Abstract
The mutant prevention concentration (MPC) of a C-8-methoxy fluoroquinolone exhibited a narrow distribution for 14 genetically diverse clinical isolates of Mycobacterium tuberculosis, indicating that results from single-isolate studies are likely to be representative. When one isolate was challenged with a variety of antituberculosis agents, C-8-methoxy fluoroquinolones were exceptional in having MPCs below the maximum concentration attained in serum by use of commonly recommended doses.
Antibiotic resistance is becoming an increasingly serious problem for many bacterial diseases (12). To help halt further selection of resistant mutants, we have defined a drug concentration threshold above which bacterial cells require the presence of two or more resistance mutations for growth (20). The simultaneous occurrence of multiple mutations is a rare event relative to the number of cells present during infection; consequently, administration of antibiotic above the concentration threshold, which we call the mutant prevention concentration (MPC), should severely restrict selection of resistant mutants. Experimentally, MPC has been taken as the drug concentration that allows no mutant to be recovered from a susceptible population of more than 1010 cells (7). For MPC to be therapeutically useful, it must be below the concentration achievable in serum or tissue with safe doses of antibiotic. Whether such situations exist has not been determined.
In earlier work we found that addition of a methoxy group to the C-8 position of N-1-cyclopropyl fluoroquinolones makes the compounds particularly effective against quinolone-resistant mutants of Escherichia coli (13, 22), Staphylococcus aureus (7, 21), Mycobacterium bovis BCG (6, 7), and Mycobacterium tuberculosis (19). For M. bovis BCG the MPC of a C-8-methoxy fluoroquinolone was only 12% of that observed of its C-8-hydrogen derivative or a clinical standard, ciprofloxacin (7). Thus, C-8-methoxy fluoroquinolone attack of a pathogenic mycobacterium could provide a good experimental system for determination of whether MPC can be lower than the serum drug concentration.
In the present study we measured the susceptibilities of 14 M. tuberculosis isolates to PD161148, the fluoroquinolone that had previously been shown to have the most activity against mycobacteria, to determine whether diverse isolates respond to fluoroquinolones in the same general way. Test isolates with different IS6110 DNA types were chosen (18). To minimize potential bias, we studied several clinical isolates from each of the three main genetic groups of M. tuberculosis (16). As an additional geographical test we examined strains collected from both the United States and Russia. We then chose one isolate, an outbreak strain from New Jersey (3), to compare the abilities of several new C-8-methoxy fluoroquinolones (Fig. 1) and conventional antituberculosis agents to restrict the selection of resistant mutants.
FIG. 1.
Fluoroquinolone structures.
Clinical isolates of M. tuberculosis were cultured as described previously (6). The MIC was determined by plating dilutions of cultures on 7H10 agar plates containing various concentrations of antibiotic (6). The concentration that reduced the number of colonies recovered by at least 99% relative to the number of untreated control colonies recovered was taken as the MIC. For mutant selection, cultures were grown to the stationary phase in liquid medium, concentrated by centrifugation (3,000 × g for 10 min), resuspended in fresh 7H9 medium (10), and applied in various amounts to agar plates containing different concentrations of antibacterial agent. More than 1010 CFU was plated for the highest antibiotic concentrations (>2 × 109 CFU to each of five agar plates). Colonies were counted after incubation at 37°C for 4 weeks, and they were retested for growth on drug-containing agar plates to confirm that they were resistant mutants.
Increasing the quinolone concentration caused colony recovery to decline sharply and then level to a plateau (Fig. 2). A concentration was reached for the C-8-methoxy compound (PD161148) at which no mutant was recovered when more than 1010 cells were applied to agar plates (arrowhead on abscissa of Fig. 2). A plot of the plateau region using a linear scale, shown as an inset in Fig. 2, illustrates restriction of mutant selection by the C-8-methoxy compound under conditions in which mutant colonies were readily recovered with its C-8-H derivative, PD160793. Similar curves were obtained for all 14 strains, although in a few cases it was necessary to extrapolate linear plots (Fig. 2, inset) to estimate the MPC. The MPCs of PD161148 ranged from 1 to 4 μg/ml, with the MPC being 1 to 3 μg/ml for 85% of the isolates (Table 1). The narrow range of MPCs indicates that measurement of MPC is likely to be useful for comparison of the potencies of fluoroquinolones against diverse isolates of M. tuberculosis.
FIG. 2.
Effect of fluoroquinolone concentration on selection of resistant mutants. M. tuberculosis isolate TN7804 was applied to agar plates containing the indicated concentrations of PD161148 (C-8-methoxy; open circles) or PD160793 (C-8-H; filled circles). After incubation, the number of drug-resistant colonies was recorded and plotted relative to the number of CFU applied to the drug-free agar plates. The arrowhead indicates the drug concentration at which no colony was recovered from plates containing C-8-methoxy fluoroquinolone when more than 1010 cells were applied. (Inset) Data for points where the fraction of cells recovered was below 10−7, replotted using a linear scale.
TABLE 1.
Effect of the C-8-methoxy fluoroquinolone PD161148 against clinical isolates of M. tuberculosis
| Isolate no. | MIC (μg/ml) | MPC (μg/ml)a | IS6110 typeb | Genotype groupc | Sourced | Antibiotic resistancee |
|---|---|---|---|---|---|---|
| TN1626 | 0.1 | 1.0 | W | 1 | N.Y. | I, S, R, Em, K |
| TN7811 | 0.1 | 1.0 | Unique | 3 | Russia | S, K |
| TN7804 | 0.1 | 1.0 | W148 | 1 | Russia | I, S, R, Et, K |
| TN6515 | 0.07 | 1.5 | W4 | 1 | N.J. | None |
| TN7791 | 0.1 | 1.5 | Unique | 2 | Russia | I |
| TN7829 | 0.1 | 2.3 | Unique | 3 | Russia | None |
| TN7834 | 0.1 | 2.3 | Unique | 3 | Russia | None |
| TN5704 | 0.2 | 2.3 | Unique | 1 | N.J. | I |
| TN5355 | 0.2 | 2.3 | Unique | 3 | N.J. | None |
| TN5410 | 0.2 | 2.3 | Unique | 2 | N.J. | None |
| TN3541 | 0.2 | 2.3 | DV | 1 | N.Y. | I, R, Em |
| TN1865 | 0.3 | 3.0f | H | 2 | N.Y. | None |
| TN2156 | 0.3 | 3.5 | CO | 2 | N.Y. | None |
| TN5702 | 0.4 | 4.0f | Unique | 1 | N.J. | I |
The MPC was determined as the PD161148 concentration required to prevent the recovery of resistant mutants when >2 × 109 CFU was applied to each of five agar plates. Each experiment was performed twice, with results similar to those shown obtained each time.
IS6110 DNA pattern as described previously (11).
Genotype group as described previously (16).
From the Public Health Research Institute Tuberculosis Center collection. Abbreviations: N.Y., New York; N.J., New Jersey.
Isolates were resistant to the indicated antibiotics: I, isoniazid; S, streptomycin; R, rifampin; Em, ethambutol; K, kanamycin; Et, ethionamide.
Determined by extrapolation from linear plots as in inset to Fig. 2.
The average MIC of PD161148 was approximately 0.2 μg/ml (Table 1), which was about half that observed for PD160793 (data not shown). For isolates for which MICs were low, MPCs tended to be low. Neither the MIC nor the MPC displayed a relationship with the genotypic group or the IS6110 DNA polymorphism type.
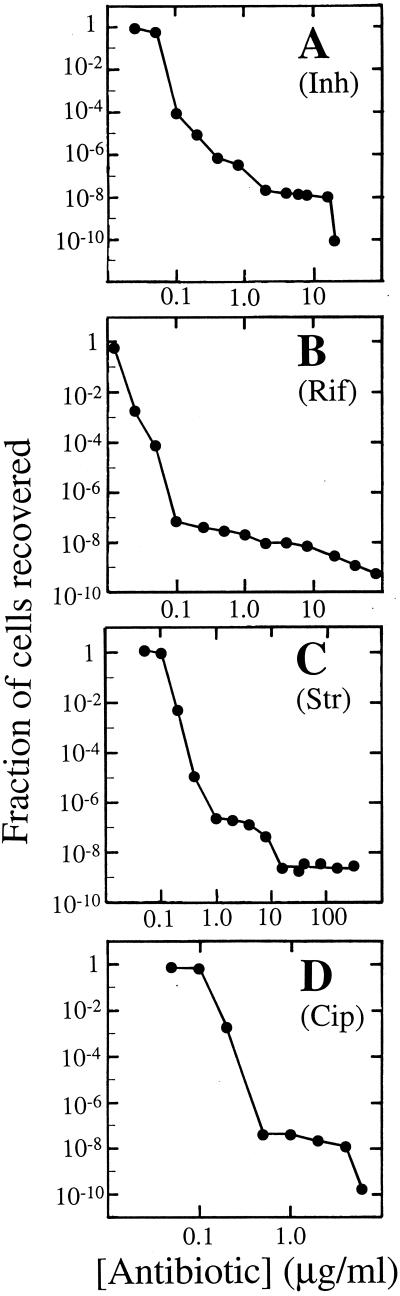
To compare antituberculosis agents for the ability to restrict selection of mutants, we recovered resistant mutants at a variety of concentrations of different drugs using M. tuberculosis strain TN6515, a pan-susceptible isolate of the W4 IS6110 DNA type (3). Mutation frequency was a complex function of drug concentration (Fig. 3). The several inflection points observed with isoniazid (Fig. 3A) and streptomycin (Fig. 3C) suggest that different resistance alleles may dominate at different antibiotic concentrations. This prediction is being tested. For ciprofloxacin (Fig. 3D), we showed previously that many different alleles are selected and that the abundance of any particular resistance allele depends on the drug concentration (22a).
FIG. 3.
Effect of antituberculosis agent concentration on selection of resistant mutants. M. tuberculosis isolate TN6515 was applied to agar plates containing the indicated concentrations of isoniazid (A), rifampin (B), streptomycin (C), and ciprofloxacin (D). After incubation, the number of drug-resistant colonies was recorded and was plotted relative to the number of CFU applied to drug-free agar plates.
The MPCs of standard antituberculosis agents and C-8-methoxy fluoroquinolones were estimated by plating more than 1010 cells on drug-containing agar and determining the concentration that allowed recovery of no colony (Table 2). These data show that MPC determinations are not limited to fluoroquinolones. However, for rifampin, streptomycin, and kanamycin we failed to find concentrations that prevented the recovery of mutants; for these compounds we obtained only minimum estimates of MPCs. Table 2 also lists the maximum concentrations of the commonly recommended doses of each compound achieved in serum. The concentrations of traditional antituberculosis agents in serum failed to exceed the MPCs; consequently, these compounds will select resistant mutants whenever they are administered as monotherapy (8, 20). In contrast, the C-8-methoxy fluoroquinolones moxifloxacin and PD135432 (gatifloxacin) had MPCs below the maximum concentration achievable in serum (Table 2).
TABLE 2.
Potencies of anti-tuberculosis agents against M. tuberculosisa
| Antibioticb | MIC99 (μg/ml)c | MPC (μg/ml)d | Dose (mg)e | Cmaxf | MPC/ Cmax | Reference for Cmax |
|---|---|---|---|---|---|---|
| Rifampin | 0.02 | >80 | 600 | 9.5 | >8 | 2 |
| Streptomycin | 0.2 | >320 | 1000 | 34 | >9 | 1, 4 |
| Isoniazid | 0.06 | 20 | 250 | 7.6 | 2.6 | 2 |
| Capreomycin | 2.0 | 160 | 1,000 | 33 | 4.8 | 4 |
| Kanamycin | 1.5 | >800 | 500 | 21 | >38 | 5 |
| Cycloserine | 14 | 70 | 750 | 35 | 2 | 23 |
| Fluoroquinolones | ||||||
| Ciprofloxacin | 0.15 | 8.0 | 750 | 4.4 | 1.8 | 9 |
| Moxifloxacin | 0.037 | 2.5 | 400 | 4.5 | 0.55 | 17 |
| PD135432g | 0.03 | 1.5 | 300 | 3.7 | 0.41 | 15 |
| Sparfloxacin | 0.075 | 2.5 | 200 | 1.4 | 1.8 | 14 |
| PD161148 | 0.07 | 1.5 | NAh |
Experiments were performed with isolate TN6515.
Unless indicated otherwise the source of all compounds was Sigma Biochemicals. The exceptions were as follows: ciprofloxacin, Miles Laboratories; moxifloxacin, Bayer AG; PD135432, Parke-Davis; sparfloxacin, Parke-Davis; and PD161148, Parke-Davis.
MIC99, MIC at which 99% of isolates are inhibited.
The MPC was determined as described in footnote a of Table 1. The MPC of each compound was determined twice, with results similar to those shown obtained each time.
The dose recommended by the manufacturer. For determination of the maximum concentration of drug in serum, streptomycin, capreomycin, and kanamycin were administered as single, intramuscular doses; all other compounds except ciprofloxacin and PD135432 were administered as oral doses once daily; ciprofloxacin and PD135432 were delivered twice daily. The initial dose of sparfloxacin was 400 mg. During treatment of tuberculosis, kanamycin is administered twice daily.
Cmax, maximum concentration of drug in serum. All are steady-state determinations except for those for streptomycin, capreomycin, and kanamycin, which were single-dose determinations.
Structure identical to that of gatifloxacin.
NA, not available.
The data described above indicate that tuberculosis may be treatable with C-8-methoxy fluoroquinolones at concentrations that will severely restrict the selection of resistant mutants. It may be possible to develop an effective dual-drug therapy by combining one of the C-8-methoxy fluoroquinolones with another antituberculosis drug: when the concentrations of both compounds are kept above their MICs and the concentration of the fluoroquinolone is above its MPC, three mutations would be required for bacterial growth. As discussed elsewhere (20), the key to preventing resistance from arising in the dual-drug situation is to have the pharmacokinetic profiles of the two compounds match such that no time exists when the concentration of only one compound is above its MIC and below its MPC. We are now examining potential partners for C-8-methoxy fluoroquinolones to create a highly effective combination therapy for the treatment of infections caused by strains of M. tuberculosis that are already resistant to isoniazid and rifampin.
Acknowledgments
We thank John Domagala, Marila Gennaro, Sam Kayman, and Tao Lu for critical comments on the manuscript. We also thank John Domagala and Glenn Tillotson for supplying fluoroquinolones.
The work was supported by grant AI35257 from the National Institutes of Health.
ADDENDUM IN PROOF
MIC99 and MPC of PD161148 were 0.06 and 2.3 μg/ml, respectively, for the laboratory strain H37Rv.
Footnotes
Publication 69 of the PHRI TB Center.
REFERENCES
- 1.Acocella G, Conti R, Luisetti M, Pozzi E, Grassi C. Pharmacokinetic studies on antituberculosis regimens in humans. Am Rev Respir Dis. 1985;132:510–515. doi: 10.1164/arrd.1985.132.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Acocella G, Nonis A, Perna G, Patane E, Gialdroni-Grassi G, Grassi C. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide administered in free combination and in a fixed triple formulation designed for daily use in antituberculosis chemotherapy. II. Two-month, daily administration study. Am Rev Respir Dis. 1988;138:886–890. doi: 10.1164/ajrccm/138.4.886. [DOI] [PubMed] [Google Scholar]
- 3.Bifani P, Mathema B, Liu Z, Moghazeh S, Shopsin B, Tempalski B, Driscoll J, Frothingham R, Musser J, Alcabes P, Kreiswirth B. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA. 1999;282:2321–2327. doi: 10.1001/jama.282.24.2321. [DOI] [PubMed] [Google Scholar]
- 4.Black H R, Griffith R S, Peabody A M. Absorption, excretion and metabolism of capreomycin in normal and diseased states. Ann N Y Acad Sci. 1966;135:974–982. doi: 10.1111/j.1749-6632.1966.tb45538.x. [DOI] [PubMed] [Google Scholar]
- 5.Cabana B, Taggart J. Comparative pharmacokinetics of BB-KS and kanamycin in dogs and humans. Antimicrob Agents Chemother. 1973;3:478–483. doi: 10.1128/aac.3.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Xu C, Zhao X, Domagala J, Drlica K. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on bacterial growth, survival, and resistance. Antimicrob Agents Chemother. 1998;42:2978–2984. doi: 10.1128/aac.42.11.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Zhao X, Domagala J, Drlica K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1756–1758. doi: 10.1128/aac.43.7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris H W. Chemotherapy of tuberculosis: the beginning. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown & Co.; 1995. pp. 745–749. [Google Scholar]
- 9.Israel D, Gillum G, Turik M, Harvey K, Ford J, Dalton H, Towle M, Echols R, Heller A, Polk R. Pharmacokinetics and serum bactericidal titers of ciprofloxacin and ofloxacin following multiple oral doses in healthy volunteers. Antimicrob Agents Chemother. 1993;37:2193–2199. doi: 10.1128/aac.37.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs W R, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems in mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 11.Kreiswirth B, Moss A. Genotyping multidrug-resistant M. tuberculosis in New York City. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown & Co.; 1995. pp. 199–209. [Google Scholar]
- 12.Levy S B. Multidrug resistance—a sign of the times. N Engl J Med. 1998;338:1376–1378. doi: 10.1056/NEJM199805073381909. [DOI] [PubMed] [Google Scholar]
- 13.Lu T, Zhao X, Drlica K. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activity by the C-8-methoxy group. Antimicrob Agents Chemother. 1999;43:2969–2974. doi: 10.1128/aac.43.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montay G. Pharmacokinetics of sparfloxacin in healthy volunteers and patients: a review. J Antimicrob Chemother. 1996;37(Suppl. A):27–39. doi: 10.1093/jac/37.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima M, Uematsu T, Kosuge K, Kusajima H, Ooie T, Masuda Y, Ishida R, Uchida H. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother. 1995;39:2635–2640. doi: 10.1128/aac.39.12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreevatsan S, Pan X, Stockbauer K, Connell N, Kreiswirth B, Whittam T, Musser J M. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan J T, Woodruff M, Lettieri J, Agarwal V, Krol G J, Leese P T, Watson S, Heller A H. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother. 1999;43:2793–2797. doi: 10.1128/aac.43.11.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Embden J D, Cave M D, Crawford J T, Dale J W, Eisenach K E, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B-Y, Pine R, Domagala J, Drlica K. Fluoroquinolone action against clinical isolates of Mycobacterium tuberculosis: effects of a C-8 methoxyl group on survival in liquid media and in human macrophages. Antimicrob Agents Chemother. 1999;43:661–666. doi: 10.1128/aac.43.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, X., and K. Drlica. A general strategy for restricting the selection of antibiotic-resistant mutants derived from fluoroquinolone studies. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 21.Zhao X, Wang J-Y, Xu C, Dong Y, Zhou J, Domagala J, Drlica K. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob Agents Chemother. 1998;42:956–958. doi: 10.1128/aac.42.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Xu C, Domagala J, Drlica K. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Zhou, J., Y. Dong, X. Zhao, S. Lee, A. Amin, S. Ramaswamy, J. Domagala, J. Musser, and K. Drlica. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J. Infect. Dis., in press. [DOI] [PubMed]
- 23.Zitkova L, Tousek J. Pharmacokinetics of cycloserine and terizidone. Chemotherapy (Basel) 1974;20:18–28. doi: 10.1159/000221787. [DOI] [PubMed] [Google Scholar]