To the editor:
Vaccination against SARS‐CoV‐2 with BNT162b2 mRNA vaccine has undoubtedly proven to be extremely important for public health. Although BNT162b2 is quite effective against COVID‐19, there is a time‐dependent decrease in neutralizing antibodies (NAbs). Even 1 month after the second BNT162b2 injection, a slight decrease in antibody titers was observed, and the time since the second vaccine dose was associated with lower neutralizing antibody activity against SARS‐CoV‐2 variants and weaker protection against COVID‐19, especially for variants like delta.1, 2 As a result, many public health agencies around the world are now recommending a third dose (booster), particularly after the appearance of variants of interest of SARS‐CoV‐2, such as delta and omicron. The aim of this study was to investigate the increase in neutralizing antibodies and anti‐SARS‐CoV‐2 spike receptor‐binding domain (anti‐SRBD) IgGs in health professionals, 1 month after vaccination with the third dose of BNT162b2 mRNA vaccine. The possible role of gender and age was further investigated.
The participants were health workers from Alexandra General Hospital in Athens, Greece, who participated in a prospective study (NCT04743388) that evaluates the efficacy of vaccination for the prevention of COVID‐19. Major inclusion criteria for participation in this study included: (i) age above 18 years; (II) ability to sign the informed consent form; and (iii) eligibility for vaccination, according to the national program for COVID‐19 vaccination. Major exclusion criteria included the presence of: (i) autoimmune disorder under immunosuppressive therapy; (ii) active malignant disease; and (iii) end‐stage renal disease.
Anti‐spike‐RBD IgG antibodies and NAbs against SARS‐CoV‐2 were measured using FDA approved methods, that is, the Elecsys Anti‐SARS‐CoV‐2 S assay (Roche Diagnostics GmbH, Mannheim, Germany) and the cPass™ SARS‐CoV‐2 NAbs Detection Kit (GenScript, Piscataway, NJ, USA), respectively, as previously described. 1
Time points for blood collection and serum isolation were day 1 (D1; first BNT162b2 dose), D22 (before the second dose), and then 2 weeks, 1, 3, 6, and 9 months after the second dose and 1 month after the booster BNT162b2 dose (1MP3D). The booster dose was administered within 1 week after the blood sampling of the 9‐month time point after the second dose. That was the time (October 2021) that the booster dose was approved for health employees in Greece. After vein puncture, serum was separated within 4 h from blood collection and stored at −80°C until the day of measurement.
Demographic data, comorbidities, and medications taken were obtained from patients after a personal interview. Body mass index (BMI) was calculated from each individual's weight and height data. Based on BMI, subjects were divided into groups: underweight with a BMI of less than 18.4; normal weight with a BMI of 18.5–24.9; overweight with a BMI of 25–29.9; and obese with a BMI of 30 or more. Participants' data were also divided into three age groups (to obtain an approximately equal number of participants), 20–40, 40–55, and ≥55 years, and the role of age in the immune response after the booster was explored.
Statistical analysis started with descriptive metrics such as mean, median, quartiles, and estimation of dispersion metrics. A normality test was performed before statistical comparison between two or more groups. To determine the normality of the data distribution, the Kolmogorov–Smirnov and Shapiro–Wilk tests and QQ plots were used. According to these tests, if the nominal normality hypothesis is rejected, the data are considered not to follow the normal distribution. It was found that the data deviated from the normal distribution in all situations of this study. Therefore, nonparametric approaches were used for the following statistical analysis. The Mann–Whitney U test was used to compare two independent groups, for example, to examine the gender effect or the influence of age groups (<50 and ≥50 years). The Wilcoxon signed‐rank test was used for pairwise group comparisons, such as neutralizing antibody levels between two occasions. For the simultaneous comparison of many groups (e.g., age groups), the Kruskal–Wallis test was utilized. The significance level was set at 5% in all cases in this study, and a result was considered significant if the calculated p value (p) was below the significance level. Python v.3.9.2 was used for statistical analysis.
The study was approved by the respective Ethical Committee of Alexandra Hospital, in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. All participants provided informed consent before entering the study.
This paper reports the results of the first 150 (57M/93F) consecutive health professionals who received the booster vaccine dose out of 308 health workers, who initially entered the study and had received two vaccine doses. The median age of all individuals was 49.6 years, whereas the median age of men was 54 years, and the median age of women was 49 years. Overall, the median BMI was 25.9 for the overall sample, with male subjects having a BMI of 27 and female subjects having a BMI of 24.
Figure 1A depicts the percent inhibition of NAbs across the time period from 2 weeks after the second immunization to 1 month after the third dose (1MP3D). One month after the booster dose, the median inhibition percentage was 97.6% (mean 95.9%), which was considerably greater than any other time point tested (Wilcoxon p value <0.001). Only one person (0.67%) had NAbs that were less than 50%, whereas the vast majority (148 subjects, or 98.7%) had very high levels of protection. Therefore, it can be clearly stated that the booster dose causes a burst in neutralizing antibody titers when this increase in NAbs levels is contrasted with the levels observed at earlier time periods. Prior to the booster, the highest levels of NAbs are recorded 2 weeks following the second vaccination, and it is at this point that a consistent decline in NAbs becomes apparent, indicating that the vaccine is working. The median NAbs levels were 96.9% 2 weeks after the second immunization, and they subsequently fell to 96.1% and 55.5% 1 and 9 months later, respectively, after the second vaccination. When compared with the previously considered maximum levels (i.e., 2 weeks after the second vaccination), the NAbs levels at 1MP3D were 151% higher, which is plausible given that the injection triggers the development of anti‐spike antibodies. High NAbs were described in convalescent COVID‐19 patients after one vaccination dose, 2 due to a rapid and strong cytokine induction the day after their vaccine dose. 3 This is possibly the reason for high NAbs after the booster dose. Similarly, the booster dose increases the NAbs titers even in patients with myeloma who did not respond to the two first vaccine doses. 4
FIGURE 1.
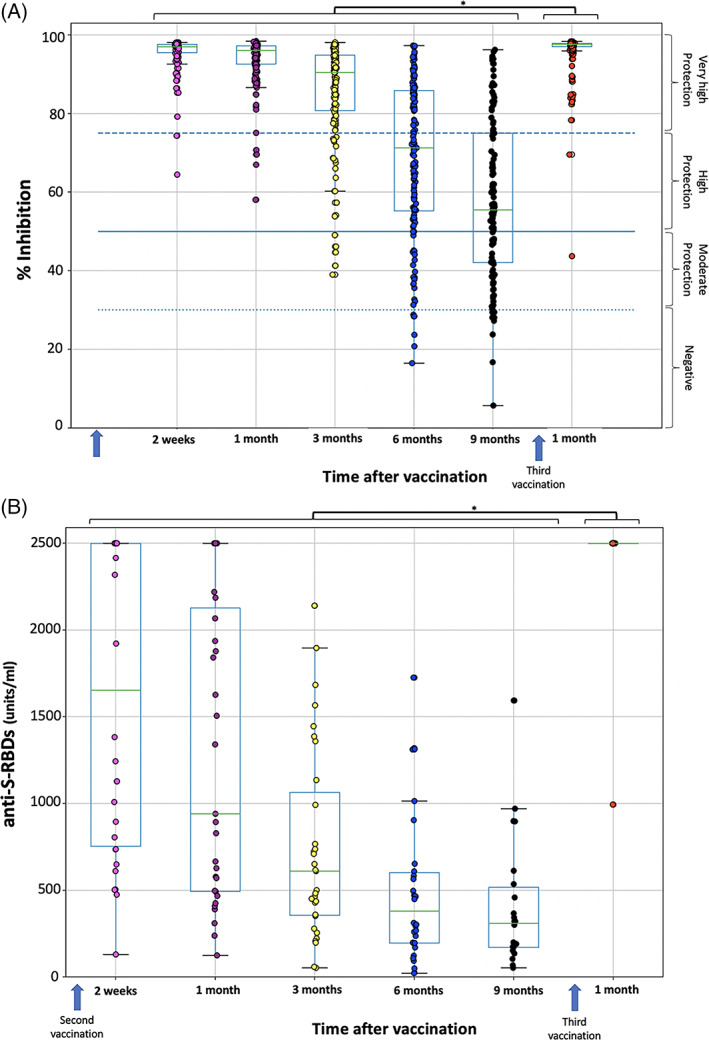
SARS‐CoV‐2 neutralizing antibodies (% inhibition) (A) and anti‐SRBD levels (B) after second and third vaccination with BNT162b2 mRNA vaccine. Antibodies were measured 2 weeks, 1, 3, 6 and 9 months after the second vaccination and 1 month after the booster dose. Asterisks (*) indicate statistically significant differences (p value <.05) between the compared groups. The boundaries of the boxplot refer to the quartiles of the distribution, while the overlaid points represent the individual NAbs or anti‐SRBD values. The dashed lines (in A) refer to the limits of inhibition, that is, 30%, 50%, and 75%
Anti‐SRBD antibodies had similar findings (Figure 1B), with antibody levels reaching the upper limit of 2500 units/mL in 149 out of 150 participants. Anti‐SRBD titers were statistically significantly greater at 1MP3D than at any prior measurement point (p value <.001), which was consistent with previous findings. A huge rise in anti‐SRBDs of 804% was observed 1 month following the third immunization, when compared with baseline levels before the booster dose (i.e., at 9 months after the second dose). A recent study also described the high anti‐S IgG antibodies in 97 Israeli individuals above the age of 65. 5
In addition, the impact of a variety of additional parameters on NAbs and anti‐SRBD titers has been examined. Several factors were examined to determine if they could influence antibody levels on each day or the reported increase in immune response as a result of the booster dosage, including age, gender, medical history (i.e., comorbidities), and BMI. For either NAbs or anti‐SRBDs, no differences were identified between males and females; the Mann–Whitney p values for NAbs and anti‐SRBDs were .803 and .670, respectively, for NAbs and anti‐SRBDs. Furthermore, when the participants were divided into three age groups, no statistically significant differences were found for either NAbs (Kruskal–Wallis p value = .230) or anti‐SRBDs (p = .779) antibodies.
A limitation of this study was the relatively small sample size, which may make it impossible to investigate specific pathophysiological situations. In addition, patients with severe concomitant diseases, such as cancer, were excluded from participation in the current study. The use of active immunosuppressive drugs has been shown to contribute significantly to poor humoral response after COVID‐19 vaccination, but such patients were not included in this study. Regarding the role of gender, it should be emphasized that unequal sample sizes were available for men and women, which should be considered. Women are almost two times more represented in the workforce than men. Although unequal sample sizes in general can lead to biased results, this is not a problem in our situation, as the imbalance is not extreme but rather normal (33.8% versus 66.2%).
We conclude that a third vaccine dose, 9 months post‐full vaccination with the BNT162b2 vaccine, produces very high NAbs and anti‐SRBD IgG titers, irrespective of age and gender. This is possibly responsible for the lower COVID‐19 rates in those who receive a third vaccine dose, 6 or for the milder COVID‐19 symptoms to those who are infected with omicron variant.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENTS
We thank Mrs Ioanna Charitaki, RN; Mrs Zoi Evangelakou, MSc; Mrs Despoina D Gianniou, MSc; Mrs Sentiljana Gumeni, PhD; Mrs Nikoletta‐Aikaterini Kokkali, RN; Mrs Christine‐Ivy Liacos, PhD; Mrs Maria S Manola, MSc; Mrs Nefeli Mavrianou, PhD; Mrs Eleni‐Dimitra Papanagnou, PhD; Mr Dimitrios Patseas, PhD; Mrs Stamatia Skourti, PhD; for administrative, technical, or material support. We also thank Roche Diagnostics GmbH (Germany) and IEMBITHEK (Greece) for partially funding this study, as well as all of the study participants for donating their time and samples.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Terpos E, Trougakos IP, Apostolakou F, et al. Age‐dependent and gender‐dependent antibody responses against SARS‐CoV‐2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96(7):E257–E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosati M, Terpos E, Agarwal M, et al. Distinct neutralization profile of spike variants by antibodies induced upon SARS‐CoV‐2 infection or vaccination. Am J Hematol. 2022;97(1):E3–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergamaschi C, Terpos E, Rosati M, et al. Systemic IL‐15, IFN‐γ, and IP‐10/CXCL10 signature associated with effective immune response to SARS‐CoV‐2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021;36(6):109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eliakim‐Raz N, Leibovici‐Weisman Y, Stemmer A, et al. Antibody titers before and after a third dose of the SARS‐CoV‐2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terpos E, Gavriatopoulou M, Ntanasis‐Stathopoulos I, et al. Booster BNT162b2 optimizes SARS‐CoV‐2 humoral response in myeloma patients; the negative effect of anti‐BCMA therapy. Blood. Published online January 05, 2022. doi: 10.1182/blood.2021014989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl. J Med. 2021;385(15):1393‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


