Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the etiological agent of coronavirus disease 2019 (COVID‐19), may manifest as a life‐threatening respiratory infection with systemic complications. Clinical manifestations among children are generally less severe than those seen in adults, but critical cases have increasingly been reported in infants less than 1 year of age. We report a severe case of neonatal COVID‐19 requiring intensive care and mechanical ventilation, further complicated by a multidrug‐resistant Enterobacter asburiae super‐infection. Chest X‐rays, lung ultrasound, and chest computed tomography revealed extensive interstitial pneumonia with multiple consolidations, associated with persistent increased work of breathing and feeding difficulties. SARS‐CoV‐2 RNA was detected in respiratory specimens and stools, but not in other biological samples, with a rapid clearance in stools. Serological tests demonstrated a specific SARS‐CoV‐2 antibody response mounted by the neonate and sustained over time. The therapeutic approach included the use of enoxaparin and steroids which may have contributed to the bacterial complication, underlying the challenges in managing neonatal COVID‐19, where the balance between viral replication and immunomodulation maybe even more challenging than in older ages.
Keywords: COVID‐19, interstitial pneumonia, neonatal sepsis, neonates, severe acute respiratory syndrome 2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) responsible for coronavirus disease 2019 (COVID‐19) was first identified in Wuhan, China, in December 2019. Since then, the virus has rapidly spread across the world due to its high transmissibility and the World Health Organization (WHO) declared COVID‐19 as a pandemic on March 11, 2020. Clinical manifestations among pediatric patients are generally less severe than those seen in adults, but the proportion of severe and critical cases reported in children inversely correlates with their age at presentation, with more severe cases reported in infants less than one year of age. 1 , 2 Nonetheless, early reports on neonatal SARS‐CoV‐2 infections were reassuring, with most cases showing an asymptomatic course or rarely symptomatic uncomplicated diseases. 3 , 4 As the pandemic evolved, however, reports of neonatal acute respiratory distress syndrome have been increasingly published, 5 , 6 , 7 , 8 , 9 , 10 raising concerns about the most appropriate diagnostic and therapeutic management of infected neonates.
This report describes a neonate with severe COVID‐19, further complicated by bacterial sepsis, overlapping the clinical, imaging and laboratory severity seen in older ages.
2. CASE REPORT
On October 2020, a 10‐day‐old female infant presented to the Emergency Department for apnea and feeding difficulties and was admitted to the Neonatal Unit. She was born at 39 + 1 weeks of gestational age via vaginal delivery and prenatal history was unremarkable. No history of contact with individuals with confirmed SARS‐CoV‐2 infection was reported, but the infant's father reported having a recent onset of cough. The infant's mother had a negative SARS‐CoV‐2 RNA nasopharyngeal swab and no anti‐SARS‐CoV‐2 antibodies detected at delivery, performed because of routine clinical practice.
Upon admission, a nasopharyngeal swab for SARS‐CoV‐2 RNA by polymerase chain reaction (PCR) testing was performed. Physical examination was initially unremarkable, with an oxygen saturation of 99% on room air, but the clinical status rapidly evolved to a decreased level of alertness.
A sepsis evaluation was performed: samples of blood, urine, and cerebrospinal fluid (CSF) were obtained for analysis. A CSF PCR panel that targets multiple pathogens causative of meningoencephalitis was performed. A qualitative PCR on the nasopharyngeal swab specimen for other respiratory bacteria and viruses was also performed. The pathogens covered by these panels are listed in Online Supporting Information. Chest‐X‐ray (CXR) showed slightly increased interstitial markings.
All the family members were tested, including both parents, a 2‐years‐old sibling, and a grandmother and all had positive SARS‐CoV‐2 RNA PCR on the nasopharyngeal swab, all showing a mild symptomatic disease. Family members had not received SARS‐CoV‐2 vaccine, as it was not yet available in Italy.
The clinical status of the baby further deteriorated, with recurrent episodes of apnea requiring intubation 6 h after admission.
Laboratory tests performed upon admission were in the normal range for age, except for mild leucopenia (WBC 5640/mmc) with lymphopenia (800 × 109/L). On the day of life (DOL) 11 the infant developed fever for 24 h (maximum 38.9°C).
CXR progressively evolved to bilateral central interstitial opacities, associated with large amount of respiratory secretions (Figure 1). A Lung Ultrasound (LUS) with a high‐frequency linear Probe (10 MHz) was performed at the bedside and showed pleural irregularities with two small subpleural consolidations and B lines, suggesting interstitial pneumonia (Figure 2). 11
Figure 1.
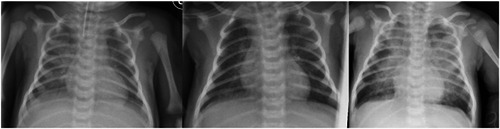
Chest X‐ray (CXR) on day of life (DOL) 12, DOL 16, and DOL 26. On DOL 12 bilateral lung opacities, particularly in the right field. On DOL 16 improving of the CXR, with increased interstitial markings and persistent opacities on the basal field of the right lung. On DOL 26 extensive bilateral lung opacities and consolidations with prevalent involvement of the paracentral fields
Figure 2.
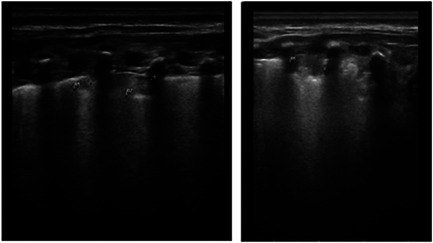
Lung ultrasound was performed scanning the lung in 14 different areas (three posteriors, two lateral, and two anterior). On day of life (DOL) 12 showing B lines and pleural irregularities, suggesting interstitial pneumonia; on DOL 26 showed a consolidation area that reached the pleura, appearing as inhomogeneous ipoechoic area, with irregular, blurred, and indistinct edges, and with lenticular echoes inside, representing air trapped.
The neonate was extubated on DOL 16 to high flow nasal cannula without supplemental oxygen. The CXR was slowly ameliorating (Figure 1). She was weaned from respiratory support on DOL 19. On DOL 23 her clinical status deteriorated, with fever, increased work of breathing (WOB), and tachypnea, requiring noninvasive respiratory support through nasal continuous positive airway pressure (nCPAP), with a 0.40 fraction of inspired oxygen. Given the clinical worsening, a blood culture was performed, which tested positive for multi‐drug resistant Enterobacter asburiae. The CXR showed a worsening of the radiological findings, characterized by extensive bilateral opacities initially in the central area and subsequently in the upper lobes (Figure 1). LUS was performed every two days and showed extension and worsening of interstitial pneumonia and of the lung infiltrates, with a consolidation area that reached the pleura likely due to bacterial super‐infection (Figure 2). Antibiotics were started (see above). The clinical conditions progressively improved and respiratory support was progressively decreased until suspension, on DOL 41. The clinical course was further complicated by feeding difficulties and recurrent episodes of increased WOB and tachypnea, even if without the requirement of oxygen supplementation. On DOL 45 a chest computed tomography (CT) was performed and revealed the presence of extensive consolidations with ground glass appearance involving the posterior fields of both lungs (Figure 3).
Figure 3.
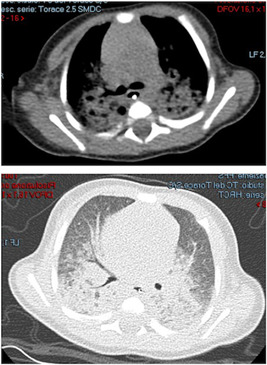
Chest computed tomography (CT) scan on day of life (DOL) 45 showing extensive consolidations with ground glass appearance involving the medium and posterior fields of both lungs, without the involvement of the anterior fields
The infant was discharged on DOL 72. After discharge, on DOL 135, cerebral magnetic resonance imaging was performed and no abnormalities were detected.
During the first days of hospitalization inflammatory markers showed only a slight increase. After an initial decline, all markers (C reactive protein [CRP], procalcitonin, interleukin‐6 [IL‐6], ferritin, D‐dimer) had a rebound during bacterial sepsis (Figure 4).
Figure 4.
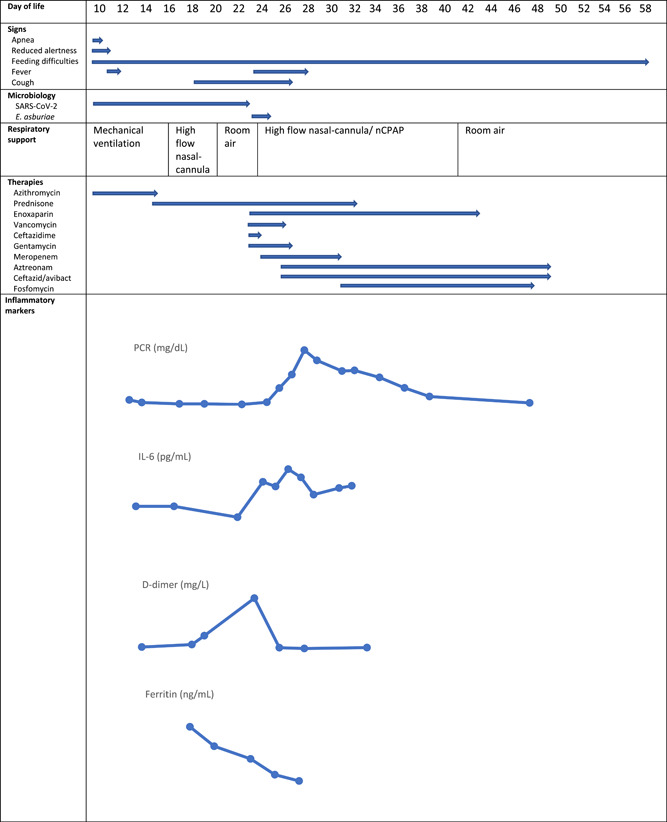
Summary of the clinical, diagnostic and therapeutic features of the present case. Inflammatory markers: C reactive protein (CRP) max value 31 mg/dL; interleukin‐6 (IL‐6) max value 418 pg/ml; ferritin max value 1275 ng/mL; D‐dimer max value >35 mg/L
2.1. Therapeutic interventions
Therapeutic interventions included broad‐spectrum antibiotics and acyclovir started at admission pending results of the microbiological tests, a 5‐day course of azithromycin at a dose of 5 mg/kg qd IV started on DOL 10, a 19‐day course of prednisone, initially at a dose of 2 mg/kg qd IV and gradually tapered until suspension on DOL 34. Because of a progressive increase of the D‐dimer till a value >35 mg/L FEU, from DOL 23 to DOL 42 subcutaneous enoxaparin was administered at a dose of 2000 IU qd. On DOL 23 vancomycin, ceftazidime, and gentamycin were initially started; based on the E. asburiae isolation, antibiotic treatment was switched to meropenem 40 mg/kg tid + aztreonam 40 mg/kg tid + ceftazidime/avibactam 50 mg/kg tid. Because of the antibiotic susceptibility profile of E. asburiae meropenem was suspended on DOL 30 and fosfomycin 65 mg/kg tid was added (Figure 4).
2.2. Microbiological tests
Detection of SARS‐CoV‐2 virus was performed by RT‐PCR following the CDC protocols. 12 This RT‐PCR assay targets SARS‐CoV‐2 virus nucleocapsid N1 and N2 genes and the human RNase P gene. Three separate master mix sets for N1, N2, and RNase P were prepared. The PCR reaction was performed using 15 μL of each master mix (SuperScript™ III Platinum™ One‐Step qRT‐PCR System, Invitrogen) and 5 μL of extracted sample. Amplification was performed on the Applied Biosystems QuantStudio 5 Real‐Time PCR System (QNS‐5; Thermo Fisher Scientific).
SARS‐CoV‐2 RNA was detected from the upper respiratory tract on DOL 10 and 22, with an amplification cycle threshold (Ct) of 24 on DOL 10, and from the lower respiratory tract on DOL 14, with a Ct of 22.4. SARS‐CoV‐2 RNA was detected in stools on DOL 17 with a Ct of 41.7, while it was undetectable on DOL 22. No SARS‐CoV‐2 RNA was detected on blood (DOL 11 and 22), CSF (DOL 11), and urine (DOL 11 and 22).
No SARS‐CoV‐2 antibodies (CLIA‐iFlash IgG and IgM, Shenzhen YHLO Biotech Co.; CLIA‐LIAISON® XL IgG, DiaSorin S.p.A.) were detected on DOL 11 and 16. On DOL 25 positive IgG with negative IgM were detected, with a quantitative result of the LIAISON® SARS‐CoV‐2 S1/S2 IgG of 42 AU/ml (cut‐off value for a positive result 15 AU/ml). The antibodies amount was monitored over time, demonstrating a progressive slight increase of anti S1/S2 IgG (65.9 AU/ml on DOL 100).
3. DISCUSSION
Literature that describes the epidemiology, clinical presentation, and prognosis of SARS‐CoV‐2 in neonates is scarce. As the number of neonatal cases around the globe continues to climb, there is the need to further fill this gap, providing some guidance to adequately manage these patients and also to counsel parents on what to expect in the clinical course of the infection.
Available data are mainly provided by single case reports or small case series. Most neonates and infants less than 90 days of life presented with no symptoms or with a variable combination of fever, feeding difficulties, neurological self‐limiting signs, acute upper respiratory tract infection, and respiratory distress with an uncomplicated course. 3 , 4 Detailed imaging studies describing the pulmonary involvement in neonates have not been published.
With limited guidelines on treatment approaches for COVID‐19 in neonates, few specific interventions were used in this newborn. Patients with COVID‐19 can rapidly progress to acute respiratory distress syndrome, as a result of the patient's own system becoming activated, with a subgroup of patients with severe COVID‐19 having a cytokine release syndrome. This hyperinflammatory state can lead to poor outcomes and is the rationale for the use of immunomodulating drugs, such as corticosteroids. 13 Nonetheless, deleterious clinical outcomes have previously been reported with the use of corticosteroids in other viral infections, including bacterial super‐infection and impaired viral clearance, which can lead to increased mortality. 14
Data on the use of corticosteroids in neonatal COVID‐19 are limited, 6 , 8 , 10 but these drugs have been used for a long time in neonatal medicine for purposes other than infections. While in two cases of neonatal COVID‐19 steroids were used late during the disease course and in combination with antiviral therapy without complications directly related to steroid treatment, 6 , 10 in another case a significant clinical, radiological, and laboratory worsening was described following two doses of dexamethasone. 8 In the present newborn the beneficial effect of weaning the neonate from mechanical ventilation was counterbalanced by the onset of bacterial sepsis and a significant worsening of the CXR, in which a contribution of both steroid‐related immunosuppression and SARS‐CoV‐2 induced immunosuppression and uncontrolled replication could have had a role. Enoxaparin was added 13 days after symptoms onset for thromboprophylaxis without short‐term side effects, because of a rapid increase of the D‐dimer. The underlying biological mechanisms of D‐dimer increase are unclear, but may reflect pulmonary vascular bed thrombosis with fibrinolysis, without systemic disseminated intravascular coagulation. 15 Whether in this case the D‐dimer increase was related to COVID‐19 or to bacterial sepsis, and their relative effects on the neonatal lungs, remains to be established. The use of other drugs, such as Remdesivir, was avoided because of the paucity of evidence of efficacy and safety in this particular age group.
SARS‐CoV‐2, like the other epidemic coronaviruses, has the potential for spread within healthcare settings, making case identification and prompt isolation crucial to protecting both healthcare workers and patients. Since admission, the infant was managed in a negative‐pressure room with droplets and contact precautions, and by using bacterial‐viral filters during respiratory support. All these measures were effective in preventing the nosocomial spread of the virus. Isolation was maintained until obtaining two consecutive negative SARS‐CoV‐2 RNA PCR nasopharyngeal swabs and a negative SARS‐CoV‐2 RNA PCR stool sample. Unlike previous reports showing a rapid virological clearance in neonatal nasopharyngeal specimen, 3 this sample remained positive for 12 days after the first detection. As expected, SARS‐CoV‐2 RNA was also detected in stools since the fifth day after disease onset but with a high Ct and with rapid clearance. This finding is in contrast with the prolonged RNA shedding previously described in stools, up to 5‐6 weeks after symptoms onset, and is even more curious considering the early use of steroids, which could have affected viral shedding. 16 Viral load kinetics and viral shedding in neonatal age remain mostly unexplored.
As previously demonstrated, this case suggests that the likely sources of SARS‐CoV‐2 infection in newborns are adult household contact. The incidence of transmission through familial exposure in pediatric SARS‐CoV‐2 infection has been estimated between 45% and 91%, 17 and it is plausible that it maybe even higher in the neonatal period. Therefore it is important to adequately inform the new parents to raise awareness about the possible household dissemination of SARS‐CoV‐2 infection, which may have relevant consequences also in neonates. Neonates born to SARS‐CoV‐2 seronegative mothers could be particularly vulnerable to severe manifestations, because of the lack of the protection usually provided through transplacental IgG transfer. Future research works are needed to establish the safety and efficacy of SARS‐CoV‐2 vaccines in pregnant women, to understand whether maternal immunization may provide passive immunity to the offspring, and to possibly determine the optimal administration schedule, in the attempt to mitigate the deleterious effect of SARS‐CoV‐2 infection in the neonate.
This case report highlights how, more than a year after the emergence of the COVID‐19 pandemic, the therapeutic approach of neonatal COVID‐19 is still challenging. Neonates are known to be vulnerable to infectious diseases, but early reassuring reports on neonatal SARS‐CoV‐2 infection made us neonatologists feel out of the storm. Unfortunately, recent literature suggests that we are not: the balance between viral replication and immunomodulation in neonatal COVID‐19 maybe even more challenging than in older age, and the clinical consequences of therapeutic approaches translated from adult medicine to neonatal age maybe unpredictable.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Informed consent has been obtained by the subject's parents.
AUTHOR CONTRIBUTIONS
Concetta Marsico and Maria Grazia Capretti provided direct care for the patient, drafted the initial manuscript, and reviewed and revised the manuscript. Arianna Aceti e Luigi Corvaglia provided direct care for the patient and critically reviewed and revised the manuscript. Filomena Carfagnini, Carla Serra, Caterina Campoli, Caterina Vocale, and Tiziana Lazzarotto analyzed the laboratory and/or imaging data, providing substantial contribution for the interpretation of data, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supporting information
Supporting information.
Marsico C, Capretti MG, Aceti A, et al. Severe neonatal COVID‐19: Challenges in management and therapeutic approach. J Med Virol. 2022;94:1701‐1706. 10.1002/jmv.27472
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Ludvigsson J. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 3. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLaren SH, Dayan PS, Fenster DB, et al. Novel coronavirus infection in febrile infants aged 60 days and younger. Pediatrics. 2020;146(3):e20201550. 10.1542/peds.2020-1550 [DOI] [PubMed] [Google Scholar]
- 5. Sinelli M, Paterlini G, Citterio M, Di Marco A, Fedeli T, Ventura ML. Early neonatal SARS‐CoV‐2 infection manifesting with hypoxemia requiring respiratory support. Pediatrics. 2020;146(1):e20201121. [DOI] [PubMed] [Google Scholar]
- 6. Trieu C, Poole C, Cron RQ, et al. Severe neonatal coronavirus disease 2019 presenting as acute respiratory distress syndrome. Pediatr Infect Dis J. 2020;39(11):e367‐e369. [DOI] [PubMed] [Google Scholar]
- 7. Wardell H, Campbell JI, VanderPluym C, Dixit A. Severe acute respiratory syndrome coronavirus 2 infection in febrile neonates. J Pediatric Infect Dis Soc. 2020;9(5):630‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frauenfelder C, Brierley J, Whittaker E, Perucca G, Bamford A. Infant with SARS‐CoV‐2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;143(3):e20201701. 10.1542/peds.2020-1701 [DOI] [PubMed] [Google Scholar]
- 9. Coronado Munoz A, Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late‐onset neonatal sepsis in a patient with COVID‐19. N Engl J Med. 2020;382(19):e49. 10.1056/NEJMc2010614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saikia B, Tang J, Robinson S, et al. Neonates with SARS‐CoV‐2 infection and pulmonary disease safely treated with remdesivir. Pediatr Infect Dis J. 2021;40(5):e194‐e196. [DOI] [PubMed] [Google Scholar]
- 11. Musolino AM, Supino MC, Buonsenso D, et al. Lung ultrasound in the diagnosis and monitoring of 30 children with coronavirus disease 2019. Pediatr Pulmonol. 2021;56(5):1045‐1052. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Diseases Control and Prevention . CDC COVID‐19 tests. Accessed on October, 25, 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html
- 13. Recovery Collaborative Group , Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID‐19 ‐ preliminary report. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406. [DOI] [PubMed] [Google Scholar]
- 15. McGonagle D, O'donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol. 2020;2:e437‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta‐analysis. Lancet Microbe. 2021;2:e13‐e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC COVID‐19 Response Team . Coronavirus disease 2019 in children – United States, February 12 – April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


