Abstract
Objective
Patients with autoimmune inflammatory rheumatic diseases receiving rituximab (RTX) therapy are at higher risk of poor COVID‐19 outcomes and show substantially impaired humoral immune response to anti–SARS–CoV‐2 vaccine. However, the complex relationship between antigen‐specific B cells and T cells and the level of B cell repopulation necessary to achieve anti‐vaccine responses remain largely unknown.
Methods
Antibody responses to SARS–CoV‐2 vaccines and induction of antigen‐specific B and CD4/CD8 T cell subsets were studied in 19 patients with rheumatoid arthritis (RA) or antineutrophil cytoplasmic antibody–associated vasculitis receiving RTX, 12 patients with RA receiving other therapies, and 30 healthy controls after SARS–CoV‐2 vaccination with either messenger RNA or vector‐based vaccines.
Results
A minimum of 10 B cells per microliter (0.4% of lymphocytes) in the peripheral circulation appeared to be required for RTX‐treated patients to mount seroconversion to anti‐S1 IgG upon SARS–CoV‐2 vaccination. RTX‐treated patients who lacked IgG seroconversion showed reduced receptor‐binding domain–positive B cells (P = 0.0005), a lower frequency of Tfh‐like cells (P = 0.0481), as well as fewer activated CD4 (P = 0.0036) and CD8 T cells (P = 0.0308) compared to RTX‐treated patients who achieved IgG seroconversion. Functionally relevant B cell depletion resulted in impaired interferon‐γ secretion by spike‐specific CD4 T cells (P = 0.0112, r = 0.5342). In contrast, antigen‐specific CD8 T cells were reduced in both RA patients and RTX‐treated patients, independently of IgG formation.
Conclusion
In RTX‐treated patients, a minimum of 10 B cells per microliter in the peripheral circulation is a candidate biomarker for a high likelihood of an appropriate cellular and humoral response after SARS–CoV‐2 vaccination. Mechanistically, the data emphasize the crucial role of costimulatory B cell functions for the proper induction of CD4 responses propagating vaccine‐specific B cell and plasma cell differentiation.
INTRODUCTION
Infectious diseases and associated complications are an important cause of morbidity and mortality in patients with autoimmune inflammatory rheumatic diseases (1). Increased susceptibility to infectious diseases in these patients is most likely due to an immunosuppressive effect of the disease itself and/or related to immunosuppressive treatment (2). COVID‐19, caused by SARS–CoV‐2, requires particular consideration in patients with autoimmune inflammatory rheumatic diseases. Rituximab (RTX), an anti‐CD20 monoclonal antibody leading to B cell depletion and used in autoimmune inflammatory rheumatic diseases like rheumatoid arthritis (RA) and antineutrophil cytoplasmic antibody–associated vasculitis (AAV), has been found to be a risk factor for poor COVID‐19–associated outcomes (3, 4). Since a suitable treatment for COVID‐19 has not been developed yet, vaccination is of crucial importance to protect these vulnerable patients. Meanwhile, various phase III clinical trials have demonstrated the efficacy and safety of messenger RNA (mRNA)–based vaccines (BNT162b2 [Pfizer‐BioNTech] [5,6] and mRNA‐1273 [Moderna] [7]) and viral vector–based vaccines (ChAdOx1 [AstraZeneca] [8] and Ad26.COV2.S [Johnson & Johnson] [9]) to prevent severe COVID‐19 disease or death.
In patients with autoimmune inflammatory rheumatic diseases, vaccination is generally regarded as safe and efficacious (10). However, in patients receiving B cell–depleting therapy with RTX in particular, hampered humoral and cellular responses following influenza, pneumococcal, and hepatitis B vaccination have been reported (11, 12, 13, 14, 15, 16). Available data on the SARS–CoV‐2 vaccine response in RTX‐treated patients with autoimmune inflammatory rheumatic diseases reveal substantially impaired humoral (17, 18, 19), but partly inducible cellular, immune responses (20). However, little is known about the complex mechanisms of interaction between T cells, B cells, and plasma cells, or the level of B cell repopulation necessary for proper vaccine response among RTX‐treated patients.
In this study, we investigated the characteristics of humoral and cellular antigen‐specific CD4/CD8 and B cell immune response upon SARS–CoV‐2 vaccination in patients treated with RTX compared to healthy controls and RA patients receiving other therapies.
PATIENTS AND METHODS
Study participants
Outpatients with rheumatic disease treated with RTX who received SARS–CoV‐2 vaccination according to federal and Berlin state recommendations between February and May 2021 were asked to participate in this study. We included 16 patients who had RA according to the American College of Rheumatology/European Alliance of Associations for Rheumatology 2010 classification criteria (21) and 3 patients with AAV defined as described by the Chapel Hill Consensus Conference Nomenclature (22), all of whom were receiving RTX. In addition, 12 patients with RA who were receiving other therapies and 30 healthy controls were included as control groups. All participants gave written informed consent in accordance with the approval of the ethics committee at the Charité University Hospital Berlin (EA2/010/21, EA4/188/20).
Peripheral blood samples were obtained using EDTA anticoagulant or serum tubes (BD Vacutainer System; BD Diagnostics) 6–9 days (referred to hereafter as day 7) after vaccination with either 2 doses of BNT162b2, 2 doses of ChAdOx1, or 1 dose of ChAdOx1 followed by 1 dose of BNT162b2. Serologic and B cell data for healthy controls have partially been published (23). Samples obtained 3–4 weeks after the second vaccination (referred to hereafter as day 21) were available for 19 RTX‐treated patients and 12 RA controls. One RTX‐treated patient (who had RA) had concomitant chronic lymphocytic leukemia (CLL); cellular data for this patient were excluded from the analyses of B cells, CD4 T cells, and the relationships between humoral immune responses, cell subsets, and demographic characteristics. Limited baseline T cell, B cell, and natural killer cell data were available for 16 of the 19 patients receiving RTX (2 AAV patients and 14 RA patients), 11 of the 12 RA patients, and 16 of the 30 healthy controls. Regarding the absolute numbers of B cells, CD4 cells, and CD8 cells, there was no difference between baseline and day 7 after the second vaccination (data not shown). Participant characteristics are summarized in Table 1, with more detailed information provided in Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060.
Table 1.
Characteristics of the study participants*
| Healthy controls (n = 30) | RA controls (n = 12) | RTX‐treated patients (n = 19)† | |
|---|---|---|---|
| Age, years | |||
| Median (IQR) | 57 (46.25–79.5) | 68 (63–79.5) | 58 (56.5–65) |
| No. <50 | 9 | 1 | 3 |
| No. 50–69 | 12 | 5 | 13 |
| No. >69 | 9 | 6 | 3 |
| Sex, no. female/male | 15/15 | 9/3 | 14/5 |
| Vaccine, no. | |||
| 2× BNT162b2 | 24 | 11 | 14 |
| 2× mRNA‐1273 | 0 | 0 | 1 |
| 2× ChAdOx1 | 3 | 1 | 1 |
| 1× ChAdOx1, 1× BNT162b2 | 3 | 0 | 3 |
| Immunosuppression, no. | |||
| MTX | – | 8 | 4 |
| Leflunomide | – | 1 | 0 |
| Sulfasalazine | – | 0 | 1 |
| AZA | – | 0 | 1 |
| JAK inhibitor | – | 4 | 2 |
| TNF inhibitor | – | 1 | 0 |
| Abatacept | – | 2 | 1 |
| Prednisolone‡ | – | 3 | 8 |
| DAS28, median (IQR) | – | 2.58 (1.70–3.99) | 2.65 (1.96–3.41) |
| Time since last RTX treatment, median (IQR) months | – | – | 9 (6–13.5) |
| Duration of RTX treatment, median (IQR) years | – | – | 3 (2–6) |
IQR = interquartile range; MTX = methotrexate; AZA = azathioprine; TNF = tumor necrosis factor; DAS28 = Disease Activity Score in 28 joints.
Included 16 patients with rheumatoid arthritis (RA) and 3 patients with antineutrophil cytoplasmic antibody–associated vasculitis.
The maximum prednisolone dosage was 5 mg/day in the RA group and 7.5 mg/day in the rituximab (RTX) group.
Enzyme‐linked immunosorbent assay and surrogate SARS–CoV‐2 neutralization test
The assays were performed according to the manufacturer's instructions, as previously described (23). Briefly, serum samples were diluted at 1:100 in sample buffer, pipetted onto strips of 8 single wells of a 96‐well microtiter plate, and precoated with recombinant SARS–CoV‐2 spike or nucleocapsid proteins. Calibrators, a positive control, and a negative control were carried out on each plate. After incubation for 60 minutes at 37°C, wells were washed 3 times and peroxidase‐labeled anti‐IgG or anti‐IgA antibody solution was added, followed by a second incubation step for 30 minutes. After 3 additional washing steps, substrate solution was added and the samples were incubated for 15–30 minutes in the dark. Optical density (OD) values were measured on a POLARstar Omega plate reader (BMG Labtech) at 450 nm and at 620 nm. Finally, OD ratios were calculated based on the sample and calibrator OD values. To identify individuals who had previously been infected with SARS–CoV‐2, we measured antibodies against the nucleocapsid protein (not a vaccine component) on day 7 after the second vaccination (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060).
Isolation and staining of peripheral blood mononuclear cells (PBMCs)
PBMCs were prepared by density‐gradient centrifugation using Ficoll‐Paque Plus solution (GE Healthcare Biosciences). For antigen‐specific T cell analysis, PBMCs were cryopreserved at −80°C. For surface staining, 1–3 × 106 cells were suspended in 50 μl of phosphate buffered saline (PBS)/0.5% bovine serum albumin/EDTA and 10 μl Brilliant Buffer (BD Horizon). Cells were stained for 15 minutes on ice and washed afterwards with Dulbecco's PBS containing 1% fetal calf serum (FCS; Biowest) (810g, for 8 minutes at 4°C). For intracellular staining after T cell stimulation, cells were first stained for 30 minutes with 1:1,000 BUV395 Live/Dead (Invitrogen) in PBS, followed by 5 minutes with 2.5 μl Fc Block (Miltenyi Biotech) in 50 μl resuspended cells. Cells were fixed in Lyse/Fix (Becton Dickinson), permeabilized with FACS Perm II solution (Becton Dickinson), and intracellularly stained.
Staining of antigen‐specific B cells
To identify receptor‐binding domain (RBD)–specific B cells, recombinant purified RBD (DAGC149; Creative Diagnostics) was labeled with either Alexa Fluor 647 or Alexa Fluor 488 as previously described (23). Double‐positive cells were considered antigen‐specific. A blocking experiment using unlabeled RBD at 100‐fold concentration was performed to ensure specificity of detection.
Peptide stimulation of antigen‐specific T cells
For each stimulation, 2 × 106 PBMCs obtained from 15 healthy controls, 12 RA controls, and 19 RTX‐treated patients were thawed and washed twice in prewarmed RPMI 1640 medium (containing 0.3 mg/ml glutamine, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 10% FCS, and 25 units/ml DNase I [Roche International]), rested for 1 hour in culture medium (RPMI 1640 with glutamine, antibiotics, and 10% FCS) and stimulated with SARS–CoV‐2 spike (PepMix SARS–CoV‐2 [S B.1.1.7]; JPT) (24) or T Cell TransAct (Miltenyi Biotech) in the presence of allophycocyanin (APC)–conjugated anti‐CD107a (clone H4A3; BioLegend) for 16 hours. Brefeldin A (10 μg/ml; Sigma‐Aldrich) was added after 2 hours. Due to cell number limitations, T Cell TransAct stimulation was not carried out for all participants. CD4 T cells coexpressing CD154 and CD137 were considered antigen‐specific. Spike‐specific CD8 T cells were identified based on activation‐dependent coexpression of CD137 and interferon‐γ (IFNγ) and CD107a and IFNγ, respectively. Subjects whose samples showed an increase in frequency after stimulation of at least 2‐fold compared to unstimulated control were defined as responders.
Analytical methods
All flow cytometric analyses were performed using a BD FACS Fortessa (BD Biosciences). To ensure comparable mean fluorescence intensities over the time of the analyses, Cytometer Setup and Tracking beads (BD Biosciences) and Rainbow Calibration Particles (BD Biosciences) were used. For flow cytometric analysis, the following fluorochrome‐labeled antibodies were used: BUV737‐conjugated anti‐CD11c (clone B‐ly6; BD), BUV395‐conjugated anti‐CD14 (clone M5E2; BD), BUV395‐conjugated anti‐CD3 (clone UCHT1; BD), BV786‐conjugated anti‐CD27 (clone L128; BD), BV711‐conjugated anti‐CD19 (clone SJ25C1; BD), BV605‐conjugated anti‐CD24 (clone ML5; BD), BV510‐conjugated anti‐CD10 (clone HI10A; BD), BV421‐conjugated anti‐CXCR5 (clone RF8B2; BD), phycoerythrin (PE)–Cy7–conjugated anti‐CD95 (clone APO‐1/Fas; ThermoFisher), PE‐CF594–conjugated anti‐IgD (clone IA6‐2; BioLegend), APC‐Cy7–conjugated anti‐CD38 (clone HIT2; BioLegend), PE–Cy7–conjugated anti‐IgG (clone G18‐145; BD), biotin‐conjugated anti‐IgA (clone G20‐359; BD), BV650‐conjugated anti‐IgM (clone MHM‐88; BD), fluorescein isothiocyanate (FITC)–conjugated anti‐HLA–DR (clone L234; BioLegend), PE‐conjugated anti‐CD21 (clone B‐ly4; BD), APC‐conjugated anti‐CD22 (clone S‐HCL‐1; BD), FITC‐conjugated anti–tumor necrosis factor (anti‐TNF) (clone Mab11; BioLegend), BV650‐conjugated anti‐IFNγ (clone 4S.B3; BD), BV786‐conjugated anti‐CD40L (clone 24‐31; BioLegend), and PE‐CF594–conjugated anti‐CD137 (clone 4B4‐1; BioLegend). The absolute number of B cells was measured with Trucount (BD), and samples were processed according to the manufacturer's instructions (B cells were defined as CD19+CD45+CD3−CD14−CD16−CD56− lymphocytes).
Sorting of plasmablasts, B cells, and T cells from peripheral blood for single‐cell analysis
As previously described (23), cells were enriched from peripheral blood using StraightFrom Whole Blood CD19, CD3, and CD138 MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions. Sorted populations were identified as plasmablasts (DAPI−CD3−CD14−CD16−CD38++CD27++), memory B cells (DAPI−CD3−CD14−CD16−CD38−CD27+), and activated T cells (DAPI−CD3+CD14−CD16−CD38++HLA–DR+). The 3 sorted populations were pooled and further processed for single‐cell RNA sequencing.
Single‐cell RNA library preparation and single‐cell transcriptome sequencing
Sequencing was performed on a NextSeq500 device (Illumina) using High Output v2 Kits (150 cycles) with the recommended sequencing conditions for 5′ GEX libraries and as previously described (23). In particular, transcriptome profiles were merged and normalized, variable genes were detected, and Uniform Manifold Approximation and Projection (UMAP) was performed with default parameter settings using FindVariableGenes, RunPCA, and RunUMAP with 30 principal components. Expression values are represented as ln (10,000 × UMIsGene) /UMIsTotal +1). Transcriptionally similar clusters were identified using shared nearest neighbor (SNN) modularity optimization, SNN resolutions ranging from 0.1 to 1.0 in 0.1 increments were computed, or gating was performed manually using Loupe Browser (10x Genomics). Data from transcriptome and immune profiling were merged using the same cellular barcodes.
Statistical analysis
All samples included in the final analyses had at least 1 × 106 events with a minimum threshold for CD19+ cells of 5,000 events, apart from RTX‐treated patients: minimum recorded CD19+ events in the RTX group were 16 and 45 events, in 2 different patients, of >1 million total recorded events. Flow cytometric data were analyzed by FlowJo software version 10.7.1 (TreeStar). GraphPad Prism software version 5 was used for statistical analysis. For comparison of multiple groups, two‐way analysis of variance with Šidák's post test for multiple comparisons or Kruskal‐Wallis with Dunn's post test was used. Spearman's correlation coefficient was calculated to detect possible associations between parameters or disease activity. P values less than 0.05 were considered significant. Data on cellular subsets for 1 patient receiving RTX who had concomitant CLL were excluded from the analyses of B cells, CD4 T cells, and the relationships between humoral immune responses, cell subsets, and demographic characteristics. For 1 patient receiving RTX and 6 healthy controls there was not enough material to perform fluorescence‐activated cell sorting T cell staining for activated T cells. A correlation matrix was calculated using the base R and corrplot package (R Foundation for Statistical Computing) using the Spearman method (n = 17 for the RTX group; 1 patient excluded due to concomitant CLL and 1 patient excluded due to partially missing T cell data).
Data and materials availability
All data, code, and materials used in the analysis are available at https://datadryad.org/stash/. In particular, the genetic data are available in a gene bank.
RESULTS
Cohorts and participant characteristics
This study recruited 19 patients receiving RTX (16 patients with RA and 3 patients with AAV [RTX group]), 30 healthy controls, and 12 patients with RA receiving other therapies as an additional control group (RA group). Most study participants were vaccinated twice with the mRNA vaccine BNT162b2; 1 RTX‐treated patient was vaccinated twice with mRNA‐1273. Three healthy controls, 1 RA control, and 1 RTX‐treated patient were vaccinated twice with the viral vector vaccine ChAdOx1. According to national recommendations, 3 RTX‐treated patients and 3 healthy controls received 1 dose of ChAdOx1 followed by a heterologous vaccination with 1 dose of BNT162b2.
Regarding demographic characteristics, healthy controls were age‐matched to RTX‐treated patients and were younger than RA patients. As is typical for patients with rheumatic diseases, the majority of RA patients and RTX‐treated patients were women. Disease activity at the time of first vaccination was comparable between the RA control group and RTX group. At the time of vaccination, median time since the last RTX treatment was 9 months. RTX‐treated patients had received B cell–depleting therapy on average for 3 years, and presented with a range of circulating B cell numbers of 0–484/μl blood. Demographic characteristics and additional treatments for all study participants are summarized in Table 1. (Further details on the patient cohorts are provided in Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060.)
Impaired humoral immune response upon SARS–CoV‐2 vaccination in RA patients and RTX‐treated patients
Antibody responses to SARS–CoV‐2 vaccines were assessed in all individuals on day 7 after the second vaccination. All healthy controls became positive for anti–spike S1 (anti‐S1) IgG and IgA and showed >90% SARS–CoV‐2 neutralization. Notably, IgA and IgG anti‐vaccine titers were significantly diminished on day 7 after the second vaccination in the RA control group, and especially in the RTX group, compared to the healthy control group (Figure 1A). Anti‐S1 IgG antibodies were detected in 8 (66.7%) of 12 patients in the RA group and 8 (42.1%) of 19 patients in the RTX group. Simultaneously, 5 (41.7%) of 12 patients in the RA group and 9 (47.4%) of 19 patients in the RTX group developed anti‐S1 IgA antibodies. Virus‐neutralizing antibodies were found in only 8 (66.7%) of the 12 RA control patients and 9 (47.4%) of the 19 RTX‐treated patients (Figure 1A).
Figure 1.
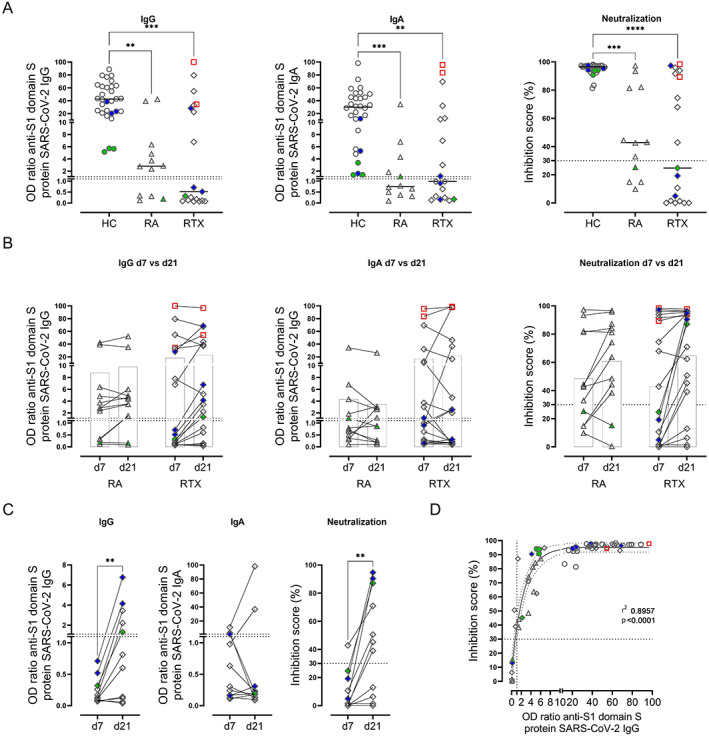
Reduced and delayed humoral immune response to SARS–CoV‐2 vaccination in patients with rheumatoid arthritis (RA) and patients treated with rituximab (RTX). A, Anti‐S1 IgG antibody titer, anti‐S1 IgA antibody titer, and inhibition score indicating antibody neutralization, determined by enzyme‐linked immunosorbent assay (ELISA) for spike protein S1 IgG, ELISA for spike protein S1 IgA, and blocking ELISA for virus neutralization, respectively, in peripheral blood samples obtained from healthy controls (HCs; n = 30), RA controls (n = 12), and RTX‐treated patients (n = 19) on day 7 after the second SARS–CoV‐2 vaccination. Symbols represent individual subjects; horizontal lines show the mean. B, Anti‐S1 IgG antibody titer, anti‐S1 IgA antibody titer, and antibody neutralization in serum samples obtained from 12 RA patients and 19 RTX‐treated patients on day 7 after (d7) and 3–4 weeks after (d21) the second vaccination. Linked symbols represent individual subjects; open bars show the mean. Two‐way analysis of variance with Šidák's post test was used for comparisons. The interaction effect was not significant. C, Delayed IgG response on day 21 in 5 of the 11 RTX‐treated patients who did not initially show seroconversion (on day 7 after the second vaccination). Linked symbols represent individual subjects. D, Significant correlation, determined by Spearman's correlation test, between IgG titers and inhibition score of antibody neutralization among RTX‐treated patients. Solid and dotted curved lines show the sigmoidal model with 95% confidence bands. Red indicates previously infected subjects; green indicates subjects who were vaccinated twice with ChAdOx1; blue indicates subjects who received 1 dose of ChAdOx1 followed by a heterologous vaccination with 1 dose of BNT162b2. Dotted lines indicate the upper limit of normal. ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001, by Kruskal‐Wallis with Dunn's post test in A, by Mann‐Whitney test in C.
Two RTX‐treated patients with unknown prior infection (identified as anti‐nucleocapsid protein positive) (Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060) developed high titers of anti‐S1 IgG, IgA, and neutralizing‐antibodies comparable with the titers in healthy controls.
Delayed serologic response in RTX‐treated patients
As previously reported (25), patients with autoimmune inflammatory rheumatic diseases may show a delayed humoral immune response after vaccination. To address this possibility, we obtained additional blood samples from patients in the RA and RTX groups 3–4 weeks (day 21) after the second vaccination (Figure 1B). Two RA patients and 5 RTX‐treated patients developed positive IgG antibodies; IgA was detected in 2 patients in the RA group and 1 patient in the RTX group 3–4 weeks after the second vaccination. Neutralizing antibodies were detected in 2 patients in the RA group and 6 patients in the RTX group at this later time point. Among the RTX‐treated patients who did not show seroconversion on day 7 after the second vaccination, there was a significant increase in IgG and neutralizing‐antibody formation at the later time point (Figure 1C). Notably, IgG titers correlated with neutralizing antibodies (r = 0.8957, P < 0.0001) (Figure 1D). Thus, and considering the delayed vaccine response, 10 (83.3%) of 12 RA patients and 13 (68.4%) of 19 RTX‐treated patients showed IgG seroconversion with neutralizing antibodies after SARS–CoV‐2 vaccination, even though at lower titers compared to those in healthy controls.
Interestingly, the data suggested that RTX‐treated patients had a potential dichotomous response: 13 of 19 RTX‐treated patients showed seroconversion to IgG (RTX IgG+), while 6 of 19 did not (RTX IgG−). To identify potential factors resulting in IgG seroconversion among RTX‐treated patients, further study addressed potential differences between the 2 groups. With regard to comedication, we found a negative correlation between prednisolone dose and IgG formation as well as B cell counts, while there was no significant relationship with the use of methotrexate (Supplementary Figures 2A–C, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060). There was no association between the Disease Activity Score in 28 joints in RA and vaccine‐induced humoral response (Supplementary Figure 2D).
Requirement of a minimum of 10 B cells per microliter in the peripheral circulation for specific IgG induction
We analyzed the B cell compartment (gating strategy shown in Figure 2A) and RBD‐specific B cells (Figure 2B) among the different groups. RTX‐treated patients presented with significantly lower relative and absolute B cell numbers compared to healthy controls and RA controls (Figure 2C). Notably, a significant difference in the frequency and absolute number of B cells was also found between IgG+ RTX‐treated patients and RTX‐treated patients who did not show seroconversion (Figure 2D). In our RTX cohort, 10 B cells per microliter in the peripheral circulation (or 0.4% of lymphocytes accordingly) was identified as the minimum needed to mount seroconversion to anti‐S1 IgG among RTX‐treated patients (Figure 2D).
Figure 2.
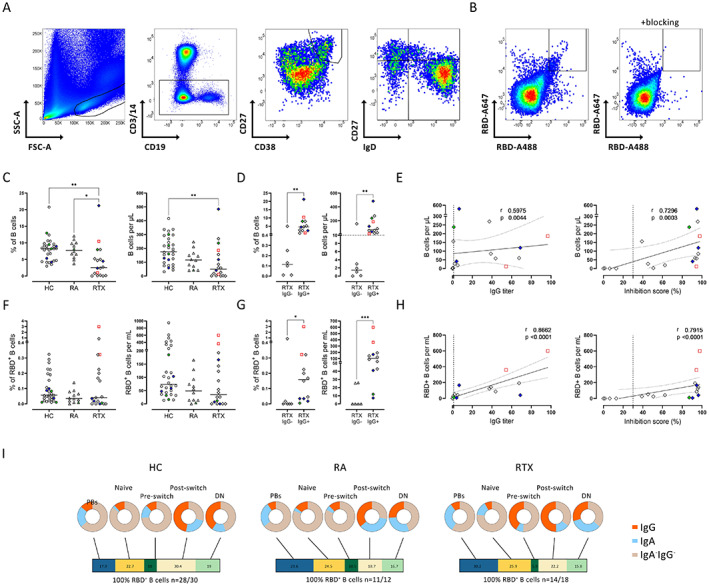
Reduction in the frequencies and numbers of total B cells and antigen‐specific B cells in RTX‐treated patients, and correlation of B cell numbers with humoral immune response. A, Representative flow cytometry plots of receptor‐binding domain (RBD)–positive B cells, plasmablasts (PBs), and non‐plasmablast B cell subsets based on IgD/CD27 classification. B, Representative flow cytometry plots of RBD+ B cells before and after blocking with unlabeled RBD. C and D, Frequency and absolute number of CD19+ B cells in healthy controls, RA controls, and RTX‐treated patients (C) and in IgG+ RTX‐treated patients compared to IgG− RTX‐treated patients (D) on day 7 after the second SARS–CoV‐2 vaccination. Dotted line in D indicates the minimum number of B cells needed to mount seroconversion to anti‐S1 IgG. E, Correlations between the number of CD19+ B cells and humoral immune response, as indicated by IgG formation and neutralizing capacity, in RTX‐treated patients. F and G, Frequency and absolute number of RBD+ cells among total CD19+ B cells in healthy controls, RA controls, and RTX‐treated patients (F) and in IgG+ RTX‐treated patients compared to IgG− RTX‐treated patients (G) on day 7 after the second vaccination. H, Correlations between the number of RBD+ B cells and humoral immune response, as indicated by IgG formation and neutralizing capacity, in RTX‐treated patients. I, Frequencies of RBD+ B cell subsets (bars) and Ig isotype distribution (pie charts) in healthy controls, RA controls, and RTX‐treated patients on day 7 after the second vaccination. DN = double negative. In C, D, F, and G, symbols represent individual subjects; horizontal lines show the mean. In E and H, vertical lines indicate the upper limit of normal; dotted lines show the 95% confidence interval. Red indicates previously infected subjects; green indicates subjects who were vaccinated twice with ChAdOx1; blue indicates subjects who received 1 dose of ChAdOx1 followed by a heterologous vaccination with 1 dose of BNT162b2. * = P < 0.05; ** = P < 0.01; *** = P < 0.001. See Figure 1 for other definitions.
In the RTX group, there was a direct relationship between B cell numbers and humoral anti‐vaccine response, as indicated by the significant correlations of the absolute number of B cells (Figure 2E) and B cell frequency (Supplementary Figure 3A, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060) with anti‐S1 IgG titers (r = 0.5975, P = 0.0044 and r = 0.6391, P = 0.0021, respectively) and the even stronger correlations of absolute number of B cells and B cell frequency with neutralizing antibodies (r = 0.7296, P = 0.0003 and r = 0.7744, P < 0.0001, respectively). These findings clearly suggest that humoral protection elicited by vaccination is dependent on the critical availability of B cells in RTX‐treated patients. In the RA and healthy control groups we did not find a significant correlation between B cell numbers and serologic response (data not shown), suggesting that the correlation between B cell numbers and IgG response is restricted to patients with B cell counts below the lower limits of normal.
Significantly lower frequencies and numbers of antigen‐specific B cells in RTX‐treated IgG nonresponders
Next, we studied SARS–CoV‐2–specific B cell responses in RTX‐treated patients, using flow cytometry to quantify RBD‐specific B cells in peripheral blood (23) (gating strategy shown in Figure 2B). While no significant difference was seen between healthy controls, RA patients, and RTX‐treated patients (Figure 2F), RTX‐treated patients who did not show seroconversion after the second vaccination (RTX IgG−) had significantly reduced frequencies and absolute numbers of RBD+ specific B cells compared to IgG+ RTX‐treated patients (Figure 2G). The number (Figure 2H) and frequency (Supplementary Figure 3A) of RBD+ B cells in RTX‐treated patients correlated significantly with the induction of IgG (r = 0.8662, P < 0.0001 and r = 0.6898, P = 0.0008 respectively) and neutralizing antibodies (r = 0.7915, P < 0.0001 and r = 0.5674, P = 0.0070, respectively).
Subsequent analyses addressed the distribution of RBD‐specific B cells among B cell subsets (5 RTX‐treated patients were excluded from the analysis due to very limited RBD+ B cell numbers, which did not permit a reliable analysis of the corresponding B cell subsets). As previously shown for healthy controls (23), RA control and RTX‐treated patients who were able to mount RBD+ B cells were also able to generate IgG+ plasmablasts upon vaccination. We found no significant difference between the groups regarding RBD+ B cell subset distribution or Ig isotypes (Figure 2I and Supplementary Figures 3B and C).
Reduced frequencies of Tfh‐like and activated CD4 and CD8 T cells in RTX‐treated IgG nonresponders
We next investigated how the dynamics of CD4/8 T cell subsets interrelate with the induction of vaccine‐specific B cells and IgG. In contrast to B cells, there was no significant difference regarding the frequency, absolute numbers, or memory formation of CD4 T cells (Supplementary Figures 4A and C, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060) and CD8 T cells (Supplementary Figures 4B and D) between healthy controls, RA controls, and RTX‐treated patients. A subsequent analysis addressed the differences between vaccine responders and nonresponders in the RTX group (representative gates shown in Figure 3A). Interestingly, patients who lacked anti‐vaccine IgG antibodies showed significantly lower frequencies of circulating Tfh‐like CD4 T cells, defined as CD4+CXCR5+PD1+, as well as of activated CD4/8 T cells coexpressing CD38+HLA–DR+ (Figure 3B). Activated CD4 T cell frequencies correlated significantly with absolute B cell numbers (r = 0.5490, P = 0.0122) (Figure 3C). These data suggest an impaired bidirectional T cell–B cell interaction in patients with gradual B cell depletion that results in insufficient vaccination‐induced humoral immunity.
Figure 3.
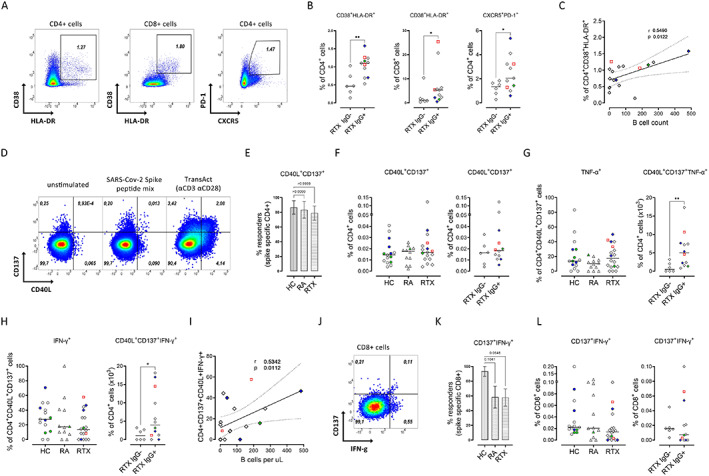
Correlation of the frequencies of activated CD4 T cells and interferon‐γ (IFNγ)–positive antigen‐specific CD4+ T cells with absolute B cell counts. A, Representative flow cytometry plots of activated CD4 and CD8 T cells and Tfh‐like CD4 T cells. Values are the percent of cells. B, Significant decrease in the frequencies of the indicated cell types in RTX‐treated patients who did not respond to SARS–CoV‐2 vaccination (RTX IgG−). C, Correlation of the frequency of activated CD4 T cells with B cell count in RTX‐treated patients. D, Representative flow cytometry plots of antigen‐specific CD4 T cells (CD137+CD40L+) in peripheral blood mononuclear cells from a healthy control, left unstimulated, stimulated with SARS–Cov‐2 spike peptide mix, or stimulated with TransAct. Values are the percent of cells. E–G, Responder rate (E) and frequency of antigen‐specific CD4 T cells (F) after stimulation with B.1.1.7 SARS–CoV‐2 spike peptide mix, and production of tumor necrosis factor (TNF)–positive (G) and IFNγ+ (H) spike‐specific CD4+ T cells in healthy controls (n = 15), RA controls (n = 12), and RTX‐treated patients (n = 18), and in IgG+ RTX‐treated patients (n = 12) and IgG− RTX‐treated patients (n = 6). I, Correlation of the frequency of IFNγ+ antigen‐specific CD4 T cells with B cell count in RTX‐treated patients. J, Representative flow cytometry plot of antigen‐specific CD8 T cells (CD137+IFNγ+). K and L, Responder rate (K) and frequency of antigen‐specific CD8+ T cells (L) after stimulation with B.1.1.7 SARS–CoV‐2 spike peptide mix. In B, F–H, and L, symbols represent individual subjects; horizontal lines show the mean. In C and I, dotted lines show the 95% confidence interval. In E and K, bars show the mean ± SD. Red indicates previously infected subjects; green indicates subjects who were vaccinated twice with ChAdOx1; blue indicates subjects who received 1 dose of ChAdOx1 followed by a heterologous vaccination with 1 dose of BNT162b2. * = P < 0.05, ** = P < 0.01. See Figure 1 for other definitions.
Impaired cytokine secretion of antigen‐specific CD4 T cells is characteristic of IgG− RTX‐treated patients and correlates with absolute B cell number
The overall occurrence of spike‐specific CD4 T cells (representative gates shown in Figure 3D and Supplementary Figure 5A, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060) in stimulated compared to unstimulated samples was found to be similar in all groups: 86.7% of healthy controls (13 of 15), 83% of RA controls (10 of 12), and 73.7% of RTX‐treated patients (14 of 19) (Figure 3E). This finding was also consistent with a comparable magnitude of response between the groups (Figure 3F) as well as similar memory subset distribution (Supplementary Figure 5E). A more detailed study of the RTX group showed that the majority of IgG+ RTX‐treated patients (10 [76.9%] of 13) versus 50% of IgG− RTX‐treated patients (3 of 6) showed an appropriate increase in antigen‐specific CD4 T cells upon stimulation. With regard to functional analyses of cytokine secretion by spike‐specific CD4 T cells, RTX‐treated patients who did not show seroconversion had significantly reduced TNF production (Figure 3G) and IFNγ production (Figure 3H) compared to IgG+ RTX‐treated responders (representative gates shown in Supplementary Figure 5B).
Since most patients in the nonseroconverted RTX group had very low circulating B cell counts, we wondered if there was a relationship between reduced B cell numbers and impaired cytokine production by antigen‐specific CD4+ T cells. Indeed, IFNγ production, but not TNF production, was significantly correlated with absolute B cell numbers (r = 0.5342, P = 0.0112 for IFNγ), suggesting the importance of B cell costimulatory functions for the proper and interactive induction of CD4 responses.
Lower antigen‐specific CD8 responses in RA patients and RTX‐treated patients
Compared to unstimulated samples, 93.3% of healthy control samples (14 of 15) but only 58.3% of RA control samples (7 of 12) and 57.9% of RTX‐treated patient samples (11 of 19) showed an increase in spike‐specific CD8 T cells coexpressing CD137 and IFNγ (representative gates shown in Figure 3J and Supplementary Figure 5C) upon stimulation (Figure 3K). To assess the degranulation function of CD8 T cells, we analyzed the coexpression of CD107a and IFNγ. The responder rate for CD8 T cells coexpressing CD107a and IFNγ after stimulation was low overall, with 60% in the healthy control group (9 of 15), 41.6% in the RA control group (5 of 12), and 42.1% in the RTX group (8 of 19) (data not shown). Regarding the amplitude of CD8 responses, the frequencies of spike‐specific CD8 T cells coexpressing CD137 and IFNγ (Figure 3L), and CD107a and IFNγ (Supplementary Figure 5D), as well as their memory subset distribution, were comparable between all groups (Supplementary Figure 5E).
Correlation of antigen‐specific and activated T cell subsets with RBD+ plasmablasts
To identify further predictive factors of IgG seroconversion in RTX‐treated patients, we constructed a correlation matrix (Figure 4) including antigen‐specific T cell and B cell subsets as well as demographic data. IgG titers and neutralizing antibodies correlated significantly with RBD+ plasmablasts and memory compartments, as we have previously shown (23). Furthermore, neutralizing antibodies correlated significantly with the frequency of activated CD38+HLA–DR+ CD4/8 T cells as well as with IFNγ‐ and TNF‐producing antigen‐specific CD4 T cells. Activated CD38+HLA–DR+ CD4/8 T cells also correlated significantly with RBD+ plasmablasts, while circulating Tfh‐like CD4 T cells correlated significantly with total RBD+ B cells. Notably, there was a significant correlation of TNF‐ and IFNγ‐producing antigen‐specific CD4 T cells with RBD+ plasmablasts and switched memory B cells. There was no significant correlation of IgG titer with age or time since the last RTX infusion.
Figure 4.
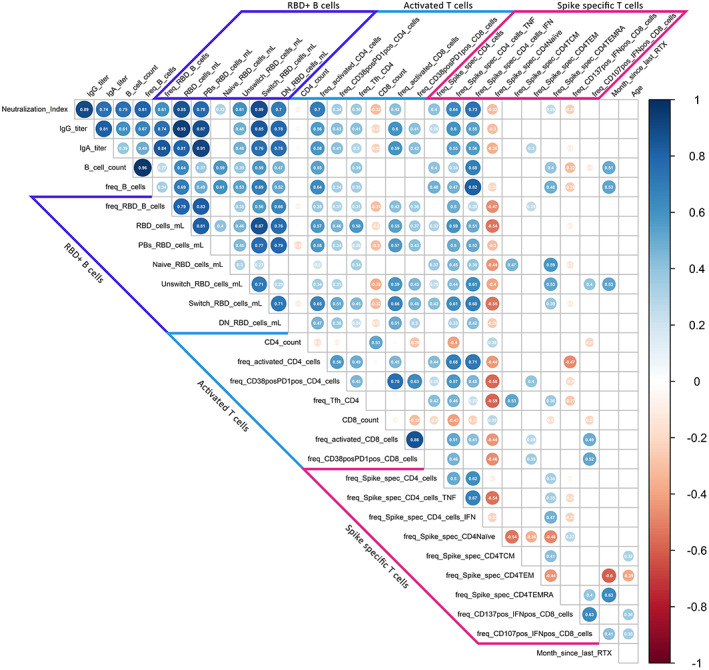
Correlation of humoral and cellular vaccine responses in rituximab (RTX)–treated patients. The Spearman's correlation matrix shows the relationships between humoral responses, receptor‐binding domain (RBD)–positive B cell subsets, activated CD4 and CD8 T cells, antigen‐specific CD4 and CD8 T cell response, month since last RTX dose, and demographic characteristics of the patients. A total of 17 RTX‐treated patients were included in the analysis (due to partial missing data for 2 patients). Red circles indicate negative correlations; blue circles indicate positive correlations. Size and color intensity indicate the strength of correlation. Values inside the circles are the correlation coefficient. Only correlations with P ≤ 0.05 are shown. PBs = plasmablasts; DN = double negative; TNF = tumor necrosis factor; IFN = interferon; TCM = central memory T cells; TEM = effector memory T cells; TEMRA = terminally differentiated effector memory T cells.
Interestingly, antigen‐specific CD8 responses induced upon stimulation did not correlate significantly with humoral immunity, or with B cell or CD4 T cell subsets, suggesting an independent, more direct antigen‐driven cellular immunity compared to the CD4–CD19 interaction required for IgG formation.
Reduced circulating follicular B cell frequency in IgG− RTX‐treated patients
To further investigate the specific differences during SARS–CoV‐2 vaccination in RTX‐treated patients, we sorted CD27++CD38++ plasmablasts, CD27+ memory B cells, and HLA–DR+CD38+ activated T cells as indicators of the ongoing adaptive immune response after vaccination. The cytometrically enriched cells were subsequently analyzed using Drop‐Seq single‐cell RNA sequencing (23). Unsupervised analysis using UMAP for Dimension Reduction identified 15 distinct clusters: 4 B cell clusters, 3 plasmablast clusters, and 8 clusters of activated T cells (Figures 5A–C). Here, clusters 3 and 5 were of particular interest: cluster 3 is enriched with circulating follicular‐like B cells expressing CXCR5 and CCR6 and cluster 5 contains CD40LG, PDCD1, and ICOS expressing Tfh‐like CD4 T cells. Follicular B cells and CD40LG+PD1+ Tfh cells were substantially reduced in RTX‐treated patients, with the reduction most pronounced among IgG nonresponders in the RTX group (Figure 5D and Supplementary Figures 6 and 7, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42060).
Figure 5.

Diminished frequencies of follicular T and B cells in RTX‐treated patients, determined by single‐cell transcriptome and Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE‐seq) analyses. A, Uniform Manifold Approximation and Projection (UMAP) clustering of peripheral blood CD27++CD38++ plasmablasts, CD27+ memory B cells, and T cells. Samples from 2 healthy controls (healthy donors [HDs]), 4 RA controls, and 5 RTX‐treated patients (3 responders [R] and 2 nonresponders [NR] to SARS–Cov‐2 vaccine) were isolated and sorted by fluorescence‐activated cell sorting for single‐cell sequencing. B, Relative expression levels of selected signature genes in the 15 identified clusters (total number of cells sequenced 38,038). Larger circles indicate higher expression. C, UMAP clustering of cells in samples from 2 healthy controls, 4 RA controls, 2 IgG− RTX‐treated patients (RTX nonresponders), and 3 IgG+ RTX‐treated patients (RTX responders) (top) and cluster frequency comparison for clusters 3 and 5 (bottom). Symbols represent individual subjects; bars show the mean. D, UMAP representation of the expression levels of CCR6, CXCR5, CD40LG, and PDCD1 in healthy controls, RA controls, and RTX‐treated nonresponders and responders. See Figure 1 for other definitions. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42060/abstract.
DISCUSSION
SARS–CoV‐2 vaccines have been approved based on their protection against COVID‐19 in clinical trials (5, 6, 7, 8). However, certain patient groups receiving immunosuppressive therapies appear to develop insufficient humoral and cellular responses (18, 23, 26, 27), but limited data about the underlying impairments are available. Protection through immunization is achieved by an orchestrated immune response between different cellular subsets of innate immunity (antigen‐presenting cells) and adaptive immunity, such as B cells and T cells. Anti–B cell therapies such as anti‐CD20 antibodies (RTX, obinutuzumab, and ocrelizumab) and inhibitors of Bruton's tyrosine kinase are associated with poor humoral immune response to SARS–CoV‐2 vaccination in patients with autoimmune inflammatory rheumatic diseases (17, 18, 19, 20), multiple sclerosis (28), and CLL (29). Since B cell depletion enhances the risks of poor COVID‐19 outcomes (3), but also can reduce anti–SARS–CoV‐2 vaccine responses, it is of utmost importance to delineate the level of B cell repopulation necessary to achieve anti‐vaccine responses and get insights into the complex relationship between antigen‐specific B cells and T cells.
Therefore, we aimed to investigate humoral and cellular responses in RTX‐treated patients versus controls. Consistent with previous data (17, 18, 19, 25), serologic IgG conversion with formation of neutralizing antibodies was significantly lower and delayed in both RA controls and RTX‐treated patients, with an even more pronounced effect in the RTX cohort, compared to healthy controls. This finding was closely linked to the availability of peripheral B cells, activated CD4/8 T cells, as well as circulating Tfh‐like cells. Another risk factor identified for developing a substantially diminished vaccination response was the prednisolone dose. Ongoing antigen exposure through mRNA vaccines seems to permit prolonged germinal center (GC) maturation (30), which might be an explanation for the further increase in antibody titers over an additional period in some patients.
Besides the IgG nonresponders in the RTX group, 2 patients in the RA group with normal B cell numbers did not develop anti‐S1 IgG antibodies. After completing the analysis, it appears that the underlying cause is most likely related to impaired T cell responses: in 1 patient due to inhibition of costimulation by abatacept, consistent with a prior report (18). The other patient, who was treated with a JAK inhibitor, lacked cytokine production by antigen‐specific T cells after receiving 2 doses of the ChAdOx1 vaccine. Even though significantly lower IgG responses were reported after 2 doses of ChAdOx1 in healthy individuals (31), it remains to be delineated whether the treatment and/or selected vaccine may account for this finding. Induction of vaccine‐specific IgG in individuals vaccinated with ChAdOx1/BNT162b2 was comparable with that in individuals vaccinated with 2 doses of BNT162b2. Interestingly, IgA formation was comparable across all groups, although the protective potency of IgA remains to be determined.
Of utmost importance, our RTX cohort showed a correlation between IgG seroconversion, neutralizing antibodies, and absolute B cell number. A minimum of 10 B cells per microliter in the peripheral circulation is a candidate biomarker for a high likelihood of an appropriate cellular and humoral vaccination response. Patients with B cell numbers below this range not only presented with lower antigen‐specific B cells, but they also showed substantially diminished circulating Tfh‐like CD4 T cells, reduced activated CD4/8 T cells coexpressing CD38 and HLA–DR, as well as impaired IFNγ secretion of spike‐specific CD4 T cells. The frequency of IFNγ‐secreting antigen‐specific CD4 T cells also correlated with the absolute number of B cells, suggesting that these cells interact to achieve proper anti‐S1 responses. Mechanistically, the current data suggest the critical role of available costimulatory B cell functions for the induction of proper CD4 Th response. This finding is consistent with observations of previously described impaired B cell–T cell crosstalk in RTX‐treated patients (32, 33, 34), leading to reduced frequencies of activated T cells (34), down‐regulation of CD40L in CD4 T cells (32, 33), and reduced antigen‐specific CD8 T cells after influenza vaccination (14).
With regard to the induction of antigen‐specific CD8 T cells upon stimulation, the RTX and RA groups both showed a tendency toward reduced responder rates compared to the healthy control group, although it was not statistically significant. However, other than for antigen‐specific CD4 T cells, neither B cell depletion nor IgG formation correlated with spike‐specific CD8 T cells, suggesting that their induction occurred independently upon SARS–CoV‐2 vaccination. It is not clear how these vaccine‐specific CD8 T cells provide antiviral protection on clinical grounds.
The debate about what correlates with protection after vaccination against SARS–CoV‐2 is ongoing, while it is widely accepted that neutralizing antibodies are a reliable surrogate of protection against virus variants (35, 36). The threshold for protective SARS–CoV‐2 IgG titer is still unknown, although non‐human primate studies suggest that it is likely already very effective at low titers (37). Our study provides evidence that detection of RBD‐specific B cells and spike‐specific CD4 T cells may provide cellular correlates of this response, while the CD8 response occurred in an independent manner. The role of these 2 lines of vaccine response needs to be further delineated.
Limitations of this study are the small number of RA and RTX‐treated patients and the heterogeneity of the groups (including different disease‐modifying antirheumatic drug [DMARD] regimens and different vaccination strategies). In this regard, Mrak et al (38) analyzed the impact of comedication on vaccination response in 74 RTX‐treated patients and did not observe differences in the levels of antibodies in the presence or absence of concomitant treatment with conventional synthetic DMARDs (csDMARDs) or prednisolone. This finding suggests that the impact of RTX on B cells is more relevant than the effect of csDMARDs.
Here, we present a first study investigating humoral as well as antigen‐specific T cell and B cell responses in RTX‐treated patients after SARS–CoV‐2 vaccination. Mechanistically, the data provide insights into the crucial role of available B cells equipped with proper costimulatory function to interactively cross‐talk with CD4 T cells. These functions likely result in GC formation, plasma cell differentiation, and vaccine‐specific IgG production. As a clinical consequence, we propose a range of absolute B cell numbers signifying expansion of vaccine responses after RTX treatment, which may support optimization of vaccination protocols among this vulnerable patient group.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Dörner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Stefanski, Rincon‐Arevalo, E. Schrezenmeier, Radbruch, Lino, Dörner.
Acquisition of data
Stefanski, Karberg, Ritter, Jahrsdörfer, H. Schrezenmeier, Ludwig, Chen, Claußnitzer, Haibel, Proft.
Analysis and interpretation of data
Stefanski, Rincon‐Arevalo, Szelinski, Jahrsdörfer, H. Schrezenmeier, Sattler, Kotsch, Guerra, Durek, Heinrich, Ferreira‐Gomes, Burmester, Mashreghi, Lino, Dörner.
Supporting information
Disclosure Form
Supplementary Table S1 Individually described patients of RA and RTX group.
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We kindly thank all study participants for taking part and all team members of the rheumatology outpatient office RheumaPraxis Steglitz, Berlin (Drs. Kirsten Karberg and Henning Christian Brandt) as well as of AGZ Rheumatology Charité Mitte Berlin (Dr. Anne Claußnitzer), for organization of patient schedules.
Dr. Stefanski's work was supported by the German Society of Rheumatology. Mr. Rincon‐Arevalo's work was supported by a COLCIENCIAS scholarship (award 727, 2015). Dr. E. Schrezenmeier's work was supported by the Federal Ministry of Education and Research (grant BCOVIT, 01KI20161), and the Berlin Institute of Health (BIH)–Charité Clinician Scientist Program funded by the Charité Universitätsmedizin Berlin and the BIH. Mr. Ritter's work was supported by an MD scholarship from the BIH. Dr. H. Schrezenmeier's work was supported by the Ministry for Science, Research, and Arts of Baden‐Württemberg, Germany (CORE‐Project) and the European Commission (award HORIZON2020 Project SUPPORT‐E, no. 101015756). Dr. Chen's work was supported by a state scholarship fund organized by the China Scholarship Council. Dr. Mashreghi's work was supported by the state of Berlin and the European Regional Development Fund (award ERDF 2014–2020, EFRE 1.8/11, DRFZ), the BIH Starting Grant Multi‐Omics Characterization of SARS‐CoV‐2 infection (Project 6, Identifying immunological targets in Covid‐19), and the Senate of Berlin (Modulation of the mucosal immune response in order to prevent severe COVID‐19). Dr. Dörner's work was supported by the German Research Foundation (TRR 130/project 24, Do491/7‐5, and Do 491/10‐1).
Dr. Stefanski and Mr. Rincon‐Arevalo contributed equally to this work.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42060&file=art42060‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Hsu CY, Ko CH, Wang JL, Hsu TC, Lin CY. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther 2019;21:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furer V, Rondaan C, Heijstek M, van Assen S, Bijl M, Agmon‐Levin N, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open 2019;5:e001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson‐Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones JM, Faruqi AJ, Sullivan JK, Calabrese C, Calabrese LH. COVID‐19 outcomes in patients undergoing B cell depletion therapy and those with humoral immunodeficiency states: a scoping review. Pathog Immun 2021;6:76–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furer V, Rondaan C, Heijstek MW, Agmon‐Levin N, van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:39–52. [DOI] [PubMed] [Google Scholar]
- 11. Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti‐tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta‐analysis. Arthritis Care Res (Hoboken) 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 12. Huang Y, Wang H, Tam WW. Is rheumatoid arthritis associated with reduced immunogenicity of the influenza vaccination? A systematic review and meta‐analysis. Curr Med Res Opin 2017;33:1901–8. [DOI] [PubMed] [Google Scholar]
- 13. Bingham CO III, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010;62:64–74. [DOI] [PubMed] [Google Scholar]
- 14. Graalmann T, Borst K, Manchanda H, Vaas L, Bruhn M, Graalmann L, et al. B cell depletion impairs vaccination‐induced CD8+ T cell responses in a type I interferon‐dependent manner. Ann Rheum Dis 2021;80:1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013;122:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richi P, Alonso O, Martín MD, González‐Hombrado L, Navío T, Salido M, et al. Evaluation of the immune response to hepatitis B vaccine in patients on biological therapy: results of the RIER cohort study. Clin Rheumatol 2020;39:2751–6. [DOI] [PubMed] [Google Scholar]
- 17. Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS‐CoV‐2 vaccination in rituximab‐treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis 2021;80:1355–6. [DOI] [PubMed] [Google Scholar]
- 18. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. LB0003 immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and general population: a multicenter study. Ann Rheum Dis 2021;80 Suppl:200–1. [DOI] [PubMed] [Google Scholar]
- 19. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El‐Qunni AA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA Vaccines to SARS‐CoV‐2 [preprint]. medRxiv 2021.
- 20. Westhoff TH, Seibert FS, Anft M, Blazquez‐Navarro A, Skrzypczyk S, Doevelaar A, et al. Correspondence on ‘SARS‐CoV‐2 vaccination in rituximab‐treated patients: evidence for impaired humoral but inducible cellular immune response.’ Ann Rheum Dis 2021;80:e162. [DOI] [PubMed] [Google Scholar]
- 21. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 22. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 23. Rincon‐Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, et al. Impaired humoral immunity to SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 2021;6:eabj1031. [DOI] [PubMed] [Google Scholar]
- 24. Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub‐Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS‐CoV2 BNT162b2 (tozinameran) prime‐boost vaccination in kidney transplant recipients. J Clin Invest 2021;131:e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simon D, Tascilar K, Fagni F, Krönke G, Kleyer A, Meder C, et al. SARS‐CoV‐2 vaccination responses in untreated, conventionally treated and anticytokine‐treated patients with immune‐mediated inflammatory diseases. Ann Rheum Dis 2021. 80:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID‐19 vaccine in immune‐mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med 2021;27:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner JS, O'Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS‐CoV‐2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021;596:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Shaw R, Stuart A, Greenland M, Dinesh T, Provstgaard‐Morys S, et al. Safety and immunogenicity report from the Com‐COV study: a single‐blind randomised non‐inferiority trial comparing heterologous and homologous prime‐boost schedules with an adenoviral vectored and mRNA COVID‐19 vaccine. Lancet 2021;398:856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Antonopoulos I, Daoussis D, Lalioti ME, Markatseli TE, Drosos AA, Taraviras S, et al. B cell depletion treatment decreases CD4+IL4+ and CD4+CD40L+ T cells in patients with systemic sclerosis. Rheumatol Int 2019;39:1889–98. [DOI] [PubMed] [Google Scholar]
- 33. Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down‐regulation of the T cell costimulatory molecule CD40 ligand: an open‐label trial. Arthritis Rheum 2005;52:501–13. [DOI] [PubMed] [Google Scholar]
- 34. Ramwadhdoebe TH, van Baarsen LG, Boumans MJ, Bruijnen ST, Safy M, Berger FH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019;58:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 36. Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJ, Simmons R, et al. SARS‐CoV‐2 infection rates of antibody‐positive compared with antibody‐negative health‐care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature 2021;590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mrak D, Tobudic S, Koblischke M, Graninger M, Radner H, Sieghart D, et al. SARS‐CoV‐2 vaccination in rituximab‐treated patients: B cells promote humoral immune responses in the presence of T‐cell‐mediated immunity. Ann Rheum Dis 2021;80:1345–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Table S1 Individually described patients of RA and RTX group.
Appendix S1: Supporting Information


