Abstract
Background
Polyethylene glycol (PEG) may elicit anaphylaxis to COVID‐19 mRNA vaccines, and guidance for patients at risk is needed.
Methods
In retrospective patients with PEG allergy collected from 2006 till 2019, clinical, skin, and basophil activation test (BAT) characteristics discriminative for PEG allergy were analyzed and compared with the literature. In 421 prospective real‐life patients asking for allergy workup for COVID‐19 vaccine hypersensitivity in 2020/2021, risk assessment was performed and tolerance of the recommended vaccination approach was assessed.
Results
Ten patients with PEG allergy were found in the retrospective cohort. Patients reacted with immediate anaphylaxis (100%) not only to PEG‐based laxatives/bowel preparations or injections, but also to cold medication, antiseptics, analgetics, or antibiotics. Skin tests ± BAT with PEG ± elicitors were positive in 10/10. Provocation tests were positive in 7/9 patients. From the prospective cohort, 370/421 patients self‐reporting increased risk for vaccine allergy lacked criteria necessitating allergy workup and were recommended for routine vaccination. A total of 51/421 patients were tested, and three (6%) with PEG allergy were identified, whereas 48 patients remained negative in skin tests. Vaccination was recommended in all those patients. No hypersensitivity reactions were reported to vaccination including six PEG‐allergic patients tolerating COVID‐19 vaccination.
Conclusions
Taking a detailed history excluded PEG allergy in most referred patients and enabled direct safe vaccination. Immediate urticaria/anaphylaxis to typical elicitors identified patients requiring PEG allergy workup. Skin tests ± BAT identified PEG allergy and helped to select the vaccine and the vaccination approach. Even PEG‐allergic patients can tolerate COVID‐19 vaccines.
Keywords: allergy, allergy testing, COVID‐19, hypersensitivity, polyethylene glycol
Lack of immediate urticaria/anaphylaxis to typical elicitors largely excludes PEG allergy and enables routine vaccination. Allergy tests identify PEG allergy and help to select the vaccination approach. PEG‐allergic patients may tolerate COVID‐19 vaccines.Abbreviations: BAT, basophil activation test; COVID‐19, coronavirus disease 2019; PEG, polyethylene glycol; SPT, skin prick test

Abbreviations
- BAT
basophil activation test
- COVID‐19
Coronavirus disease 2019
- IDT
intradermal test
- IgE
Immunoglobulin E
- MW
molecular weight
- OPT
oral provocation test
- PEG
polyethylene glycol
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
- SPT
skin prick test
1. INTRODUCTION
Polyethylene glycols (PEGs, synonym macrogols) comprise a family of hydrophilic polymers produced by polymerization of ethylene oxide. 1 PEGs are ubiquitously used in medicine such as in laxatives and intestinal cleaning solutions (molecular weight (MW) 3350–4000), in injection solutions or liquid cosmetics (MW<800), emulsions (varying MW), as auxiliary materials in tablets (MW ≥ 6000) and for chemical, biological, industrial, cosmetic, and recreational uses. 1 Anaphylaxis to PEG has been described. 2 , 3 , 4 , 5 PEG 2000 is also a component in the artificial lipid layer in SARS‐CoV‐2 mRNA vaccines of Moderna (mRNA‐1273) and Pfizer‐BioNTech (BNT162B24, Comirnaty®), whereas the DNA vaccines of AstraZeneca (Vaxzevria®) and Johnson & Johnson (Janssen®) are vector‐based vaccines containing polysorbate 80 (PS80) and trometamol. 2 , 6 PEG is the only excipient in COVID‐19 mRNA vaccines that clearly demonstrated to cause delayed‐type and immediate‐type allergic reactions, while the role of trometamol and PS80 as relevant allergens in DNA vaccines remain more questionable. 2 , 3 , 4 , 7 Cases with anaphylaxis to mRNA vaccines for COVID‐19 have been reported with an incidence of 4.7 cases/10 6 doses for the Comirnaty® and 2.5/10 6 doses for Moderna vaccinations, about 2–4 times higher than that expected for other vaccinations. 8 , 9 , 10 , 11 Such reports fueled public concern about the allergenic potential of PEG. 3 , 12 , 13 , 14 , 15 Several individuals report alleged hypersensitivity reactions to the first dose of COVID‐19 vaccination. Additionally, those with previous severe allergic reactions and anaphylaxis to a variety of elicitors including medications seek allergy testing before vaccination. 16 , 17 Recommendations on how to manage patients at risk for PEG allergy have been published but have not been evaluated for their safety in real‐life patients. 18 , 19 , 20 , 21 , 22
The objective of this study is to (a) identify clinical criteria in our patients with PEG allergy and compare these with criteria reported in the literature, (b) to apply these criteria to 421 prospective real‐life patients who were asking for allergy workup for COVID‐19 vaccine hypersensitivity, and (c) to report the outcome of the recommendations for patient management according to individual risk assessment.
2. METHODS
2.1. Identification of criteria indicating PEG allergy in retrospective patients
Patients with immediate‐type allergic reactions to PEG between 2006 and 2019 were retrospectively retrieved from the medical charts of two large allergy units in southern Germany (Table 1). Clinical data including history, skin prick (SPT), intradermal (IDT) and oral provocation test (OPT) results were extracted from the patient charts. Main clinical criteria were identified and compared with those published in the literature. 18 , 19 , 20
TABLE 1.
Immediate‐type hypersensitivity reactions to polyethylene glycol observed in two allergy units in South Germany between 2006 and 2019
| Age | Sex | Exposure | Immediate‐type reaction | PEG test results | Oral challenge test (OCT) | Further observation |
|---|---|---|---|---|---|---|
| 77 | M |
(1) Colon cleansing solution (Klean‐Prep®, is PEG 3350) (2) Accidentally exposed to colon cleansing solution of PEG 4000 in hospital setting |
Generalized urticaria, bronchial obstruction, unconsciousness Generalized urticaria, bronchial obstruction |
SPT: Pos. to Klean‐Prep®, PEG 1500, 3350, 4000 Neg. to PEG 400, PEG 600 |
OCT with Klean‐Prep®: generalized urticaria, shortness of breath 40 min after drinking 10 mL of the solution (cumulative dose ~590 mg) | PEG as excipient was found in patient's long‐term medication—Blopress® (PEG 8000), Gallobeta® (PEG 4000), and calcium/vitamin D tablet. The patient was advised to continue the intake |
| 35 | F |
(1) Colon cleansing solution (Endofalk Classic®, is PEG 3350) (2) Disinfectant (Paroex®, containing PEG) for dental treatment (3) Vaginal application of PVP iodine solution (containing PEG 400 and PEG 4000) |
Generalized urticaria, angioedema, vomiting 10 min after drinking Generalized urticaria, tachycardia, and unconsciousness Generalized urticaria |
SPT: Pos. to Endofalk Classic®, Paroex® solution, PVP‐Jod‐ratiopharm®, and PEG 4000 solution BAT Pos. to PEG 4000 |
OCT with magnesium capsules containing PEG 4000 (Magno Sanol® capsules): Generalized urticaria after two capsules (~60 mg PEG 4000) | As permanent medication. an oral contraceptive (Swingo 30®, active ingredients levonorgestrel, and ethinylestradiol) containing PEG 4000 was taken daily and tolerated without developing allergic reactions |
| 19 | F | Fourth subcutaneous injection of depot contraceptive medroxyprogesterone acetate (Sayana®, containing PEG 3350) | Generalized urticaria, rhinitis 3–5 min after injection |
SPT: Neg. to Sayana® IDT: No cutaneous reaction, but tingling of the tongue and sneezing/rhinorrhea BAT: Neg. to PEG 4000 |
SCT 0.1 mL Sayana®: oral tingling, generalized urticaria, sneezes OCT with PEG 3350‐containing laxative Movicol® (1.3 g PEG 3350): generalized urticaria after 20 min OCT with medroxy‐progesterone acetate and the methyl‐4‐hydroxy benzoate negative |
Contraception was changed to desogestrel pills (Cyprella®) containing povidone k 30 (linear polymer of n‐vinyl‐2‐pyrrolidone) and was tolerated without allergic reaction |
| 28 | F | Depot contraceptive medroxy‐progesterone acetate (Depot‐clinovir®, contains PEG 3350) | Generalized erythema, pruritus, shortness of breath, vertigo, hypotension |
Pos. to depot‐clinovir®, PEG 4000 Neg. to medroxy‐progesterone acetate |
Labial provocation with a knife tip (~10 mg) of PEG 4000 tolerated | Tolerated two shots of Comirnaty® with antihistamine prophylaxis |
| 46 | M | Prednisolone injection (Rotexmedica®, contains PEG 4000) | Generalized urticaria, bronchial obstruction | SPT: Negative to Prednisolon 25 mg Rotexmedica® | Intragluteal provocation with Rotexmedica®: sneezes and mild bronchial obstruction. Alternative preparation with same active ingredient and excipients except PEG tolerated | Tolerated one shot of Vaxzevria® and cross‐over to Comirnaty® without prophylaxis |
| 47 | F |
(1) Oral analgesic metamizole (Novalminsulfon Lichtenstein® contains PEG 6000) (2) Effervescent calcium tablet (Calcium Sandoz forte®, containing PEG 4000) |
Generalized urticaria Generalized urticaria |
SPT: Neg. to Calcium Sandoz forte®, PEG 4000, analgesics including metamizole |
OCT with Calcium Sandoz forte effervescent tablet and metamizole (Novalgin®, with PEG 4000 and PEG 8000): Generalized urticaria after 15–30 min OCT with PEG 3350 solution: generalized urticaria (eliciting dose 500 mg) |
Tolerated two shots of Vaxzevria® without prophylaxis |
| 21 | F | Different oral analgesics (paracetamol, ibuprofen, ASS) | Generalized urticaria, angioedema of the lips, bronchial obstruction | SPT: Pos. to PEG 4000 | OCT with PEG 4000: After application of a second dose of 10 mg erythema and urticaria | OCT with celecoxib, paracetamol, ibuprofen, carboxymethylcellulose neg |
| 24 | F |
(1) Cough lozenge (Mucoangin® contains PEG 6000) (2) Cephalexin® (contains PEG 6000) + metamizole (Novalgin®, contains PEG 4000 & PEG 8000) |
Generalized urticaria, pruritus, headache, nausea Generalized urticaria, hypotension, headache, nausea |
SPT: Pos. to Mucoangin® and PEG 4000 Neg. to Cephalexin and Novalgin® |
OCT with PEG 4000: After application of 10 mg swelling and erythema of both hands | OCT with paracetamol, tramadol, celecoxib, doxycycline, ciprofloxacin, roxithromycin negative |
| 12 | M | Erythromycin syrup (Infectomycin®, contains PEG 6000) | Generalized urticaria, angioedema of the eyelids, dizziness, pruritus in the throat, dyspnea after 30 min |
SPT: Pos. PEG 4000 After SPT systemic reaction with itching at the palate and dizziness |
Labial and OPT tolerated until maximal dose 30 mg of PEG 4000, discontinued because of systemic reaction in SPT | |
| 76 | F | Cold medication effervescent tablet (Multinorm®, contains PEG) | Generalized urticaria, pruritus, dyspnea, hypotension after 30 min |
2007: SPT: Pos. to PEG 4000; Neg. to Multinorm® tablet 2021: SPT: Neg. to PEG 2000, PEG 4000, PEG 6000, PS 80, Comirnaty® IDT: Neg. to PEG 2000, PEG 4000, PEG 6000, PS 80 BAT: Pos. to Comirnaty®, Moderna Neg. to PEG 2000, 3350, 4000, 6000, PS 80, Vaxzevria® |
No OCT performed | Tolerated two shots of Vaxzevria® under emergency preparedness |
Abbreviations: BAT, basophil activation test; IDT, intradermal test; mi, minutes; Neg, negative; OCT, oral challenge test, SCT, subcutaneous challenge test; PEG, polyethylene glycol; Pos, positive; PS80, polysorbate 80; SPT, skin prick test.
2.2. Management of patients asking for allergy workup for COVID‐19 vaccine hypersensitivity
Patients referred to the allergy unit from April 2020 until April 2021 because of self‐reported adverse reactions after the first dose of a mRNA (Comirnaty® or Moderna vaccine), or DNA (Vaxzevria® or Janssen®) COVID‐19 vaccine, or reporting possible allergy against a vaccine ingredient were assessed and managed prospectively as outlined in Figure 1. Testing was carried out with vaccines, PEG, PS80, and trometamol, the latter only if relevant in the patient history (reaction to Moderna® or other preparation containing trometamol). Patients without clinical criteria for PEG allergy (Table 3) were referred for direct vaccination without allergy tests, 1 , 6 , 18 as recommended by the algorithm of the German Federal Institute for Vaccines and Biomedicine (Paul Ehrlich Institute) and German allergy associations (Figure S1), 21 , 22 which mirrors other published recommendations. 18 , 19 , 20 Mastocytosis without criteria for vaccine excipient allergy was no indication for testing. 23 Unclear cases were handled by shared decision‐making discussing the case between the allergy resident, allergy supervisors, and the department director. Patients with suspicion for possible allergy to vaccine ingredient(s) were skin tested, and in patients with PEG allergy, the basophil activation test (BAT) was added as described and outlined in Table S1 and in Supplementary Methods.
FIGURE 1.
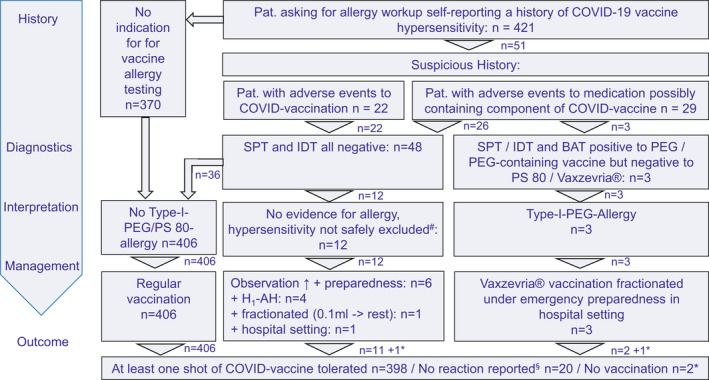
Flow chart of patients evaluated requesting allergy diagnostics regarding vaccination against COVID‐19 and tolerating vaccination. # Patients with immediate urticaria or anaphylaxis‐like reaction as a precaution for safety in case of non‐allergic hypersensitivity; *patients who did not want to be vaccinated; §patients not reporting despite call to report any hypersensitivity reaction
TABLE 3.
Patients for which polyethylene glycol allergy testing is required before vaccination
| Clinical suspicion of PEG allergy: |
| Patients with severe systemic allergic reactions or anaphylaxis within 1–2 h to |
| ‐ PEG‐based laxatives/bowel preparations or PEG‐containing drug injections |
| ‐ PEG‐containing cough medication, lozenges or vaginal suppositories |
| ‐ PEG‐containing products after sensitization to active ingredients have been excluded or only certain brand names of the same drug containing PEG |
| Individually consider clinical possibility of PEG allergy: |
| Patients with severe doctor‐confirmed or doctor‐treated allergic reactions anaphylaxis to structurally different drugs |
2.3. Outcome of vaccination
Outcome of vaccinations with regard to hypersensitivity reactions was recorded by patient interview asking for events occurring within 1 hour and by telephone interview of those 51 patients in whom we performed allergy tests. We asked the patients without need of allergy workup to report to us, if such reactions occur.
3. RESULTS
3.1. Retrospective patient series with PEG allergy
Ten patients with confirmed IgE‐mediated allergic reactions to PEG were identified in our two allergy units within the last 14 years (Table 1). This report includes the highest number of allergy‐tested PEG‐allergic patients in the literature. Nevertheless, our study spanning a 14‐year period shows that PEG allergies are rare. In the medical history of PEG‐allergic patients, reports of repeated reactions were frequent (40%), but not in the majority of patients. The common clinical manifestation was anaphylaxis according to the NIAID/FAAN criteria for anaphylaxis by Sampson et al (10/10; 100%), 24 and with generalized skin reaction (urticaria in 9/10 and generalized erythema in 1/10) in all patients (Table 1). Two patients developed unconsciousness (patients 77 m, 35 f). The onset of the allergic reactions was rapid, within few minutes in case of injections and within 60 minutes following oral uptake of the allergen. A common feature was that the systemic allergic reactions occurred after exposure to PEG 3350 or PEG 4000 in the dissolved form in most cases. The medications containing PEG were intestinal cleansing preparations, laxatives, lozenges, effervescent tablets, and solutions for injection. PEG uptake was oral, via the intestinal or genital mucosa or via subcutaneous tissue following injections. SPT with the suspected drugs and/or PEG solutions (PEG 3350 or PEG 4000) were positive in 8/10 cases. Skin test reactivity was negative in 2/2 patients to PEGs of lower MW, as has been reported in the literature. 1 Mild systemic symptoms (tingling of the tongue or palate, sneezing/rhinorrhea, and dizziness), similar to those observed during OPT, were observed in 2/10 (19 f, 12 m) individuals following skin tests. Whereas in two patients, OPT was stopped because of safety concerns after having received a knife tip (~10 mg) or 30 mg of PEG 4000, respectively, before a reaction developed, in 7/9 patients (77 m, 35 f, 19 f, 46 m, 47 f, 21 f, and 24 f), the allergic reaction could be reproduced by OPT using the appropriate PEG preparation; two patients developed bronchial obstruction or shortness of breath (77 m and 46 m). The criteria we found for PEG allergy in the history in these 10 patients were in accordance with and confirmed those published in reviews and recommendations (Tables 2 and 3). 2 , 6 , 12 , 18 , 19
TABLE 2.
Parameters indicative of PEG allergy in patients in our study and comparison to a review and a large case series in the literature
| Parameter | PEG allergy in this study | Wenande et al. 1 | Bruusgaard et al. 6 |
|---|---|---|---|
| Gender (f/m) | 7/3 | 14/23 | 6/4 |
| Age (y: median, range) | 38.5 (12–77) | 24–86 | 35 (18–64) |
| Time interval a (min) | 18.5 (4–30) | “Rapid in onset within minutes” | <10 min |
| Elicitors b (n) |
Laxatives/bowel preparations (3) Analgetic tablets (2) Injectable contraceptive (2) Antibiotic tablets/sirup (2) Calcium or vitamin effervescent tablet (2) Cold medicine lozenge/syrup (2) Injectable corticosteroid (1) Iodine solutions (1) Chlorhexidine disinfectant (1) |
Laxatives/bowel preparations 20/37 (54%), corticosteroids, vitamins/minerals, throat lozenges, ultrasound gels, disinfectants, antiepileptics, antiemetics, anticoagulants, antidepressants, analgesics, antibiotics, anti‐inflammatory drugs, reflux medication toothpaste, dental floss, creams, shampoos, paint, wound dressings, tissue sealants, hydrogels |
Oral medication (analgesics, antacids, antibiotic tablets) Injections Laxatives Cough medicine Cream/ointment, shaving products, hand soap, toothpaste, mouth wash, dental floss, hair products (shampoo, coloring), make‐up, make‐up remover, vaseline, epoxy, cleaning agent |
| Patients >1 reaction n (%) | 4 (40) | 16 (43) | 7 (70) |
| Severe anaphylaxis c n (%) | 2 (13) | 14 (38) | 8 (80) |
| Anaphylaxis n (%) | 15 (100) | 28 (78) | 8 (80) |
| Urticaria b n (%) | 14 (93) | 44 (66) | ns |
| Skin/mucosal reaction b n (%) | 15 (100) | 62 (92) | ns |
| Most frequent symptoms b n (%) | Generalized skin reaction 15 (100), dyspnea 6 (60), angioedema 3 (20) | Pruritus, tingling, flushing, urticaria, angioedema, hypotension, and bronchospasm | Urticaria, itching, flushing, discomfort, angioedema, breathlessness, burning sensation, and fainting |
Abbreviation: ns, not specified.
Time interval from exposure to reaction.
Individual episodes counted separately.
Severe anaphylaxis according to Ring and Messmer grades III and IV.
3.2. Patients self‐reporting a history of COVID‐19 vaccine hypersensitivity
In total, 421 e‐mails and telephone calls were received from patients or referring doctors self‐reporting a history of COVID‐19 vaccine hypersensitivity or alleged increased allergy risk for vaccination against COVID‐19 (Figure 1). Cases were assessed for signs indicative of PEG allergy or for immediate‐type hypersensitivity to vaccination against COVID‐19 (see section 2). Most patients reported unspecific and/or delayed reactions not indicating immediate‐type allergy, such as swellings at the reaction site, delayed urticaria, flu‐like symptoms, 25 or a history of other drug, insect venom, or food allergies not qualifying for further allergy workup for PEG allergy. Thus, exclusion of criteria indicative of PEG allergy (Table 3) and published recommendations (Figure S1) were helpful to recommend direct vaccination without allergy test or additional precaution in 370 patients (88%). Hence, the vast majority of patients suspecting allergic reactions could be encouraged to proceed to vaccination without allergy tests by history alone (Figure S1, Table 3).
Other 51 patients (12%) were invited for allergy skin testing, when it was felt that an allergy to a vaccine ingredient could not be safely excluded by history alone. In three patients (6%), PEG allergy was diagnosed: In two patients, PEG allergy was newly diagnosed by us and in one patient externally 8 years ago. They were identified by their typical history and by positive SPT and IDT results (Table 4). BAT was positive in 3/3 PEG‐allergic patients but not in controls (Figure 2, Table 4, Tables S2 and S3). One patient showed pronounced reactions (max. %CD63‐positive basophils) to Comirnaty®, Moderna vaccine, PEG 4000, and PEG 6000 (34%, 35%, 16%, and 36%), one patient to Moderna® (16.1%), and one patient to both mRNA vaccines (37% and 42%).
TABLE 4.
Characteristics and vaccination management of new patients with confirmed PEG allergy
| Age | Sex | Elicitor | Reaction | Skin test results | Interpretation | Management |
|---|---|---|---|---|---|---|
| 73 | M | Colon cleansing solution (=PEG 3350) | 30 min after third or fourth cup of PEG‐preparation palmar erythema, generalized itch, dizziness |
SPT: Pos. to PEG 3350, 4000 Neg. to: PEG 2000, PEG 6000, PS 80 IDT: Pos. to PEG 3350, 4000, 6000. After IDT generalized itch and palmar erythema Neg. to PEG 2000 and PS 80 BAT: Pos. to PEG 4000, 6000, Comirnaty®, Moderna Neg. to PEG 2000, 3350, PS80, Vaxzevria® |
No OCT was performed because of systemic reaction after skin test High risk for reaction to mRNA‐containing COVID‐19 vaccines. Low risk for reaction to Vaxzevria® because of negative skin tests and BAT and thus unlikely cross‐reactivity to polysorbate 80 |
mRNA vaccination avoidance DNA vaccination against COVID‐19 indicated. Tolerated first shot of fractionated Vaxzevria® (0.1 mL; 0.4 mL) under inpatient emergency preparedness, without premedication |
| 59 | F |
(1) Colon cleansing solution Movicol Orange® (=PEG 3350) (2) Corticosteroid +local anesthetic injection (lidocaine/triamcinolone, + PEG) |
(1) 1 h after intake of Moviprep Orange® swelling of hands, generalized urticaria, nausea, vomiting, diarrhea (2) Few min after injection of Lidocaine/Triamcinolon‐ preparation generalized urticaria, dyspnea, dizziness |
SPT: Pos. to PEG 4000, 6000 Neg. to PEG 2000, PS80, Moviprep Orange After SPT nausea and dizziness BAT: Pos. to Moderna‐vaccine, Neg. to PEG 2000, 3350, 4000, 6000, PS80, Comirnaty®, Vaxzevria® |
No OCT was performed because of systemic reaction after skin test High risk for reaction to mRNA‐containing COVID‐19 vaccines. Low risk for reaction to Vaxzevria® because of negative skin tests and BAT and thus unlikely cross‐reactivity to polysorbate 80 |
Subcutaneous challenge to triamcinolone, articaine negative. mRNA vaccination avoidance. DNA vaccination against COVID‐19 recommended as in other patients but not yet done |
| 28 | F |
(1) Cold medication sirup (PEG 6000) (2) Local anesthetic injection (mepivacaine, contains PEG) (3) Colon cleansing solution (Movicol®, is PEG 3350) (4) Iodide solution (cont. PEG) |
(1) Few min after intake throat swelling, dyspnea, generalized pruritus (2) Few min after injection urticaria, dyspnea, drop in blood pressure, runny nose (3) 5 min after intake urticaria, dyspnea, drop in blood pressure. (4) One hour after application (foot) urticaria |
2013: SPT: Pos. to: PEG 4000 After SPT generalized urticaria 2021: SPT Neg. to: PEG 2000, 4000, 6000, PS 80, Comirnaty® IDT Pos. to: Comirnaty®. Generalized urticaria 1 h after IDT Neg. to: PEG 2000, 4000, 6000, PS 80, Vaxzevria® BAT: Neg. to PEG 2000, 4000, 6000, PS 80, Vaxzevria® Pos. to: Comirnaty®, Moderna‐vaccine |
No OCT was performed because of systemic reaction after skin test High risk for reaction to mRNA‐containing COVID‐19 vaccines. Low risk for reaction to Vaxzevria® because of negative skin tests and BAT and thus unlikely cross‐reactivity to polysorbate 80 |
mRNA vaccination avoidance DNA vaccination against COVID‐19 indicated. Tolerated first shot of fractionated Vaxzevria® (0.1 mL; 0.4 mL) under inpatient emergency preparedness, without premedication |
Abbreviations: BAT, basophil activation test; IDT, intradermal test; mi, minutes; Neg, negative; OCT, oral challenge test; PEG, polyethylene glycol; Pos, positive; PS80, polysorbate 80; SPT, skin prick test.
FIGURE 2.
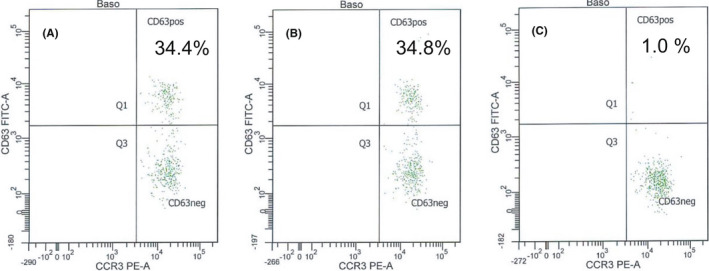
Skin test and basophil test results (A: Comirnaty® diluted 1:5, B: Moderna diluted 1:5, C: Vaxzevria® diluted 1%) in a patient with skin test‐confirmed PEG allergy who was successfully vaccinated with Vaxzevria®
In 48/51 patients (94%), no hint for PEG allergy could be identified by skin tests. Depending on the history of the reaction and backed up by lacking skin test reactivity, a risk assessment for future COVID‐19 vaccine reactions was performed by shared decision‐making. Vaccination was recommended in all patients; however, different management measures were selected for vaccination according to the risk assessment (Figure 1).
3.3. Management and outcome of COVID‐vaccination
In the retrospective cohort of 10 patients, one elderly patient died of cancer unrelated to allergy, three could not be contacted, two were not yet vaccinated, and four patients tolerated vaccination against COVID‐19: Two patients were vaccinated with Vaxzevria®, but without our consultation also one patient tolerated two shots of Comirnaty® with prophylactic antihistamine and one a Vaxzevria®‐Comirnaty® cross‐over vaccination.
In the 3/51 prospective skin test‐identified PEG‐allergic patients, skin tests and BAT with Vaxzevria® were negative and Vaxzevria® vaccination was tolerated in the first two patients, first vaccination under inpatient conditions in the absence of premedication with fractionated doses (0.1 mL; 0.4 mL; separated by 2 hours) and the third received the full second dose under emergency preparedness at a vaccination center (Table 4). Thus, PEG‐allergic patients could be vaccinated using a COVID‐19 vaccine that does not contain PEG, such as a DNA vaccine.
A total of 36 of the 48/51 skin test‐negative patients received routine vaccination without allergy‐specific precautions and in the remaining 12 patients, non‐allergic hypersensitivity reactions/skin‐test negative allergic reactions could not be excluded. Therefore, depending on the history, the risk for COVID‐19 vaccine anaphylaxis was assessed by shared decision‐making and different individual vaccination approaches were proposed (Figure 1). Vaccinations were tolerated in 48/48 patients without immediate hypersensitivity reaction.
Of note, the 370 patients who were not allergy tested reported no event of immediate allergic reactions. Two patients reported delayed reactions (urticaria, nausea, stiffness, and palsy). This indicates that shared decision‐making for vaccination management based on medical history and supported by skin tests is a helpful and safe approach.
4. DISCUSSION
PEG allergy has been widely discussed as cause of anaphylaxis to mRNA vaccines for COVID‐19 protection. 4 , 26 Consequently, in patients with PEG allergy, withholding vaccination with PEG‐containing vaccines is recommended, but, in real life, many more miss to be vaccinated based on pure suspicion. 12 , 18 , 19 , 21 Based on our data, we propose a more personalized approach showing that (a) most patients can be vaccinated without allergy tests just based on detailed history taking, that (b) allergy testing identifies patients with PEG allergy and may help in selecting the right approach for vaccination, and that (c) patients with PEG allergy can be vaccinated against COVID‐19.
An analysis of criteria indicating PEG allergy in our patients confirmed previously published clinical signs (Tables 2 and 3). 2 , 3 , 5 , 6 , 27 Position papers on how to handle vaccinations against COVID‐19 in patients at risk for allergic reactions are important guidelines. 12 , 21 , 22 In contrast, collections on real‐life data on patients asking for allergy workup concerning COVID‐19 vaccine allergy are just in the process of being published and future recommendations need to include these and adjust the guidelines, if applicable. 28 , 29 There is consensus on the following: (a) Testing of “all” is not feasible nor necessary, (b) procedures should aim at allowing vaccinations to be carried out over avoiding vaccination, and (c) selected patients should be tested prior to vaccination. 12 , 22 In addition, a delay from COVID‐19 protection due to allergy tests that are actually not needed may cause harm to individual patients.
To this end, we analyzed how many patients are eligible for vaccination just based on detailed history taking and found that 370/421 patients with concerns in regard to vaccine hypersensitivity could be referred to vaccination without further allergy workup (Figure S1, Table 3). In another 48/51 patients with negative skin tests, an allergy was largely excluded, which aided to select a less controlled vaccination approach. Of note, PEG hypersensitivity despite negative skin tests has been described and was seen also in one of our patients. 30 As we could not safely exclude non‐allergic hypersensitivity (e.g., chronic or stress‐associated urticaria and non‐IgE‐mediated hypersensitivity reactions) in 10 patients with immediate allergy‐like and in 2 with anaphylaxis‐like reactions despite negative skin test, we individually recommended prolonged observation, increased emergency preparedness and antihistamine premedication (for those with prominent urticaria), and/or fractionated vaccination ± inpatient setting for those with anaphylaxis‐like reactions (Figure 1). As none of our patients reported adverse reactions after taking the recommended shot, we conclude that the combined test regimen has a tolerable sensitivity. 25 A study from the United States reported good tolerance of the second vaccination against COVID‐19 in 80% of 159 patients with allergy‐like reaction to the first dose, however, after excluding patients with PEG allergy and those with severe symptoms, which indicates that that a decision‐making according to personalized history can augment the amount of patients receiving vaccinations. 29
Our allergy tests identified three new patients with PEG allergy, which is similar to data from the literature. 12 , 16 , 28 , 31 Confirming suspected IgE‐mediated PEG allergy with skin tests needs application of special variants of PEGs, since PEGs of lower molecular weight (MW) tend to be unresponsive. 2 , 4 Of note, PEG 2000 is declared to be part of the mRNA vaccines (2‐[(polyethylene glycol)‐2000]‐N,N‐ditetradecylacetamide (Comirnaty®) or PEG 2000 dimyristoyl glycerol (Moderna vaccine)); however, using PEG with MW of 4000 also produced positive skin test results in our patients. 2 , 4 , 12 Although false‐positive skin test results to PEG 4000 cannot be ultimately excluded, our results are in agreement with those of other groups describing positive responses only in patients with PEG allergy. 1 , 4 Similar results were obtained with BAT also showing positive reactions to both mRNA vaccines and negative results with Vaxzevria® (Figure 2, Table S2). Also, Troelnikov et al. presented patients positive in BAT to Comirnaty® and PEGylated liposomal doxorubicin but negative to Vaxzevria®. 32 Therefore, it can be assumed that only the special PEG arrangement on the surface of these lipid nanoparticles leads to a sufficient cross‐linking of IgE on basophils in some PEG‐allergic patients. 32 However, future provocation tests with PEG and mRNA vaccines would be necessary to confirm the clinical relevance of these findings.
Despite the ongoing discussion about the safety of IDT and OPTs in PEG allergy, we used these procedures to confirm a suspicion of PEG allergy and found that they can be performed with caution. 1 , 12 , 18 , 20 Indeed, systemic reactions may occur to SPT as described in the literature and as seen in our patients. 1 , 5 Therefore, it is recommended to start skin tests and OPT with low concentrations and doses, respectively, and under emergency preparedness. Skin tests to vaccines still remain unsatisfyingly validated, with sensitivity and specificity not yet being determined and vaccines, in general, may elicit test reactions of uncertain significance, for example, based on previous immunity or vaccine adjuvants. 11 OPT is the gold standard in drug allergy and PEG‐hypersensitive patients with negative SPT have been described. 30 Thus, IDT and OPT should be considered in SPT‐negative patients with clinical suspicion of PEG allergy. 1 , 5 , 33 IDT with PEG‐containing vaccines should be considered in patients with reactions after vaccination with such vaccines, as it has been suggested that PEGylation itself might have an effect on test response to PEG products. 32 In OPT, doses between 500 mg‐1.5 g PEG 3350 and 10–60 mg PEG 4000 elicited reactions and confirmed PEG allergy, demonstrating reactivity to lower amounts of PEG compared to the available data in the literature (55 g PEG 3350 and 7.1 g PEG 4000). 1
Allergy to PEG is a contraindication for applying PEG‐containing vaccines. 2 , 3 , 5 , 8 , 12 , 18 , 19 , 21 However, unexpectedly two of our historical patients with PEG allergy were ignorant of the risk despite allergy pass and tolerated Comirnaty® vaccination. In a Danish study, a further patient with isolated delayed skin reaction to Comirnaty® and positive skin test to PEG tolerated revaccination. 28 The reasons for tolerance remain unclear. Their PEG allergy may have been lost over the years, the amount of PEG in mRNA vaccines was too small, the MW was too low (MW 2000), or it may be a combination of different factors. Until further clarification, PEG‐responsive patients may receive DNA vaccines containing PS80 under emergency preparedness, as did four of our patients, who tolerated Vaxzevria®. This vaccination was given under close observation and emergency preparedness but without premedication for better monitoring of symptoms, and not all patients received fractionated dosing, indicating that these additional safety measures may not be needed in skin test‐negative patients in this setting. Although skin test cross‐reactivity between PEG and PS80 has been reported, the relevance of this observation remains unknown and was not confirmed in our patients. 1 , 20 Likewise, negative BAT to PS80 in our four PEG‐allergic patients argues against cross‐reactivity. 34
In conclusion, in this largest allergy‐tested series of patients with PEG allergy, we confirmed clinical criteria indicating PEG allergy (Table 3), attested the practicability of existing recommendations (Figure S1) 21 , 35 , demonstrated the value of allergy tests for detecting PEG allergy, and safely vaccinated all patients, including those with PEG allergy with a DNA vaccine, by giving specific individual recommendations for the vaccination approach according to risk analysis and with shared decision‐making.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information
Fig S1
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
This publication would not have been possible without the helpful cooperation of doctors Johann Wilhelm Weidringer, Philip Kampmann, and Felix Jonas from the Munich Vaccination Center. We are also grateful to Margitta Worm (Charité Berlin, Germany) for suggesting and discussing skin test concentrations/allergy workup to all German Comprehensive Allergy Centers, and Vera Mahler (Paul Ehrlich Institute, Abt. Allergologie) and other contributors from participating institutes/associations for creating the algorithm in Figure S1 and to our coworkers in our allergy department. Open access funding enabled and organized by Project DEAL.
Brockow K, Mathes S, Fischer J, et al. Experience with polyethylene glycol allergy‐guided risk management for COVID‐19 vaccine anaphylaxis. Allergy. 2022;77:2200–2210. doi: 10.1111/all.15183
Funding information
B. Eberlein received support from the company BÜHLMANN Laboratories AG outside the submitted work. This work was supported by grants from Bundesministerium für Bildung und Forschung, grant/award number: 01EA2106A project ABROGATE to K. Brockow; Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and CRC 1335 P17 and CRC 1371 P06; and bilateral funding from Luxembourg National Research Fund (FNR) project C17/BM/11656090—DFG DACH‐Lead‐Agency BI696/12‐1 to T. Biedermann. The other authors declare no external sources of funding.
Contributor Information
Knut Brockow, Email: knut.brockow@tum.de.
Tilo Biedermann, Email: tilo.biedermann@tum.de.
REFERENCES
- 1. Wenande E, Garvey LH. Immediate‐type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907‐922. [DOI] [PubMed] [Google Scholar]
- 2. Wenande E, Kroigaard M, Mosbech H, Garvey LH. Polyethylene glycols (PEG) and related structures: overlooked allergens in the perioperative setting. A A Case Rep. 2015;4:61‐64. [DOI] [PubMed] [Google Scholar]
- 3. Stone CA Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(1533–40):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine. Clin Exp Allergy. 2021;51(6):861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sellaturay P, Nasser S, Ewan P. Polyethylene glycol‐induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract. 2021;9:670‐675. [DOI] [PubMed] [Google Scholar]
- 6. Bruusgaard‐Mouritsen MA, Johansen JD, Garvey LH. Clinical manifestations and impact on daily life of allergy to polyethylene glycol (PEG) in ten patients. Clin Exp Allergy. 2021;51:463‐470. [DOI] [PubMed] [Google Scholar]
- 7. Caroli UM, Berner D, Volz T, Rocken M, Biedermann T. Delayed‐type hypersensitivity dermatitis to ethylene oxide. Contact Dermatitis. 2005;53:303‐304. [DOI] [PubMed] [Google Scholar]
- 8. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID‐19 vaccines in the US‐December 14, 2020‐January 18, 2021. JAMA. 2021;325:1101‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Team CC‐R, Food, Drug A . Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer‐BioNTech COVID‐19 Vaccine – United States, December 14‐23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNeil MM, DeStefano F. Vaccine‐associated hypersensitivity. J Allergy Clin Immunol. 2018;141:463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilsson L, Brockow K, Alm J, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628‐640. [DOI] [PubMed] [Google Scholar]
- 12. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID‐19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garvey LH, Nasser S. Anaphylaxis to the first COVID‐19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126:e106‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worm M, Bauer A, Wedi B, et al. Practical recommendations for the allergological risk assessment of the COVID‐19 vaccination – a harmonized statement of allergy centers in Germany. Allergol Select. 2021;5:72‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID‐19 vaccine‐associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paoletti G, Racca F, Piona A, et al. Successful SARS‐CoV‐2 vaccine allergy risk‐management: the experience of a large Italian University Hospital. World Allergy Organ J. 2021;14:100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blumenthal KG, Robinson LB, Camargo CA Jr, et al. Acute allergic reactions to mRNA COVID‐19 vaccines. JAMA. 2021;325:1562‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbaud A, Garvey LH, Arcolaci A, et al. Allergies and COVID‐19 vaccines: an ENDA/EAACI position paper. Allergy. 2022. 10.1111/all.15241 [DOI] [PubMed] [Google Scholar]
- 19. Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to SARS‐CoV‐2 vaccines and recommended evaluation and management: a systematic review. meta‐analysis: GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruusgaard‐Mouritsen MA, Jensen BM, Poulsen LK, Duus Johansen J, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 21. Klimek L, Bergmann KC, Brehler R, et al. Practical handling of allergic reactions to COVID‐19 vaccines: a position paper from German and Austrian allergy societies AeDA, DGAKI, GPA and OGAI. Allergo J Int. 2021;30(3):79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Worm M, Ring J, Klimek L, et al. Covid‐19 vaccination and risk of anaphylaxis – recommendations for practical management. MMW Fortschr Med. 2021;163:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonadonna P, Brockow K, Niedoszytko M, et al. COVID‐19 vaccination in mastocytosis: recommendations of the european competence network on mastocytosis (ECNM) and American initiative in mast cell diseases (AIM). J Allergy Clin Immunol Pract. 2021;9(6):2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sampson HA, Munoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391‐397. [DOI] [PubMed] [Google Scholar]
- 25. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID‐19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76:1617‐1618. [DOI] [PubMed] [Google Scholar]
- 27. Cox F, Khalib K, Conlon N. PEG that reaction: a case series of allergy to polyethylene glycol. J Clin Pharmacol. 2021;61:832‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasmussen TH, Mortz CG, Georgsen TK, Rasmussen HM, Kjaer HF, Bindslev‐Jensen C. Patients with suspected allergic reactions to COVID‐19 vaccines can be safely revaccinated after diagnostic work‐up. Clin Transl Allergy. 2021;11:e12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krantz MS, Kwah JH, Stone CA Jr, et al. Safety evaluation of the second dose of messenger RNA COVID‐19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anton Girones M, Roan Roan J, de la Hoz B, Sanchez CM. Immediate allergic reactions by polyethylene glycol 4000: two cases. Allergol Immunopathol (Madr). 2008;36:110‐112. [DOI] [PubMed] [Google Scholar]
- 31. Rojas‐Perez‐Ezquerra P, Crespo Quiros J, Tornero Molina P, Ochoa B, de Ocariz ML, Zubeldia Ortuno JM. Safety of new mRNA vaccines against COVID‐19 in severely allergic patients. J Investig Allergol Clin Immunol. 2021;31:180‐181. [DOI] [PubMed] [Google Scholar]
- 32. Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in PEG allergic patients. J Allergy Clin Immunol. 2021;148(1):91‐95. [DOI] [PubMed] [Google Scholar]
- 33. Stone BD. PEG skin testing for COVID‐19 vaccine allergy. J Allergy Clin Immunol Pract. 2021;9:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen‐based IgE testing and basophil activation test including data about cross‐reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130:155‐161. [DOI] [PubMed] [Google Scholar]
- 35. Weisser K, Kling K, Huth C, Keller‐Stanislawski B, Mahler V. Was ist bei positiver Allergieanamnese vor einer Impfung gegen COVID‐19 zu beachten? Bulletin Zur Arzneimittelsicherheit. 2021;12:23‐26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Fig S1
Table S1
Table S2
Table S3


