelae of COVID‐19 (PASC) is provisionally defined as persistence or worsening of symptoms for >4 weeks or new symptoms attributable to COVID‐19, affects 20–60% of patients. 1 Common complaints are fatigue, dyspnea, brain fog, and orthostatic intolerance, and a variety of systemic complaints. 2
PASC is a multisystem disorder, however, the exact pathophysiology remains poorly understood. Potential mechanisms include post‐infectious inflammation, immune‐mediated vascular dysfunction, thromboembolism, lung dysfunction, and nervous system dysfunction due to occult neuronal injury during SARS‐CoV‐2 infection. 3 , 4
Our case report 5 suggested that PASC can be associated with autoimmune‐mediated multisystem abnormalities. A patient with PASC developed intravenous immunoglobulin‐responsive symptoms suggestive of cerebral hypoperfusion due to cerebrovascular arteriolar dysfunction, small fiber neuropathy (SFN), and dysautonomia. 6 We aimed to confirm these findings in a group of patients with PASC. Postural tachycardia syndrome (POTS) is characterized by symptoms that are similar to PASC, including chronic fatigue, orthostatic intolerance, and brain fog linked to reduced orthostatic cerebral blood flow and dysautonomia. 7 , 8 , 9 Patients with POTS thus provide a useful comparison group aside from healthy controls, to enable identification of pathophysiology distinct to PASC.
We hypothesize that PASC is associated with autoimmune/inflammatory mediated widespread cerebrovascular and metabolic dysregulation and neuropathic changes.
Methods
Study Design
Patient Population and Study Design
The study was approved by the Brigham and Women's Hospital (BWH) Institutional Review Board as a minimal risk study.
This was a single‐center, retrospective study of consecutive patients who presented to BWH Autonomic Laboratory for evaluation of PASC. Inclusion criteria for the study were: (1) adult patients 18 years of age or older; (2) documented diagnosis of COVID‐19 disease by history of positive reverse transcriptase‐polymerase chain reaction (PCR) positive for SARS‐CoV‐2; (3) completion of comprehensive autonomic testing with skin biopsies; (4) availability of electronic health care records; (5) satisfying inclusion criteria for PASC; (6) for the POTS controls, matched by age and sex, satisfying criteria for POTS 10 ; and (7) age and sex matched asymptomatic healthy controls were used from our previous University of Massachusetts (UMASS) study. 11 Testing at the UMASS was identical to the Brigham protocol 12 used in this study except electrochemical skin conductance (ESC) 13 was used instead of quantitative sudomotor axon reflex test (QSART) for sudomotor evaluations. ESC correlates with loss of sweat gland nerve fibers and therefore can be used as a proxy for sudomotor function. 13 In addition, the inflammatory markers were not available for controls.
Exclusion criteria were: (1) significant metabolic, and acid–base disarrangement and anemia that can influence autonomic and respiratory system and/or cerebral blood flow; (2) evidence of cardiac or pulmonary disease; (2) presence of disorders associated with secondary SFN or dysautonomia (diabetes, pre‐diabetes, parkinsonism, Parkinson's disease, history of heavy alcohol use, B12 and/or folate deficiency, thyroid disease, celiac disease, hepatitis C, HIV infection, chemotherapy exposure and/or cancer diagnosis, history of autoimmune disease, and any comorbid conditions or use of medications that have been associated with SFN); (3) evidence of large fiber neuropathy on neurological examination (loss of tendon reflexes or loss of proprioception) or on nerve conduction studies; (4) the use of medications that may affect the autonomic nervous system during testing; and (5) inability to complete the Brigham protocol.
Definition of PASC
Fatigue, brain fog, and dyspnea are characteristic for PASC. 2 To grade fatigue, we used the Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF‐NRS, V2 Revised), 10 a validated self‐rating scale which measures fatigue dimensions: severity (range: 0–10), effect on life (range: 0–10), and coping ability (range: 0–10).
There is no validated brain fog scale (BFS). For the purpose of this study, we defined the 11‐point scale from 0 (no brain fog) to 10 (severe disabling brain fog, always present). The scale is based on a brain fog scale from 0 to 100 in 10‐point intervals showing brain fog in POTS correlated with fatigue. 7
At present, there are no established criteria for PASC. 3 Therefore, we followed the current guidelines 14 and provisional definition of PASC. 15 In general, PASC is described as signs and symptoms that develop during or after an infection consistent with COVID‐19, continue for weeks and are not explained by an alternative diagnosis. Furthermore, neurological disease occurring in the 6‐week interval after acute infection is associated with autoimmune mechanisms. 15
For the purpose of this study, we defined the inclusion criteria for PASC by the following: (1) presence of chronic (>4 weeks) fatigue (grade 3 or more on each of the BRAF‐NRS parts), and brain fog (grade 3 or more on BFS) which developed within the 6‐week interval after acute COVID‐19 infection.
Patients with POTS were defined using standard criteria.10,16
Additional validated neurologic symptom surveys were completed at the time of autonomic testing. Sensory complaints were surveyed by the Neuropathy Total Symptom Score‐6 (NTSS), which is a validated instrument for evaluation of neuropathic pain features. 17 The NTSS quantifies sensory symptoms (aching pain, allodynia, burning pain, lancinating pain, numbness, and prickling sensation). A score >0 indicate the presence of sensory symptoms, the NTSS range is 0 to 21.96. Autonomic symptoms were obtained from the Survey of Autonomic Symptoms (SAS), which is a validated instrument for evaluation of autonomic complaints. The symptoms were grouped into orthostatic, sudomotor, vasomotor, gastrointestinal, urinary, and sexual (for men) categories. 18 The SAS score ranges from 0 to 60 for men and 0 to 55 for women with the higher number representing a more severe severity of symptoms. A modified Borg scale, which is based on category‐ratio scale (CR‐10), with score 0 (no dyspnea) to 10 (unbearable dyspnea) was used for grading dyspnea. 19
Definition of SFN
SFN is defined as combination of symptoms and signs. 20 The criteria for SFN were SAS or NTSS >1 and abnormal skin biopsies or abnormal sudomotor testing. 13 , 20
Autonomic Testing
The Brigham protocol, 12 which consists of functional autonomic testing and skin biopsies for evaluation of small fibers, was used in this study. Autonomic testing included deep breathing, Valsalva maneuver, 10 minutes of the tilt test, and sudomotor evaluation. We described details of testing previously. 12 Electrocardiogram, continuous and intermittent blood pressure, end tidal CO2, finger pulse oximetry, and cerebral blood flow velocity (CBFv) in the middle cerebral artery (MCA) using transcranial Doppler were recorded. Normative data were published previously. 12 For the CBFv, the lower limit for women/men are 82.2–0.45 (cm/s)*age (years)/72.09–0.38 (cm/s)*age (years). 12 In healthy subjects, orthostatic CBFv is either unchanged or may decline slightly. The normal decline in orthostatic CBFv from baseline is equal or less than 10% (first minute), 11% (fifth minute), and 15% (10th minute) of the tilt test. 12
Brigham protocol results were graded using Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and Small Fibers from Skin Biopsies (QASAT). 12 QASAT is an objective instrument for grading severity of dysautonomia, small fiber neuropathy, and cerebral blood flow. Scores are calculated for each domain, including heart rate, blood pressure, cerebral blood flow, and end tidal CO2. The score equal to 0 is normal, above 0 is abnormal with the severity proportional to the number.
CO2 has a profound effect on CBFv. Hypocapnia/hypercapnia decreases/increases diameter of cerebral arterioles 21 which decreases/increases CBFv due to increase/decrease in cerebrovascular resistance. Generally, in a healthy cerebral vasculature, a decrease of end tidal CO2 by 1 mmHg decreases cerebral blood flow by about 3%. 22 We separated the CO2‐induced CBFv changes as follows. First, we assessed the QASAT CBFv tilt test response score from raw CBFv (QASAT‐CBFvtilt). Second, we calculated the adjusted QASAT CBFv score (QASAT‐Adjusted CBFvtilt) from the CBFv adjusted for the effect of CO2 by calculating the how much orthostatic drop of CBFv is due to the drop of ET‐CO2. The proportion of the CBFv changes due to the effect of CO2 was then calculated as:
For example if the QASAT‐CBFvtilt = 10 (100%) and QASAT‐Adjusted CBFvtilt = 3 (30%), than 70% ([10–3]*100) of the reduced CBFv is due to effect of CO2 and 30% is due to cerebral arteriolar vasoconstriction.
The percentage of the cerebral arteriolar dysfunction is referred as arteriolar orthostatic index (AOI).
All data were recorded using Labchart system (ADInstruments, AU), sampled at 400 Hz. The data for final statistical analyses were obtained after 10 minutes of baseline period, and at each minute of the tilt test.
The blood pressure at the level of MCA was calculated from arm pressure by adjustment for the hydrostatic gradients. 23 With average MCA‐arm distance the MVA pressure was obtained by subtracting 20 from arm pressure. That reduction is ~2 mmHg for 2.5 cm of vertical displacement. Cerebral vascular resistance index (CVRi) was calculated as mean blood pressure at the level of MCA divided by mean CBFv. 6
We also monitored the blood oxygenation by pulse oximetry from a finger.
Skin Biopsy
Epidermal nerve fiber density (ENFD) and sweat gland nerve fiber density (SGNFD) were obtained using established standards. 20 , 24 Skin samples were taken from the proximal thigh 20 cm distal to the iliac spine, and from the calf 10 cm above the lateral malleolus using a 3‐mm circular punch tool. Skin samples were immunoperoxidase‐stained for the axonal marker PGP 9.5. Skin processing and fiber counting was done at Therapath (New York, NY). Thresholds for normal values for proximal thigh were 8.3 fibers/mm (ENFD) and 37.8 fibers/mm (SGNFD) and 36.5 fibers/mm (SGNFD) for the calf, were established by Therapath. The age and gender adjusted ENFD normative values at the calf were also obtained from Therapath.
Grading of Small Fiber Neuropathy, Dysautonomia, and Cerebral Blood Flow
Test results were graded using the QASAT. 12 QASAT is an objective instrument for grading severity of dysautonomia, small fiber neuropathy, and cerebral blood flow. For each domain (heart rate, blood pressure, cerebral blood flow, and end tidal CO2), the score equal to 0 is normal, above 0 is abnormal with the severity proportional to the number.
Inflammatory Panel
We measured the following serum inflammatory markers 25 : high resolution C‐reactive protein (normative value < = 3 mg/L), tumor necrosis factor‐alpha (TNF‐α, < = 2.8 pg/ml), interleukin (IL) IL‐6 (<7.1 pg/ml), IL‐10 (<2 pg/ml), IL‐1ß (<7.1 pg/ml), leptin (3.3–18.3 ng/ml), adiponectin (2.4–17.9 ug/ml), trisulfated heparin disaccharide (TS‐HDS) antibody (<10,000 titers), 26 fibroblast growth factor receptor 3 (FGFR3) antibody (<3,000 titers), 26 acetylcholine receptor binding antibody (< = 0.02 nmol/L), ganglionic acetylcholine receptor antibody (< = 0.02 nmol/L), 27 neuronal VGKC antibody (< = 0.02 nmol/L), calcium channel P/Q binding antibody (<= 0.02 nmol/L), myoglobin (<= 71 ng/ml), 28 and human growth hormone (0.01–3.61 ng/mL). 28 We also evaluated norepinephrine supine (70–750 pg/ml) and standing (200–1,700 pg/ml), which is useful in the assessment of the hyperadrenergic form of POTS. 10 The TS‐HDS and FGFR3 antibodies were obtained from Washington University School of Medicine (St. Louis, MO), remaining antibodies as well as TNF‐α, IL‐10, human growth hormone were obtained from Mayo Clinic laboratories (Rochester, MN). IL‐6 was obtained at Mayo Clinic laboratories (Rochester, MN) or at BWH Clinical laboratories (Boston, MA). IL‐1ß was obtained at Sunquest (Tucson, AZ). Leptin was obtained at Esoterix Endocrinology (Calabasas Hills, CA). Remaining of laboratory tests were obtained at BWH Clinical laboratories (Boston, MA) or at Quest Diagnostics (Secaucus, NJ).
Statistical Analysis
A one‐way analysis of variance (ANOVA) for continuous variables and chi‐squared test for categorical variables were used to determine the differences between subject's groups. A Tukey's adjusted post hoc test or t test (when data from healthy controls were not available) and contingency tables with Fisher's exact test were used for PASC and POTS comparisons for continuous and categorical variables, respectively. Repeated‐measures ANOVA was used to evaluate the effect of time during the tilt test. Statistical analysis was obtained using R statistical (r-project.org) and tableOne python package. 29
Results
Demographics and Symptoms
Sixteen patients with PASC were screened (Fig 1). Active pressor medication excluded 2 individuals, and a history of SFN or dysautonomia excluded 5 individuals from the study. Nine patients with PASC (age 35.8. ± 7.3 years, 9 women, all White) were age and sex‐matched with 10 patients with POTS (36.3 ± 9.8, 9 women, all White) and 15 controls (40.1 ± 11.6, 13 women, all White) were included in this study (see Table 1 and Supplementary Table S1). All patients with PASC had mild COVID‐19 infection with conservative home management with one individual treated with supplemental home oxygen (see Supplementary Material Table S1). No patients with PASC included in this study received the COVID‐19 vaccine and no one had a history of severe acute respiratory distress syndrome, acute kidney, or liver failure, or reported use of corticosteroids or hydroxychloroquine.
FIGURE 1.
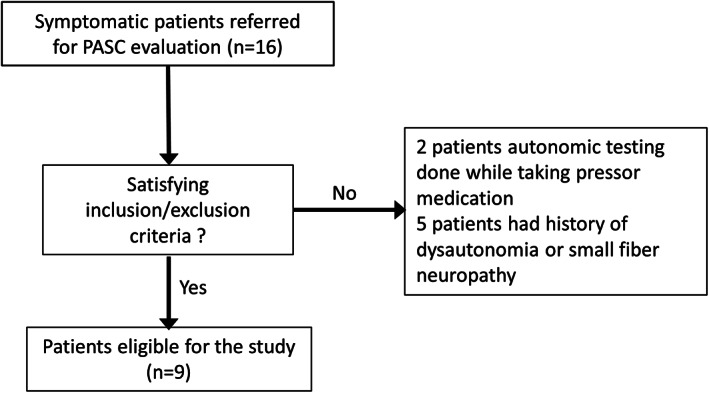
Flow chart of the study. PASC = post‐acute sequelae of coronavirus disease 2019.
TABLE 1.
Demographic Characteristics of the Study
| Diagnosis | ||||||
|---|---|---|---|---|---|---|
| Controls | POTS | PASC | ||||
| N | 15 | 10 | 9 | 95% CI, F, and p (Controls‐POTS‐PASC) | p (POTS‐PASC) | |
| Age, years, mean (SD) | 40.1 (11.6) | 36.3 (9.8) | 35.8 (7.3) | 33.7–41.0, F (2, 31) = 0.661, p = 0.524 | 0.993 | |
| Gender, n (%) | F | 13 (86.7) | 9 (90.0) | 9 (100) | p = 0.531 | 0.999 |
| M | 2 (13.3) | 1 (10.0) | ||||
| BMI, kg/m2, mean (SD) | 25.3 (5.9) | 23.6 (4.8) | 26.4(7.3) | 22.90–27.1, F (2, 31) = 0.508, p = 0.607 | 0.578 | |
| Symptom duration, years, mean (SD) | 0.0 (0.0) | 7.7 (4.3) | 0.8 (0.3) | 2.01–3.68, F (2, 31) = 35.7, p < 0.001 | <0.001 | |
BMI = body mass index; CI = confidence interval; F = bioavailability; PASC = post‐acute sequelae of coronavirus disease 2019; POTS = postural tachycardia syndrome.
The length of time from initial documented symptoms to autonomic testing was longer in POTS compared to PASC (p < 0.001). No acute symptoms indicative of COVID‐19 disease was exhibited up to 72 hours before and up to 5 weeks after Brigham protocol testing.
Clinical Symptoms
By definition, healthy controls were absent of chronic pain, autonomic symptoms, dyspnea, brain fog, or fatigue (Table 2). Patients with PASC and POTS reported neuropathic pain, fatigue, dyspnea, and symptoms in all surveyed autonomic domains. Several patients with PASC and POTS had a history of migraine headaches, but no one had a migraine during the testing. Autonomic complaints were not significantly different between PASC and POTS, whereas the tilt test induced dizziness and exacerbated fatigue and brain fog in both cohorts.
Autonomic Testing
Profiles of CBFv, end tidal CO2, heart rate, and blood pressure at baseline and during the tilt test across all groups is shown in Figure 2A‐F. Tables 2, 3, 4 summarize the test results. QASAT scores in healthy controls (parasympathetic, sympathetic, and sudomotor) were zero.
FIGURE 2.
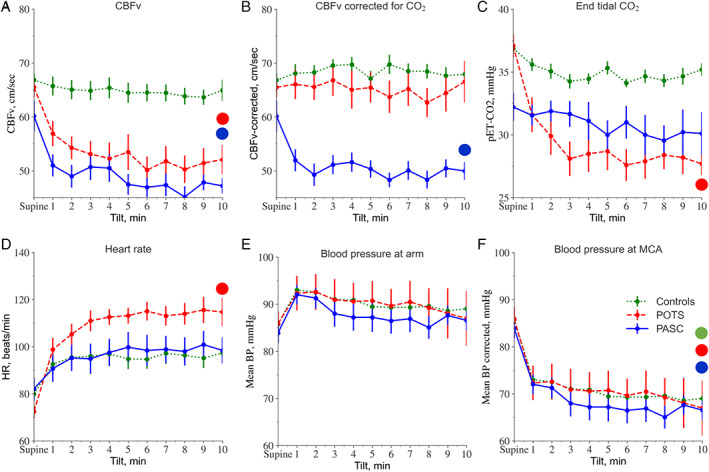
Profile of cerebral blood flow velocity (CBFv), CBFv corrected for the effect of CO2, end tidal CO2, heart rate (HR), and blood pressure (BP) during supine baseline and the tilt test for post‐acute sequelae of coronavirus disease 2019 (PASC), postural tachycardia syndrome (POTS), and controls. Large dots indicate significance at p = 0.001 level for respective data obtained by repetitive measures analysis of variance (ANOVA). [Color figure can be viewed at www.annalsofneurology.org]
TABLE 2.
Surveys Results
| Diagnosis | 95% CI, F and p | p | |||||
|---|---|---|---|---|---|---|---|
| Controls | POTS | PASC | PASC‐POTS‐Controls | PASC‐Controls | PASC‐POTS | ||
| N | 15 | 10 | 9 | ||||
| Pain symptoms, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| NTSS score, range 0–21.96, mean (SD) | 0.0 (0.0) | 10.9 (5.4) | 10.0 (5.1) | 5.58–8.36, F (2, 31) = 30.53, p < 0.001 | <0.001 | 0.703 | |
| Autonomic symptoms, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100.0) | |||||
| SAS score, range 0–60 men, 0–55 women, mean (SD) | 0.0 (0.0) | 24.9 (5.8) | 25.9 (11.2) | 14.60–19.26, F (2, 31) = 63.6, p < 0.001 | <0.001 | 0.942 | |
| Orthostatic symptoms, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| Orthostatic survey, range 0–5, mean (SD) | 0.0 (0.0) | 3.9 (1.0) | 3.9 (1.1) | 2.32–2.87, F (2, 31) = 110.8, p < 001 | <0.001 | 0.999 | |
| Sudomotor symptoms, n (%) | No | 15 (100.0) | 9 (100) | <0.001 | <0.001 | 0.999 | |
| Yes | 10 (100.0) | ||||||
| Sudomotor survey, range 0–20, mean (SD) | 0.0 (0.0) | 7.1 (2.8) | 8.0 (5.0) | 3.98–6.09, F (2, 31) = 27.8, p < 0.001 | <0.001 | 0.783 | |
| Vasomotor symptoms, n (%) | No | 15 (100.0) | 1 (10.0) | 1 (11.1) | <0.001 | <0.001 | 0.999 |
| Yes | 9 (90.0) | 8 (88.9) | |||||
| Vasomotor survey, range 0–10, mean (SD) | 0.0 (0.0) | 5.0 (3.0) | 5.2 (3.7) | 2.52–4.30, F (2, 31) = 17.7, p < 0.001 | <0.001 | 0.979 | |
| Gastrointestinal symptoms, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| Gastrointestinal survey, range 0–15, mean (SD) | 0.0 (0.0) | 7.4 (3.1) | 7.4 (3.6) | 4.01–5.83, F (2, 31) = 38.4, p < 0.001 | <0.001 | 0.999 | |
| Urinary symptoms, n (%) | No | 15 (100.0) | 7 (70.0) | 4 (44.4) | <0.001 | 0.003 | 0.370 |
| Yes | 3 (30.0) | 5 (55.6) | |||||
| Urinary survey, range 0–5, mean (SD) | 0.0 (0.0) | 1.0 (1.8) | 1.3 (1.5) | 0.34–1.21, F (2, 31) = 3.96, p < 0.001 | 0.037 | 0.823 | |
| Sexual symptoms, men, n (%) | No | 1 (100.0) | <0.001 | 0.999 | 0.999 | ||
| Yes | 1 (100.0) | ||||||
| Sexual survey, range 0–5, men, mean (SD) | 0.0 (nan) | 5.0 (nan) | NA | NA | 0.999 | ||
| Brain fog, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| Brain fog score, mean (SD) | 0.0 (0.0) | 5.3 (1.4) | 6.2 (2.4) | 3.31–4.36, F (2, 31) = 66.08, p < 0.001 | <0.001 | 0.363 | |
| Fatigue, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100.0) | |||||
| Total fatigue score, mean (SD) | 0.0 (0.0) | 20.9 (4.0) | 21.3 (4.0) | 13.02–15.14, F (2, 31) = 213.7, p < 0.001 | <0.001 | 0.945 | |
| Dyspnea, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| Dyspnea score, mean (SD) | 0.0 (0.0) | 4.5 (2.2) | 5.4 (1.0) | 2.84–3.78, F (2, 31) = 61.56, p < 0.001 | <0.001 | 0.270 | |
| Dizziness during the tilt test, n (%) | No | 14 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 8 (100.0) | 9 (100) | |||||
CI = confidence interval; F = bioavailability; NA = not applicable; NTSS = Neuropathy Total Symptom Score; PASC = post‐acute sequelae of coronavirus disease 2019; POTS = postural tachycardia syndrome.
TABLE 3.
Testing Results
| Diagnosis | 95% CI, F, and p | p | |||||
|---|---|---|---|---|---|---|---|
| Controls | POTS | PASC | PASC‐POTS‐controls | PASC‐controls | PASC‐POTS | ||
| N | 15 | 10 | 9 | ||||
| Inflammatory markers, number, mean (SD) | 0.8 (0.6) | 1.3 (0.8) | NA | NA | 0.578 | ||
| Inflammatory markers, presence, n (%) | No | 3 (30.0) | 3 (33.3) | NA | NA | 0.999 | |
| Yes | 7 (70.0) | 6 (66.7) | |||||
| Norepinephrine, supine pg/ml, mean (SD) | 355.7 (123.6) | 556.9 (245.2) | 371.0 (161.4) | 336.6–519.1, F (2, 18) = 2.53, p < 0.107 | 0.989 | 0.196 | |
| Norepinephrine, standing pg/ml, mean (SD) | 435.8 (131.2) | 723.2 (272.1) | 501.0 (262.9) | 438.1–668.5, F (1, 2) = 3.03, p < 0.075 | 0.893 | 0.240 | |
| ENFD, n (%) | Abnormal | 5 (55.6) | 5 (55.6) | p = 0.001 | 0.001 | 0.999 | |
| Normal | 15 (100.0) | 4 (44.4) | 4 (44.4) | ||||
| ENFD‐proximal, fibers/mm, mean (SD) | 14.7 (4.0) | 12.1 (7.1) | 9.3 (4.1) | 10.20–13.90, F (2, 30) = 3.23, p < 0.053 | 0.006 | 0.491 | |
| ENFD‐distal, fibers/mm, mean (SD) | 10.4 (2.8) | 7.4 (5.7) | 5.9 (5.1) | 6.31–9.51, F (2, 31) = 3,62, p < 0.048 | 0.032 | 0.737 | |
| SGNFD, n (%) | Abnormal | 2 (25.0) | 5 (62.5) | p = 0.004 | 0.002 | 0.315 | |
| Normal | 14 (100.0) | 6 (75.0) | 3 (37.5) | ||||
| SGNFD‐proximal, fibers/mm, mean (SD) | 54.7 (10.2) | 64.3 (13.9) | 35.6 (23.1) | 44.61–58.44, F (2, 19) = 5.09, p < 0.017 | 0.072 | 0.013 | |
| SGNFD‐distal, fibers/mm, mean (SD) | 50.5 (10.0) | 49.7 (36.0) | 45.3 (22.2) | 39.73–57.3, F (2, 25) = 0.156, p = 0.856 | 0.849 | 0.922 | |
| ESC (sudomotor), n (%) | Abnormal | 7 (70.0) | 5 (55.6) | NA | NA | 0.650 | |
| Normal | 3 (30.0) | 4 (44.4) | |||||
| ESC‐feet, ug/kg, mean (SD) | 1.2 (0.3) | 1.2 (0.3) | 1.09–1.36, F (2, 19) = 0.264, p = 0.770 | 0.879 | |||
| Baseline mean CBFv, cm/s, mean (SD) | 66.8 (5.1) | 65.5 (6.4) | 60.1 (9.6) | 61.70–66.63, F (2, 31) = 2.755, p = 0.079 | 0.070 | 0.225 | |
| Mean CBFv @ 10 min of the tilt test, cm/sec, mean (SD) | 64.9 (8.2) | 52.1 (9.6) | 47.2 (4.8) | 51.90–57.58, F (2, 31) = 16.28, p < 0.001 | <0.001 | 0.382 | |
| Mean CBFv drop @ 10 min of the tilt test, %, mean (SD) | −3.0 (7.5) | −20.3 (15.1) | −20.0 (13.4) | −18.64 to −10.20, F (2, 31) = 8.88, p < 0.001 | 0.005 | 0.997 | |
| Mean CBFv @ 10 min of the tilt test corrected for pET‐CO2, cm/s, mean (SD) | 67.9 (6.9) | 66.6 (13.5) | 50.0 (5.6) | 58.23–64.73, F (2, 31) = 12.28, p < 0.001 | <0.001 | 0.001 | |
| Baseline pET‐CO2, mmHg, mean (SD) | 36.9 (1.4) | 37.1 (3.2) | 32.2 (3.8) | 34.41–36.38, F (2, 31) = 9.77, p < 0.001 | 0.001 | 0.001 | |
| pET‐CO2‚ @ 10 min of the tilt test, mmHg, mean (SD) | 35.2 (2.1) | 27.7 (3.1) | 30.1 (5.7) | 29.70–32.30, F (2, 31) = 13.94, p < 0.001 | 0.001 | 0.329 | |
| AOI, %, mean (SD) | 100 (0.0) | 23.0 (26.7) | 86.7 (21.5) | 63.4–76.3, F (2, 31) = 57.51, p < 0.001 | 0.101 | <0.001 | |
| CVRi supine, mmHg/cm/sec, mean (SD) | 1.3 (0.2) | 1.3 (0.2) | 1.4 (0.3) | 1.27–1.43, F (2, 31) = 1.164, p = 326 | 0.302 | 0.540 | |
| CVRi @ 10th min of tilt test, mmHg/cm/sec, mean (SD) | 1.1 (0.2) | 1.4 (0.6) | 1.4 (0.2) | 1.16–1.56, F (2, 31) = 2.935, p = 0.068 | 0.001 | 0.930 | |
AOI = arteriolar orthostatic index; CBFv = cerebral blood flow velocity; CI = confidence interval; CVRi = cerebrovascular resistance index; ENFD = epidermal nerve fiber density; ESC = electrochemical skin conductance; F = bioavailability; NA = not applicable; NTSS = Neuropathy Total Symptom Score; PASC = post‐acute sequelae of coronavirus disease 2019; POTS = postural tachycardia syndrome; SGNFD = sweat gland nerve fiber density.
TABLE 4.
QASAT Grading Results
| Diagnosis | 95% CI, F, and p | p | |||||
|---|---|---|---|---|---|---|---|
| Controls | POTS | PASC | PASC‐POTS‐controls | PASC‐controls | PASC‐POTS | ||
| N | 15 | 10 | 9 | ||||
| QASAT‐Sympathetic sudomotor score (ESC), range 0–6, mean (SD) | 0.0 (0.0) | 1.4 (1.6) | 1.3 (1.4) | 0.05–1.77, F (2, 18) = 0.799, p = 0.465 | NA | 0.994 | |
| QASAT‐Parasympathetic cardiovagal score (deep breathing), range 0–3, mean (SD) | 0.0 (0.0) | 0.2 (0.4) | 0.4 (0.5) | 0.09–0.34, F (2, 31) = 4.53, p = 0.018 | 0.014 | 0.297 | |
| QASAT‐Increased heart rate response to tilt test, range 0–10, mean (SD) | 0.0 (0.0) | 8.5 (1.4) | 1.6 (2.4) | 2.84–3.86, F (2, 31) = 112.7, p < 0.001 | 0.088 | <0.001 | |
| QASAT‐Sympathetic adrenergic score (Valsalva maneuver), range 0–3, mean (SD) | 0.0 (0.0) | 1.6 (1.2) | 1.7 (0.7) | 0.82–1.35, F (2, 31) = 21.11, p < 0.001 | <0.001 | 0.978 | |
| QASAT‐Sympathetic adrenergic score (tilt test), range 0–10, mean (SD) | 0.0 (0.0) | 0.4 (1.3) | 0.9 (2.7) | −0.11 to ‐0.97, F (2, 31) = 0.973, p = 0.389 | 0.358 | 0.764 | |
| QASAT‐Cerebral blood flow response to tilt test score, range 0–10, mean (SD) | 0.0 (0.0) | 7.6 (1.8) | 6.7 (2.4) | 4.19–5.32, F (2, 31) = 16.28, p < 0.001 | <0.001 | 0.382 | |
| QASAT‐Cerebral blood flow, total score, range 0–11, mean (SD) | 0.0 (0.0) | 8.1 (1.7) | 7.4 (2.1) | 4.67–5.69, F (2, 31) = 125.4, p < 0.001 | <0.001 | 0.582 | |
| QASAT‐pET‐CO2‚ score, range 0–10, mean (SD) | 0.0 (0.0) | 6.6 (3.2) | 0.9 (2.0) | 1.78–3.21, F (2, 31) = 35.41, p < 0.001 | 0.547 | <0.001 | |
| QASAT‐Autonomic failure, n (%) | No | 15 (100.0) | <0.001 | <0.001 | 0.999 | ||
| Yes | 10 (100.0) | 9 (100) | |||||
| QASAT‐Autonomic failure score, range 0–24, mean (SD) | 0.0 (0.0) | 12.6 (3.9) | 7.1 (4.6) | 5.44–7.70, F (2, 31) = 49.48, p < 0.001 | <0.001 | 0.002 | |
| QASAT‐ENFD, range 0–8, mean (SD) | 0.0 (0.0) | 2.6 (3.2) | 2.4 (2.7) | 0.88–2.45, F (2, 31) = 5.628, p = 0.008 | 0.028 | 0.993 | |
| QASAT‐SGNFD, range 0–8, mean (SD) | 0.0 (0.0) | 1.0 (1.9) | 2.2 (2.8) | 0.43–1.74, F (2, 27) = 4.52, p = 0.020 | 0.015 | 0.318 | |
| QASAT‐Total score, range 0–54, mean (SD) | 0.0 (0.0) | 30.5 (4.6) | 20.7 (9.7) | 15.07–19.04, F (2, 31) = 98.96, p < 0.001 | <0.001 | 0.015 | |
CI = confidence interval; ENFD = epidermal nerve fiber density; ESC = electrochemical skin conductance; F = bioavailability; PASC = post‐acute sequelae of coronavirus disease 2019; POTS = postural tachycardia syndrome; QASAT = Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and small fibers from skin biopsies; SGNFD = sweat gland nerve fiber density.
PASC had mild but widespread dysautonomia. Total QASAT score and autonomic failure score was lower in PASC compared to POTS (QASAT total score p = 0.015, QASAT autonomic failure score p = 0.002).
Parasympathetic dysfunction was observed in 4 patients (44%) with PASC and 2 POTS patients (20%). The QASAT parasympathetic score was similar to POTS.
Adrenergic dysfunction associated with abnormal blood pressure responses to the Valsalva maneuver was observed in 9 patients (100%) with PASC and 7 patients (70%) with POTS. The QASAT sympathetic score was similar between PASC and POTS. Sudomotor dysfunction was detected in 5 patients (56%) with PASC and 7 patients (70%) with POTS, the QASAT sudomotor score was similar. Each patient, in both groups, had at least one abnormal autonomic function test. Orthostatic hypotension, a marker of more advanced adrenergic failure, was not detected in any patient. Supine and orthostatic norepinephrine levels were similar in PASC and POTS.
Tilt Test
Baseline supine CBFv, heart rate, and blood pressure were similar among all three groups (Fig 2A–F, Tables 3 and 4). During the tilt test, the CBFv declined in both PASC and POTS groups compared with controls (p < 0.001), the decline was similar in PASC compared with POTS (p = 0.997). Orthostatic CBFv corrected for end‐tidal CO2 remained lower in PASC (p < 0.001) but was normalized in POTS (p = 0.001, comparison PASC vs POTS). In the PASC group, all patients had abnormally reduced orthostatic CBFv. Reduced orthostatic CBFv in PASC included both subjects without (n = 6) and with (n = 3) orthostatic tachycardia. The orthostatic CBFv decline in 6 patients with PASC without orthostatic tachycardia was consistent with orthostatic cerebral hypoperfusion syndrome (OCHOS) 6 and in the remaining 3 patients with PASC with orthostatic tachycardia was consistent with POTS.
Supine CVRi was similar across all groups. Orthostatic CVRi was elevated in PASC. AOI was higher in patients with PASC compared with patients with POTS. Overall, the PASC cohort had orthostatic heart rate responses similar to controls (p = 0.088). Orthostatic heart rate increase was more pronounced in POTS compared to PASC (p < 0.001). Orthostatic blood pressure was normal and QASAT adrenergic orthostatic tilt test score was equal in all studied groups (p = 0.973).
End Tidal CO2
Baseline supine end tidal CO2 was reduced in the PASC group as compared to the POTS group (p = 0.001) and controls (p = 0.001). During the tilt test, the end tidal CO2 was stable in the controls, declined in the patients with POTS and remained low but stable in the patients with PASC. The end tidal CO2 was similar at the end of the 10 minutes of the tilt test in the PASC and POTS groups (p = 0.329). The oxygen saturation was normal (>96%) in all patients throughout testing.
Skin Biopsies
Skin biopsies were normal in healthy controls. Seven patients (78%) with PASC were diagnosed with SFN on skin biopsy. One patient with normal skin biopsy had abnormal sudomotor testing consistent with SFN, thus, a total of 8 patients with PASC (89%) had SFN. ENFD was abnormal in 5 patients (56%); and SGNFD was abnormal in 5 patients (63%). One patient with normal ENFD had abnormal SGNFD. In comparison, 5 of the patients with (50%) POTS had biopsy‐confirmed SFN. ENFD was abnormal in 5 patients (50%), SGNFD and ENFD were abnormal in 2 patients (20%).
One patient with POTS declined the skin biopsy but had abnormal sudomotor testing consistent with SFN. Frequency of SFN diagnosed by skin biopsy was similar between PASC and POTS (p = 0.999 [ENFD] and p = 0.315 [SGNFD]).
Inflammatory Markers
Inflammatory markers were common but heterogeneous (Tables 3 and 5). In the patients with PASC, 6 (67%) individuals showed elevation in at least one inflammatory marker, compared to 7 (70%) of patients with POTS. The TS‐HDS antibody, found in 4 patients (44%) with PASC and one (10%) in a patient with POTS, was the most commonly elevated marker.
TABLE 5.
Inflammatory Markers for Each Subject. Only Abnormal Markers are Shown
| # | Diagnosis | Inflammatory markers |
|---|---|---|
| 1 | PASC | TS‐HDS: 11000 |
| 2 | PASC | TS‐HDS: 22000, FGFR3: 10000 |
| 3 | PASC | |
| 4 | PASC | CRP: 12.74 mg/L |
| 5 | PASC | TS‐HDS: 29000, TNF‐alpha: 3.2 pg/ml |
| 6 | PASC | TS‐HDS: 14000, IL‐10, TNF‐alpha: 2.9 pg/ml, growth hormone: 8.71 ng/ml |
| 7 | PASC | |
| 8 | PASC | |
| 9 | PASC | FGFR3: 5200 |
| 1 | POTS | |
| 2 | POTS | CRP: 6.1 mg/L |
| 3 | POTS | |
| 4 | POTS | TS‐HDS: 22000 |
| 5 | POTS | Adiponectin 19.4 ug/ml, TNF‐alpha: 3.9 pg/ml |
| 6 | POTS | Ganglionic acetylcholine receptor antibody: 0.09 nmol/L |
| 7 | POTS | CRP: 9.2 mg/L |
| 8 | POTS | |
| 9 | POTS | FGFR3: 18000 |
| 10 | POTS | Acetylcholine receptor binding antibody: 0.41 nmol/L |
FGFR = fibroblast growth factor receptor; PASC = post‐acute sequelae of coronavirus disease 2019; POTS = postural tachycardia syndrome; TS‐HDS = trisulfated heparin disaccharide.
Discussion
This study showed widespread multisystem dysregulation affecting cerebrovascular, peripheral neural/autonomic, respiratory systems, and evidence of low‐grade inflammation in PASC.
Cerebral Blood Flow Dysregulation
Orthostatic CBFv was abnormally reduced in PASC. The degree of CBFv reduction (~20%) is clinically significant and similar to POTS, where it has been linked to cerebral hypoperfusion. 8 Although the reduced CBFv was observed in both patients with PASC and patients with POTS, there is etiological difference.
The CBFv decline in PASC is largely independent of hypocapnia, whereas in POTS it is mainly driven by hypocapnia. This CBFv decline occurs with normal blood pressure (eg, without orthostatic hypotension), in both conditions. Cerebral blood flow primarily depends on blood pressure and CO2. Hypocapnia decreases diameter of cerebral arterioles 21 with corresponding changes in vascular resistance and blood flow. In healthy subjects, the end tidal CO2 changes only slightly during orthostasis. In upright posture, gravity alters blood flow causing increased pressure in the feet, and decreased pressure in the brain (~16 mmHg; see Fig 2F), forming hydrostatic gradients. 23 A normal response to the tilt test is cerebral arteriolar vasodilatation, which maintains relatively constant cerebral blood flow.
In PASC, orthostatic CBFv decline is independent of CO2 because the orthostatic end tidal CO2 did not decline. Therefore, reduction of orthostatic CBFv is likely due to failure to actively dilate cerebral arterioles to counteract reduced orthostatic blood pressure in the brain vasculature. The CBFv passively followed pressure drop in the MCA due to a downstream vascular resistance as cerebral arterioles remained relatively constant. This pattern is consistent with OCHOS. 6 In contrast to PASC, orthostatic CBFv decline in POTS is induced mainly by hypocapnic hyperventilation, 8 , 9 the mechanisms also observed in this study.
The cause of vascular dysregulation in PASC is unclear. Cerebral vasodilatory responses are primary determined by myogenic and metabolic factors and to a lesser extent by autonomic innervation. 30 Therefore, numerous factors affect cerebrovascular regulation. Low‐grade vascular inflammation may play a role given the association of low‐grade inflammation with greater arterial stiffness mediated by multiple elements in the vascular wall. 31 Reduced cerebral blood flow, increased cerebrovascular resistance, inflammation, and damage of the vascular bed may interfere with brain tissue oxygenation and has been linked to cognitive deficit. 32
Clinically, PASC resembles myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in many respects, including women's preponderance, symptoms overlap, and postviral sequale. 33 Patients with ME/CFS have vascular dysregulation associated with cerebral hypoperfusion and orthostatic intolerance, findings which are similar to our PASC cohort. Hence, both PASC and ME/CFS can follow a common postviral trajectory associated with endothelial dysfunction. Endotheliitis caused by infection or inflammation of endothelial cells around the brainstem has been already considered as a driver for PASC. 34
Small Fiber Neuropathy, Dysautonomia, and Pain
Direct evidence of SFN on skin biopsy was found in 78% of PASC cases. Our findings are consistent with reported loss of small fibers in patients with PASC using corneal confocal microscopy. 35 Sudomotor dysfunction was reported in 26% of patients who recovered from COVID‐19, 36 which is less than in our study (56%). The difference may be due to differences in the patients' selection and normative values. SFN can be an underlying cause of dysautonomia, which could explain the multi‐organ symptoms. Our study detected frequent dysautonomia in PASC affecting several autonomic systems (eg, parasympathetic, sympathetic adrenergic and sudomotor), in most cases, confirming a recent report. 37 Dysautonomia was mild in both patients with PASC and patients with POTS, as evidenced by lack of orthostatic hypotension.
A variety of pain symptoms were reported in PASC. 2 , 3 All our patients with PASC suffered from chronic pain, however, in most cases, the pain was moderate. Here, we observed damage of small sensory fibers on skin biopsies in the majority of patients with PASC, a loss which can be an etiological source of pain. 20
The origin of SFN and related dysautonomia in PASC remains to be clarified. In general, COVID‐19 may cause SFN and related dysautonomia and sensory complaints de novo, or can exacerbate or unmask the underlying condition. 37
Although the testing in our patients with PASC corresponds to one timepoint, the abnormal findings could be the result of COVID‐19 as history of symptoms and signs indicative of SFN or dysautonomia were absent and no patient complained of brain fog and fatigue before the onset of the COVID‐19.
Hypocapnic Hyperventilation
Patients with PASC reported chronic dyspnea and had lower supine baseline end tidal CO2 compared with both patients with POTS and controls suggesting the presence of hyperventilation‐induced hypocapnia. Orthostatic hypocapnia typically accompanying POTS, 8 was also noted in our PASC cohort. Chronic hyperventilation, however, can be detrimental to brain functioning because it increases pH and alters excitability of the cell membranes. 38 , 39
Persistent respiratory symptoms have been reported in patients with PASC and exercise hyperventilation has been described as a major limiting factor in COVID‐19 survivors. 4 The origin of hyperventilation is unknown. The respiratory symptoms and hyperventilation in PASC can be due to pathophysiology of organ damage during the initial phase of the disease or it is part of PASC, or both. In our cohort, no patient had significant metabolic or acid–base disarrangement, oxygen desaturation, or cardiorespiratory disease that may explain hypocapnia. We did not perform comprehensive pulmonary testing, however, it was shown that, in patients with PASC, respiratory complaints cannot be accounted for by impairment of pulmonary function alone. 4 We speculate that the abnormal ventilation control in PASC is compensatory and may stem from cerebral hypoperfusion by either stimulating automatic ventilator control or suppressing the inhibitory systems.
Inflammatory Markers
Elevated inflammatory markers were identified in most of PASC cases in this study, suggesting that chronic inflammation due to autoimmunity may play a role in our findings. The most common was the TS‐HDS antibody, present in 4 patients (44%) with PASC but only in one patient (10%) with POTS. TS‐HDS is an abundant disaccharide component of heparin sulfate found at peripheral nerve sites. 26 Anti‐TS‐HDS is associated with thickened basal lamina and deposition of the terminal C5b‐C9‐lytic complex on endoneural capillaries. Anti‐TS‐HDS were found previously in a subset of patients with SFN and dysautonomia. 26 , 40 Capillary pathology associated with TS‐HDS points to immune mediated vasculitis as an underlying substrate of SFN and dysautonomia in patients with anti‐TS‐HDS. Nevertheless, TS‐HDS antibodies were detected only in 44% of patients with PASC, and therefore its significance has to be confirmed in a larger cohort.
We observed multiple autoantibodies in PASC. We also found heterogeneous, although different antibodies in the POTS group which is consistent with the literature. 41 The reason for the mixed pattern of detectable autoantibodies for PASC and POTS syndrome is unclear. It is possible that the presence of autoantibodies represents overall immune system dysfunction, susceptibility to autoimmunity, or it is a proxy for chronic low‐grade inflammation. However, it is inherently difficult to detect chronic low grade inflammation as no reliable chronic inflammatory markers exist. 25 However, the preponderance of women in PASC, also observed in other studies, 42 is consistent with underlying autoimmune mechanisms, perhaps due to the immunomodulatory and vasodilatory effect of estrogen. 43 False positive results need to be considered particularly for antibodies with low titers. 44 Recent autopsy studies of hospitalized patients with fatal COVID‐19 showed evidence of immune‐mediated inflammation of muscles 45 , 46 and peripheral nerve tissue 46 with abnormal expression of a type 1 interferon‐inducible protein compared to hospitalized cohorts. Whereas an activated innate immune system response can be an underlying mechanism of SFN, none of our patients with PASC had evidence of myopathy on clinical examination; or had a muscle or nerve biopsy to determine the levels of inflammatory infiltrate. Creatine kinase was also normal in all 7 patients with PASC with available laboratory testing. The degree to which dysautonomia is observed in patients with PASC is a result of impaired inflammatory response and remains to be fully defined.
Furthermore, 78% of patients with PASC had a history of anxiety/depression before COVID‐19, therefore neuropsychiatric vulnerability for PASC may exits.
Limitations
The number of patients in each group was relatively small, although the groups were well matched. Thus, generalization of results to other patients with PASC will need to be confirmed. PASC remains a poorly defined clinical syndrome, and more studies are needed to understand its pathophysiology. This was a retrospective study and subject to referral and selection biases. We evaluated the CBFv as a proxy for cerebral blood flow, as well as for cerebral perfusion. This approach is based on the assumption that the diameter of middle cerebral artery is constant during insonation. Orthostasis does not change the middle cerebral artery diameter 47 implying that CBFv is a good substitute for cerebral blood flow measurements. We used nonspecific immune markers. Future studies may utilize more sensitive and specific inflammatory markers induced by SARS‐CoV‐2 virus. We did not perform cardiovascular magnetic resonance (CMR) studies to assess myocarditis that has been reported in PASC and may cause tachycardia and chest pain. 48 CMR should be performed when cardiovascular autonomic testing does not lead to diagnosis or an electrocardiogram (ECG) shows typical changes, 49 which was not seen in our cases. We also did not perform full pulmonary assessment in our PASC cohort.
Conclusions
This study detected multisystem abnormalities in cerebrovascular, respiratory, neuronal, and autoimmune systems in PASC. We suspect that low grade inflammation of small vessels plays a role. Larger prospective studies are needed to confirm our findings.
Author Contributions
P.N., D.S., and D.F. contributed to the conception and design of the study. P.N., S.S.M., H.A.S., D.S., S.P.M., D.F., W.J.M., and D.M.P. contributed to the acquisition and analysis of data. P.N., S.S.M., H.A.S., D.S., S.P.M., D.F., W.J.M., and D.M.P. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
The authors declared no conflict of interest.
Supporting information
Table S1.Details of Patients With PASC and POTS
Acknowledgment
We thank Karen Downing for help with data collection.
Summary
Post‐acute sequelae of coronavirus disease 2019 (COVID‐19; PASC) is a complication of COVID‐19 disease associated with chronic fatigue, brain fog and orthostatic intolerance. Pathophysiology of PASC is poorly understood.
This study evaluated patients with PASC following mild COVID‐19 infection using standardized autonomic assessments, including Valsalva maneuver, deep breathing, sudomotor and tilt tests with cerebral blood flow velocity (CBFv) measurements, and skin biopsies for small fiber neuropathy (SFN).
This study showed that PASC following mild COVID‐19 is associated with multisystem involvement, including cerebrovascular and respiratory dysregulation, small fiber neuropathy, and related dysautonomia and chronic inflammation.
Autonomic testing expanded for cerebrovascular measurements and skin biopsies can explain some of the PASC symptoms and could guide treatment.
References
- 1. Dixit NM, Churchill A, Nsair A, Hsu JJ. Post‐acute COVID‐19 syndrome and the cardiovascular system: what is known? Am Heart J Plus 2021;5:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maltezou HC, Pavli A, Tsakris A. Post‐COVID syndrome: an insight on its pathogenesis. Vaccine 2021;9:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation: a possible explanation for long‐lasting exercise intolerance in mild COVID‐19 survivors? Front Physiol 2020;11:614590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novak P. Post COVID‐19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci 2020;21:100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Novak P. Orthostatic cerebral Hypoperfusion syndrome. Front Aging Neurosci 2016;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ross AJ, Medow MS, Rowe PC, Stewart JM. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 2013;23:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novak V, Spies JM, Novak P, et al. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 1998;29:1876–1881. [DOI] [PubMed] [Google Scholar]
- 9. Stewart JM, Pianosi P, Shaban MA, et al. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc 2018;7:e008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 11. Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology 2011;76:2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novak P. Autonomic testing. New York: Oxford University Press, 2019. [Google Scholar]
- 13. Porubcin MG, Novak P. Diagnostic accuracy of electrochemical skin conductance in the detection of Sudomotor fiber loss. Front Neurol 2020;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Overview | COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19 | Guidance | NICE [Internet]. [date unknown]. Available at: https://www.nice.org.uk/guidance/ng188. Accessed August 22, 2021
- 15. Li H‐F, Hao H‐J, Chen X‐J. Provisional case definitions for COVID‐19‐associated neurological disease. Lancet Neurol 2020;19:890–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 2006;291:H904–H913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bastyr EJ, Price KL, Bril V, MBBQ Study Group . Development and validity testing of the neuropathy total symptom score‐6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther 2005;27:1278–1294. [DOI] [PubMed] [Google Scholar]
- 18. Zilliox L, Peltier AC, Wren PA, et al. Assessing autonomic dysfunction in early diabetic neuropathy: the survey of autonomic symptoms. Neurology 2011;76:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc 1994;26:1078–1081. [PubMed] [Google Scholar]
- 20. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence of different CO2 tensions. Neuroradiology 1976;10:257–262. [DOI] [PubMed] [Google Scholar]
- 22. Immink RV, Pott FC, Secher NH, van Lieshout JJ. Hyperventilation, cerebral perfusion, and syncope. J Appl Physiol (1985) 2014;116:844–851. [DOI] [PubMed] [Google Scholar]
- 23. Petersen LG, Petersen JCG, Andresen M, et al. Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol‐Regul Integr Comp Physiol 2016;310:R100–R104. [DOI] [PubMed] [Google Scholar]
- 24. Gibbons CH, Illigens BMW, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical‐pathologic correlation. Neurology 2009;72:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine TD, Kafaie J, Zeidman LA, et al. Cryptogenic small‐fiber neuropathies: serum autoantibody binding to trisulfated heparan disaccharide and fibroblast growth factor receptor‐3. Muscle Nerve 2020;61:512–515. [DOI] [PubMed] [Google Scholar]
- 27. Shouman K, Sandroni P. Antibody testing for suspected autoimmune autonomic dysfunction and small fiber neuropathies. J Clin Neurophysiol 2021;38:274–278. [DOI] [PubMed] [Google Scholar]
- 28. Medic Spahic J, Ricci F, Aung N, et al. Proteomic analysis reveals sex‐specific biomarker signature in postural orthostatic tachycardia syndrome. BMC Cardiovasc Disord 2020;20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollard TJ, Johnson AEW, Raffa JD, Mark RG. Tableone: an open source python package for producing summary statistics for research papers. JAMIA Open 2018;1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiong L, Liu X, Shang T, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry 2017;88:520–531. [DOI] [PubMed] [Google Scholar]
- 31. van Bussel BC, Schouten F, Henry RM, et al. Endothelial dysfunction and low‐grade inflammation are associated with greater arterial stiffness over a 6‐year period. Hypertension 2011;58:588–595. [DOI] [PubMed] [Google Scholar]
- 32. Yew B, Nation DA. Alzheimer's disease neuroimaging initiative. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain J Neurol 2017;140:1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Visvabharathy L, Hanson B, Orban Z, et al. Neuro‐COVID long‐haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv 2021; 2021.08.08.21261763. [Google Scholar]
- 34. Bisaccia G, Ricci F, Recce V, et al. Post‐acute sequelae of COVID‐19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis 2021;8:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bitirgen G, Korkmaz C, Zamani A, et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol 2021;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hinduja A, Moutairou A, Calvet J‐H. Sudomotor dysfunction in patients recovered from COVID‐19. Neurophysiol Clin 2021;51:193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID‐19 infection: an early experience. Clin Auton Res 2021;31:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peebles KC, Ball OG, MacRae BA, et al. Sympathetic regulation of the human cerebrovascular response to carbon dioxide. J Appl Physiol (1985) 2012;113:700–706. [DOI] [PubMed] [Google Scholar]
- 39. Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg 1991;75:731–739. [DOI] [PubMed] [Google Scholar]
- 40. Trevino JA, Novak P. TS‐HDS and FGFR3 antibodies in small fiber neuropathy and Dysautonomia. Muscle Nerve 2021;64:70–76. [DOI] [PubMed] [Google Scholar]
- 41. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019;285:352–366. [DOI] [PubMed] [Google Scholar]
- 42. Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid‐19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur 2021;8:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 44. Ebright MJ, Li S‐H, Reynolds E, et al. Unintended consequences of Mayo paraneoplastic evaluations. Neurology 2018;91:e2057–e2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aschman T, Schneider J, Greuel S, et al. Association between SARS‐CoV‐2 infection and immune‐mediated myopathy in patients who have died. JAMA Neurol 2021;78:948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Suh J, Mukerji SS, Collens SI, et al. Skeletal muscle and peripheral nerve histopathology in COVID‐19. Neurology 2021;97:e849–e858. [DOI] [PubMed] [Google Scholar]
- 47. Serrador JM, Picot PA, Rutt BK, et al. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000;31:1672–1678. [DOI] [PubMed] [Google Scholar]
- 48. Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin‐positive COVID‐19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021;42:1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ståhlberg M, Reistam U, Fedorowski A, et al. Post‐Covid‐19 tachycardia syndrome: A distinct phenotype of post‐acute Covid‐19 syndrome. Am J Med 2021;134:1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Details of Patients With PASC and POTS


