To the Editor:
The SARS‐CoV‐2 vaccines have been widely distributed based on remarkable efficacy in immunocompetent patients. Unfortunately, there is a growing body of literature indicating decreased efficacy in patients with lymphoma, particularly those receiving B‐cell‐directed therapy. 1 Given high rates of morbidity and mortality, improving vaccine strategies is a critical area of unmet need. Studies have shown improved immunogenicity in solid organ recipients with a third mRNA vaccine. 2 This benefit was not as apparent in patients who lacked detectable antibodies after the first two mRNA doses. While the Centers for Disease Control and Prevention (CDC) currently recommends a third mRNA vaccine for immunocompromised patients, there is considerable interest in alternative regimens. Due to concerns for thrombotic adverse events and global supply limitations, some countries have utilized heterologous vaccination strategies. Researchers in Europe and more recently, the United States, have demonstrated significant increases in antibody levels with viral vector/mRNA vaccine combinations in healthy individuals. 3 Data with heterologous vaccinations in people with defects in humoral and cellular immunity, however, are limited. We have previously reported on successful seroconversion with the Ad26.COV2.S viral vector vaccine (J&J) in a lymphoma patient after inadequate response to two doses of the BNT162b2 mRNA vaccine (Pfizer/BioNTech). 4 Here, we present a larger series of B‐cell lymphoma patients who obtained J&J vaccines after the standard two‐dose mRNA vaccine series. The majority of patients subsequently received another mRNA vaccine for a total of four vaccinations.
As part of an IRB‐approved trial conducted at the University of Washington/Fred Hutchinson Cancer Research Center, seven patients with low‐grade B‐cell lymphomas who had initially received the two‐dose mRNA vaccination series and subsequently obtained a J&J viral vector vaccine were identified. Patients had independently sought out heterologous vaccination based on the lack of sufficient spike antibody response to the initial two‐dose mRNA series, as assessed by the Roche Elecsys Anti‐SARS‐CoV‐2 S, a semiquantitative total antibody assay against the spike protein receptor binding domain.
The median age was 62 years (range 41–79). Four were men and three were women. Lymphoma subtypes included Waldenstrom's macroglobulinemia (WM, n = 3), follicular lymphoma (FL, n = 3), and chronic lymphocytic leukemia (CLL, n = 1). (Table S1). Median spike antibody level after completion of the initial two‐dose mRNA vaccination was < 0.4 AU/mL (range < 0.4–0.6 AU/mL) as measured by the Roche Elecsys Anti‐SARS‐CoV‐2 S assay; reference interval for a negative result was < 0.8 AU/mL. The median time between the second dose of the mRNA vaccine and the J&J vaccine was 97 days (range 70–173).
Patients underwent one blood sample collection after receiving the J&J vaccine. Median time from J&J to collection was 38 days (range 21–94). The median IgG, absolute lymphocyte count, and normal CD19+ B‐cell counts were 597 mg/dL (range 180–1007), 0.97 × 103/mL (range 0.61–2.56), and 0.6 cells/mL (range 0–121), respectively. Nucleocapsid antibody was nonreactive in all patients, indicating no evidence of prior SARS‐CoV‐2 infection. Utilizing the same Roche assay for semiquantitative anti‐spike binding antibody assessment, three patients remained undetectable (< 0.4 AU/mL), two had a modest yet positive response (2.6 and 5.3 AU/mL), and two experienced a greater seroconversion (118 and 207 AU/mL). (Figure 1). Positive responses were seen in a WM patient receiving zanubrutinib, a CLL patient receiving ibrutinib, a FL patient 8 months after rituximab monotherapy, and a FL patient 3 months after ibrutinib + venetoclax. Lack of response to J&J was seen in two WM patients receiving zanubrutinib and an FL patient who completed obinutuzumab and bendamustine 5 months prior. Patients who remained seronegative had a median normal B‐cell count of 0 cells/mL (range 0–0.4) compared to 2.3 cells/mL (range 0.6–121) in patients who became positive.
FIGURE 1.
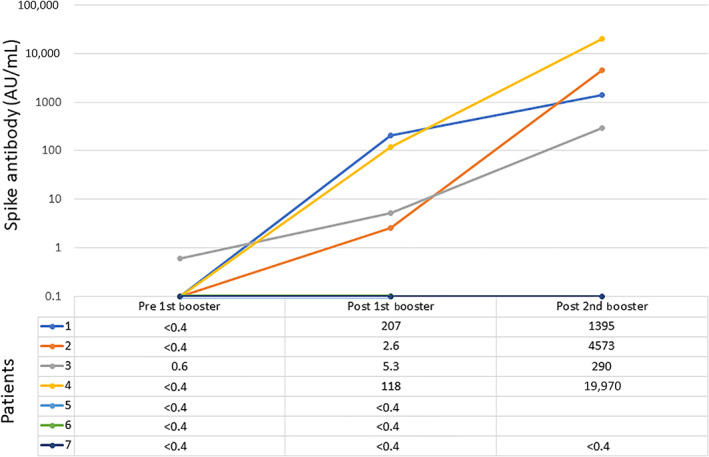
Serologic response with sequential vaccinations
After the CDC's recommendation for a third mRNA vaccine for immunocompromised patients, five of the seven patients obtained a third mRNA vaccine and a fourth overall SARS‐CoV‐2 vaccine. Among this subgroup, four had seroconverted with J&J and one had not. Patients underwent a second sample collection a median of 16 days after the fourth vaccine (range 9–27). The four patients who originally seroconverted with J&J experienced further increase in antibody level (range 290–19 970 AU/mL, Figure 1). The one patient who did not seroconvert with J&J remained undetectable. None of the patients reported significant adverse events.
It has become increasingly evident that the SARS‐CoV‐2 vaccines are ineffective for many patients with lymphoid malignancies, due to their innate immune dysfunction and/or receipt of B‐cell‐directed therapies. Given the increased risk of complications and the prolongation of the pandemic, efforts must be focused on predicting who these individuals are as well as understanding the complexities of their immune response or lack thereof. A number of trials are investigating these questions, including collaborations sponsored by the Leukemia and Lymphoma Society (LLS) and the CLL Global Research Foundation (NCT04852822).
Here we present the first case series evaluating the use of a heterologous mRNA/vector/mRNA vaccination strategy in patients with lymphoma. By serology assessment alone, the use of a viral vector vaccine after the two‐dose mRNA series was successful in inducing a response. Our results mirror a recent report from the LLS in which 9 of 17 seroconverted with J&J after mRNA vaccination; resulting antibody levels ranged from 2.3 to 157 AU/mL using the same Roche assay. 5 A more robust response was seen in three who were seropositive prior to J&J (> 2500 AU/mL). Interestingly, we also found that the addition of a fourth dose allowed for further augmentation of the serologic response.
These findings are promising for vulnerable patients struggling to understand how to exist in their environment. Determining exactly who this strategy will benefit, however, is not possible from this small cohort. A number of variables such as type of B‐cell directed therapy, length of and time from exposure, sequence of and duration between vaccinations, and underlying disease biology may have had an impact. There was a suggestion, though, that an absence of normal B‐cells may be predictive of inability to mount an appropriate serologic response.
Given the paucity of data we look to the transplant literature for insight into other vaccine‐induced immunologic changes, recognizing the differences in mechanisms of immunosuppression, that is, calcineurin inhibitors and antimetabolites. In a German study of solid organ recipients, researchers found that the vector/mRNA vaccine schedule led to statistically significant increases in spike antibody levels, neutralization antibody activity, and SARS‐CoV‐2‐reactive CD4 T‐cells, as well as a trend toward increased numbers of CD8 T‐cells. 6 Unfortunately, the scope of our analysis did not allow for assessment of cellular immune response.
In light of our findings, we are challenged with new questions. What are the immunologic changes that allow for a viral vector/mRNA approach to be effective after failed response to homologous mRNA vaccination? Does the improvement in serologic response correlate with actual clinical benefit? For whom should a heterologous approach be considered before a homologous approach? Is one type of vaccine better utilized for priming and is that dependent on patient‐specific clinical features? And lastly, how can we improve outcomes for a patient population who is often excluded from vaccine trials?
CONFLICT OF INTEREST
CU has received consultancy/honorarium from Abbvie, Astrazeneca, Atara, TG therapeutics, Epizyme, Jansen, Pharmacyclics. She receives research funding from Abbvie, Pharmacyclics, Astrazeneca, Kite/Gilead, Loxo/Lilly, Adaptive Biotechnologies. AG receives contract revenue from Abbott and research funding from Gilead and Merck. MS receives consultancy/honoraria or participates in advisory boards, steering committees or data safety monitoring committees from Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron and Atara Biotherapeutics; research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, GenMab. JH received consultancy/honoraria from Gilead Sciences, Amplyx, Allovir, Allogene therapeutics, CRISPR therapeutics, CSL Behring, OptumHealth, Octapharma, and Takeda; and research funding from Takeda, Allovir, Karius, and Deverra Therapeutics, and Gilead. RL receives research funding from Juno therapeutics, TG Therapeutics, Incyte, Bayer, Cyteir, Genentech, SeaGen, RAPT and received consultancy from Morphosys. EW has no conflicts of interest. AG receives research funding from Merck, I‐Mab bio, IgM Bio, Takeda, Gilead, Astra‐Zeneca, Agios, Janssen, BMS, SeaGen, Teva; and consultancy/Honoraria from Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Nurix Inc, Cellectar, Janssen, SeaGen, Epizyme, I‐Mab bio, Gilead, Genetech, and has equity ownership in Compliment Corporation.
Supporting information
Table S1 Patient disease characteristics and vaccinations.
ACKNOWLEGMENTS
We acknowledge that receipt of extra vaccines is a luxury primarily afforded to those who live in the United States. We recognize the inequity of the situation, as millions of people around the world lack access to any type of SARS‐CoV‐2 vaccines. Eradication of the virus is a global effort in which all must have the opportunity to participate. This project was funded through philanthropic support from Frank and Betty Vandermeer, Tom and Sonya Campion, Doug and Marsha Holbrook, Dick and Diane Hoch and Dean and Gwenn Polik.
Funding information Dean and Gwenn Polik; Dick and Diane Hoch; Doug and Marsha Holbrook; Frank and Betty Vandermeer; Tom and Sonya Campion
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Greenberger LM, Saltzman LA, Senefeld JW, et al. Antibody response to SARS‐CoV‐2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Normark J, Vikström L, Gwon YD, et al. Heterologous ChAdOx1 nCoV‐19 and mRNA‐1273 vaccination. N Engl J Med. 2021;385(11):1049‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill JA, Ujjani CS, Greninger AL, et al. Immunogenicity of a heterologous COVID‐19 vaccine after failed vaccination in a lymphoma patient. Cancer Cell. 2021;39(8):1037‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenberger LM, Saltzman LA, Senefeld JW, et al. Anti‐spike antibody response to SARS‐CoV‐2 booster vaccination in patients with B cell‐derived hematologic malignancies. Cancer Cell. 2021;S1535‐6108(21):00490‐00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector‐based COVID‐19 vaccine regimens in solid organ transplant recipients. Am J Transplant. Published online August 28, 2021. doi: 10.1111/ajt.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Patient disease characteristics and vaccinations.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


