A 78‐year‐old woman with no personal or family history of abnormal bleeding noticed a bruise on her thigh 2 weeks after her second COVID‐19 (SARS‐CoV‐2) mRNA vaccination (Pfizer‐BioNTech; day 1; not shown). Otherwise, she had no serious adverse events following vaccination. Her skin bruise persisted. On day 38, a new skin bruise appeared on her left hand. On day 50, she visited a hospital because of pain and swelling in the left hand (Figure 1A). She had no evidence of any malignancies or autoimmune disorders and no medication intake. Her SARS‐CoV‐2 antigen was negative. The hematoma was removed to treat her compartment syndrome of the left hand. However, she had persistent postoperative bleeding and red blood cell units were transfused (2 units/day for 3 days). On day 55, she was transferred to our hematology department.
FIGURE 1.
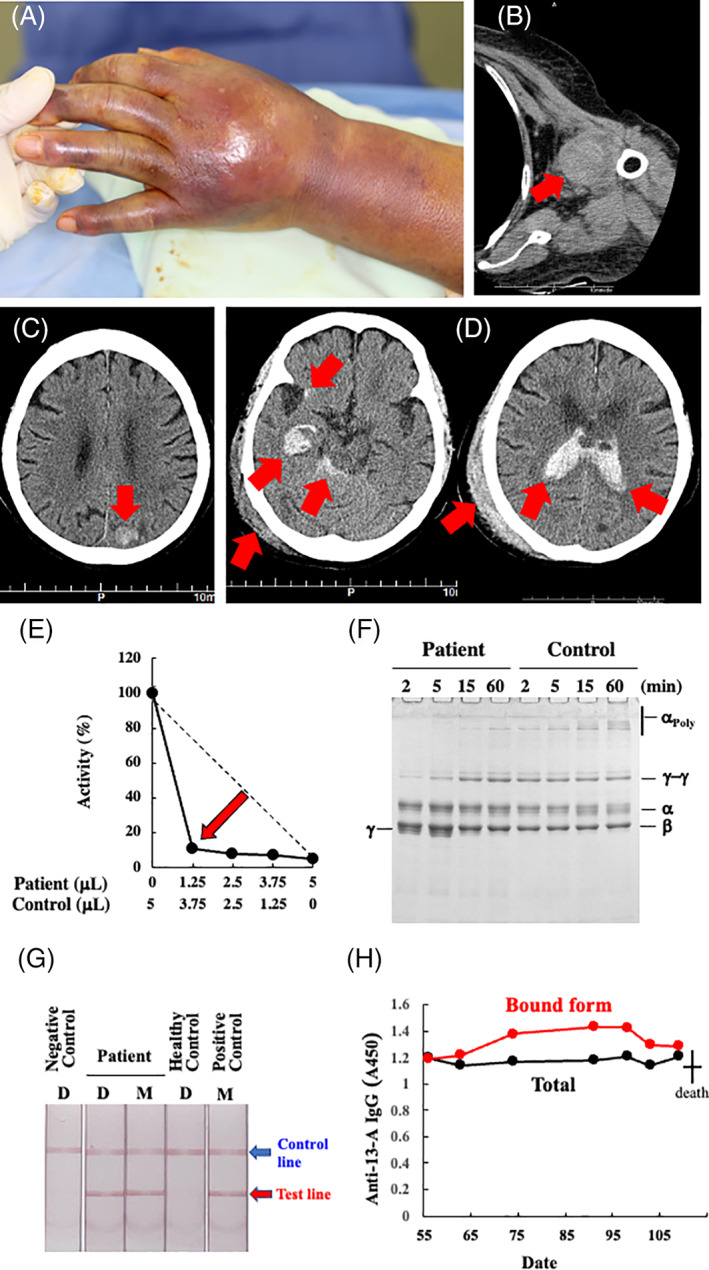
The major bleeding symptoms/sites on day 55 (A–C) and day 109 (D); the results of an experimental work‐up for AiF13D on day 56 (E–H). (A) Ecchymosis involving the left hand and forearm. She had pain and numbness in her fingers. (B) Computed tomography scan showing her left shoulder joint bleeding. (C) central nervous system (CNS) bleeding in her left posterior parietal lobe. (D) Computed tomography scan showing right subcortical and subarachnoid CNS hemorrhages, that had perforated into the ventricles. She also had a subcutaneous hematoma on the right side of the head. (E) The five‐step dilution cross‐mixing test by an amine incorporation assay was performed using the patient's plasma at the ratios of 0:1, 1:3, 1:1, 3:1, and 1:0 with a normal plasma. The mixed samples were incubated at 37°C for 2 h, before the assay. The patient's sample showed a downward concave “inhibitor” pattern. A straight broken line depicts a theoretical “deficient” pattern. (F) Fibrin cross‐linking reaction. Gamma‐chain dimerization was significantly retarded and γ‐chain monomer remained even after 5 min. Alpha‐chain polymerization was almost absent. (G) Immunochromatographic test for anti‐FXIII‐A subunit autoantibodies with (spiked; M) or without (direct; D) mixing patient's and normal control's plasma showed positive results. (H) Enzyme‐liked immunosorbent assay for anti‐FXIII‐A subunit autoantibodies (day 56–day 91). Despite the administration of Prednisolone and IVIg, the amounts of both F13‐bound (Bound Form) and total anti‐F13 autoantibodies in our case remained almost unchanged. Filled‐black and ‐gray circles depict bound forms and total (free and bound forms) amounts of anti‐FXIII‐A subunit IgG, respectively [Color figure can be viewed at wileyonlinelibrary.com]
Her physical examination revealed ecchymosis on her hind upper arms and left knee (not shown). Computed tomography (CT) scans showed intra‐articular bleeding in her left shoulder (Figure 1B) and asymptomatic central nervous system (CNS) bleeding (Figure 1C). Accordingly, the patient received 2 units of fresh frozen plasma for 4 days with no apparent effect.
Blood tests revealed normocytic anemia (red cell count 2.40 × 106/μL, reference range 3.86–4.92 × 106/μL; hemoglobin 7.8 g/dL, 11.6–14.8 g/dL; hematocrit 22.8%, 35.1%–44.4%) with normal platelet count (159 × 103/μL, 158–348 × 103/μL), prothrombin time (10.9 s, 9.5–13.5 s), and activated partial thromboplastin time (25.9 s, 25.0–38.0 s). Fibrinogen level was 288 mg/dL (155–415 mg/dL). Factor VIII/8 (F8) activity was >201%, (62%–145%), von Willebrand factor (VWF) activity 241% (50%–150%), and its antigen level was >201% (50%–150%). F8 inhibitor was negative. Hepatitis B surface antigen, Hepatitis C antibody, and HIV‐1 and ‐2 antibodies were negative or nonreactive. No monoclonal protein was detected by initial screening.
While factor XIII/13 (F13) antigen level was slightly reduced (59%, reference range >70%), its activity was below the detection limit (<3%, 70%–140%; measured by an ammonia release assay using a Berichrom FXIII Kit (Sysmex Corporation, Kobe, Japan) at a commercial laboratory service (SRL Ltd., Hachioji, Japan). Administration of F13 concentrates (1200 units/day for 5 days) significantly improved her symptoms, and she was discharged home on day 74. In an experimental work‐up conducted by the Japanese collaborative research group for autoimmune coagulation factor deficiencies (AiCFD), a 5‐step dilution mixing test with a healthy control plasma showed an F13 inhibitor pattern (Figure 1E) and anti‐F13‐A subunit autoantibodies were detected by immunochromatography and enzyme‐liked immunosorbent assay (Figure 1G,H, respectively). Therefore, we made a definite diagnosis of autoimmune F13 deficiency (AiF13D), according to the ISTH/SSC criterion 2015. 1 Immediately, prednisolone (0.5 mg/kg daily) was administered orally on day 91, but she noticed a new bruise on her right hand on day 100. Since F13 activity was still low (14%), she was re‐hospitalized on day 103. Screening CT did not detect any internal bleeding. Prednisolone was increased to 1 mg/kg, and intravenous (IV) immunoglobulin (IVIg, 400 mg/kg for 5 days) was administered. However, she was found lying on the floor in the early morning on day 109. She had a mild disturbance of consciousness but did not complain of headache. She had no elevated blood pressure, vomiting, paralysis, or other local neurological deficits. She was given 1200 units of F13 concentrates and her F13 activity increased to 33%; however, she died of CNS bleeding (i.e., cerebral hemorrhage and subarachnoid hemorrhage) in the afternoon on the same day after approximately 10 h (Figure 1D). Since the patient's family refused to autopsy, the exact cause and/or trigger of CNS bleedings remained unknown.
Various autoimmune diseases have been reported to develop after SARS‐CoV‐2 mRNA vaccination. To date, seven cases of autoimmune F8 deficiency (AiF8D; or acquired hemophilia A), which is the most frequent AiCFD, have been reported. 2 , 3 , 4 , 5 , 6 On the contrary, AiF13D is an extremely rare AiCFD, and this is the world's first case of AiF13D that is likely to have been triggered by SARS‐CoV‐2 mRNA vaccination. The exact mechanism(s) responsible for these immuno‐hematological complications is unknown, but the onset time of our case is similar to other immune‐related adverse events such as nephritis, vasculitis, etc. following vaccination. Although the temporal relationship of the development of AiF8D following SARS‐CoV‐2 mRNA vaccination may suggest a possible etiologic contribution, 6 it is difficult 2 and challenging 5 to establish the causal relationship between the vaccine and AiF8D, and the emergence of F8 inhibitors post vaccination is most likely to be a coincidence, 2 , 3 , 5 as the authors of previous reports have acknowledged. Nevertheless, it should be noted that the world's first case with AiF8D recurrence after SARS‐CoV‐2 infection has also been reported, suggesting a close link between SARS‐CoV‐2 virus and the hemostatic mechanism. 7
The age of our AiF13D patient (78 years) and the onset time from vaccination was similar to those of the seven AiF8D cases (67–86 years; 14 days vs. 4–21 days after the first or second dose, respectively). However, her bleeding symptoms, that is, CNS hemorrhages, were much more severe than all the AiF8D cases except for one patient with hemothorax caused by an accidental fall with chest and shoulder contusion. 3 There were no clear reasons or triggers for neither the first CNS bleeding episode on day 55 nor the second episode on day 109 in our patient. It is well known that cases of congenital F13 deficiency frequently present with CNS bleeding, which is the leading cause of death. Therefore, the attending physician of any AiF13D patient should pay close attention to the development of CNS bleeding.
Like our AiF13D patient, an AiF8D patient also died of active arterial bleeding due to acute gall bladder rupture. 3 This may be because he had denied any further intervention following arterial coiling, which only transiently stopped the bleeding. Although two AiF8D patients had cessation of bleeding without using any hemostatic agents, most patients achieved hemostasis by administration of the so‐called bypassing medicines such as recombinant activated factor VII/7, activated prothrombin complex concentrates, and F8 inhibitor bypassing activity. On the contrary, since there is no bypassing drug in F13 replacement therapy, our AiF13D patient continued to receive plasma‐derived F13 concentrates. Although the recombinant F13‐A subunit preparation has been on the market for a long time, it cannot be used in Japan (or even worldwide) because it is not covered by public medical insurance, which is an important problem to be solved in the future.
Six out of seven AiF8D cases recovered with corticosteroids alone or in combination with rituximab or cyclophosphamide. One AiF8D patient was given IVIg, which was considered effective for autoimmune VWF deficiency (or autoimmune VW disease), probably due to its simultaneous relapse. 4 In this case, a large hemorrhage plaque occurred as early as the fourth day of the second inoculation, and her hemostasis was obtained by F8/VWF replacement therapy. Our AiF13D patient was less responsive to IVIg infusions and eventually developed fatal bleeding, that is, CNS hemorrhage. As mentioned above, 4 recrudescence of autoimmune hematologic disorders is not uncommon, and the attending physician should closely follow‐up on such patients.
About 10 years ago, we formed our Japanese collaborative research group on AiCFD and have been conducting a nationwide survey to make a definitive diagnosis of patients with unexplained bleeding disorders in Japan. There are many patients with thrombosis related to SARS‐CoV‐2 infection, which has been a worldwide pandemic for the past 2 years; however, very few patients have bleeding. In particular, AiCFD after SARS‐CoV‐2 vaccination is an extremely rare disease, and it is difficult to diagnose and treat; therefore, clinicians may need to raise awareness of this disease.
In conclusion, AiF13D should be differentiated from bleeding due to other causes after SARS‐CoV‐2 mRNA vaccination. This is because the disease requires special laboratory tests, such as the ammonia release assay and amine incorporation assay, and specific hemostatic therapy by employing F13 concentrates along with immunosuppressants.
This study was approved by the institutional review boards of Yamagata University of Medicine and Oji General Hospital. All procedures were conducted in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Saori Shimoyama collected clinical data and samples, wrote a draft, and proofread the manuscript. Yuji Kanisawa and Kento Ono collected clinical data and proofread the manuscript. Masayoshi Souri carried out experimental examinations and proofread the manuscript. Akitada Ichinose designed the study, wrote, edited, and proofread the manuscript.
PATIENT CONSENT STATEMENT
Informed consent was obtained from the patient and her family.
ACKNOWLEDGMENT
This study was approved by the institutional review boards of Yamagata University of Medicine and Oji General Hospital. All procedures were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the patient and her family. This study was supported by research aids to Akitada Ichinose from the Japanese Ministry of Health, Labor, and Welfare (21FC1008). The authors are indebted to Dr Hiroto Horiguchi of Sapporo Medical University for his help in writing the manuscript as well as Dr Tomoya Harada of Kinikyo Tomakomai Hospital for his help in collecting clinical data and samples.
Shimoyama S, Kanisawa Y, Ono K, Souri M, Ichinose A. First and fatal case of autoimmune acquired factor XIII/13 deficiency after COVID‐19/SARS‐CoV‐2 vaccination. Am J Hematol. 2022;97(2):243-245. doi: 10.1002/ajh.26426
Funding information Japanese Ministry of Health, Labor, and Welfare, Grant/Award Number: 21FC1008
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- 1. Ichinose A, Kohler HP, Philippou H. Factor XIII and fibrinogen SSC Subcommittee of the ISTH. Recommendation for ISTH/SSC Criterion 2015 for autoimmune acquired factor XIII/13 deficiency. Thromb Haemost. 2016;116:772‐774. [DOI] [PubMed] [Google Scholar]
- 2. Radwi M, Farsi S. A case report of acquired hemophilia following COVID‐19 vaccine. J Thromb Haemost. 2021;19:1515‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cittone MG, Battegay R, Condoluci A, et al. The statistical risk of diagnosing coincidental acquired hemophilia A following anti‐SARS‐CoV‐2 vaccination. J Thromb Haemost. 2021;19:2360‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portuguese AJ, Sunga C, Kruse‐Jarres R, Gernsheimer T, Abkowitz J. Autoimmune‐ and complement‐mediated hematologic condition recrudescence following SARS‐CoV‐2 vaccination. Blood Adv. 2021;5:2794‐2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farley S, Ousley R, Van Wagoner N, Bril F. Autoimmunity after coronavirus disease 2019 (COVID‐19) vaccine: a case of acquired hemophilia A. Thromb Haemost. 2021;121:1674‐1676. doi: 10.1055/a-1579-5396 [DOI] [PubMed] [Google Scholar]
- 6. Lemoine C, Giacobbe AG, Bonifacino E, Karapetyan L, Seaman C. A case of acquired haemophilia A in a 70‐year‐old post COVID‐19 vaccine. Haemophilia. Published online Oct 28, 2021. doi: 10.1111/hae.14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchini M, Glingani C, De Donno G, et al. The first case of acquired hemophilia A associated with SARS‐CoV‐2 infection. Am J Hematol. 2020;95:E197‐E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


