Abstract
Objective
The relative risk of SARS–CoV‐2 infection and COVID‐19 disease severity among people with rheumatic and musculoskeletal diseases (RMDs) compared to those without RMDs is unclear. This study was undertaken to quantify the risk of SARS–CoV‐2 infection in those with RMDs and describe clinical outcomes of COVID‐19 in these patients.
Methods
We conducted a systematic literature review using 14 databases from January 1, 2019 to February 13, 2021. We included observational studies and experimental trials in RMD patients that described comparative rates of SARS–CoV‐2 infection, hospitalization, oxygen supplementation/intensive care unit (ICU) admission/mechanical ventilation, or death attributed to COVID‐19. Methodologic quality was evaluated using the Joanna Briggs Institute critical appraisal tools or the Newcastle‐Ottawa scale. Risk ratios (RRs) and odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated, as applicable for each outcome, using the Mantel‐Haenszel formula with random effects models.
Results
Of the 5,799 abstracts screened, 100 studies met the criteria for inclusion in the systematic review, and 54 of 100 had a low risk of bias. Among the studies included in the meta‐analyses, we identified an increased prevalence of SARS–CoV‐2 infection in patients with an RMD (RR 1.53 [95% CI 1.16–2.01]) compared to the general population. The odds of hospitalization, ICU admission, and mechanical ventilation were similar in patients with and those without an RMD, whereas the mortality rate was increased in patients with RMDs (OR 1.74 [95% CI 1.08–2.80]). In a smaller number of studies, the adjusted risk of outcomes related to COVID‐19 was assessed, and the results varied; some studies demonstrated an increased risk while other studies showed no difference in risk in patients with an RMD compared to those without an RMD.
Conclusion
Patients with RMDs have higher rates of SARS–CoV‐2 infection and an increased mortality rate.
INTRODUCTION
The SARS–CoV‐2 pandemic has resulted in unprecedented morbidity and mortality due to COVID‐19. In the general population, risk factors associated with poor COVID‐19 outcomes include older age, sex, and chronic diseases (1, 2).
Patients with rheumatic diseases may be at an increased risk of infection as a result of underlying disease, associated comorbidities, and use of potentially immunosuppressive treatments (3). Furthermore, concern exists regarding whether individuals with rheumatic diseases potentially experience more severe COVID‐19 disease and poorer outcomes. However, 1 year after the first cases of COVID‐19 were described, the applicability of this heuristic to SARS–CoV‐2 infection, and the magnitude of any such heightened risk in these patients, remains unclear. Data directly addressing these questions are limited and lack clarity because of the rapid publication of many small studies during the pandemic, and these studies are frequently underpowered to show clinically significant effects.
The present systematic review and meta‐analysis are aimed at quantifying the risk of contracting SARS–CoV‐2 infection and describing COVID‐19 outcomes in patients with rheumatic and musculoskeletal diseases (RMDs).
PATIENTS AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement for reporting was used in this study (4). The study protocol was registered with PROSPERO a priori (no. CRD42020205668).
Data sources and literature search
A systematic search of the literature was conducted by a medical librarian (AAG and a second librarian) in the BioRxiv, China National Knowledge Infrastructure, Cochrane Library, Disaster Lit, Global Health, Google Scholar, LitCovid, medRxiv, Ovid Embase, Ovid Medline, PubMed, Scopus, Wanfang Data, and Web of Science Core Collection databases to find relevant articles published from January 1, 2019 to February 13, 2021. Databases were searched using a combination of controlled vocabulary and free‐text terms for COVID‐19 and rheumatic diseases. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (5). Details of the full search strategy are listed in Supplementary Table 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Details regarding included RMDs are shown in Supplementary Table 2 (http://onlinelibrary.wiley.com/doi/10.1002/art.42030). The bibliographies of included studies were reviewed to identify additional relevant literature.
Study selection eligibility criteria
Citations from all databases were imported into an EndNote x9 Library (Clarivate Analytics), where duplicates were removed. The unduplicated results were imported into Covidence v2627 for screening and data extraction. Two independent screeners reviewed titles and abstracts, and a third screener resolved disagreements. The full texts of the collected articles were then reviewed for inclusion by 2 independent screeners with a third screener to resolve disagreements.
Observational studies and experimental trials were eligible for inclusion if data regarding adult and/or pediatric patients with rheumatic diseases were reported and SARS–CoV‐2 infection or the subsequent clinical course were included as outcomes. Publications were excluded if they did not include quantifiable data regarding patients with rheumatic diseases, did not include original primary data, did not focus on human subjects, did not report data regarding outcomes related to SARS–CoV‐2 infection or its associated clinical course, included duplicate or retracted data, were case reports or series, or were not yet published as a full‐text study. There were no restrictions regarding language, and individuals fluent in a particular foreign language reviewed the articles in that language. Outcomes of interest included SARS–CoV‐2 infection and COVID‐19 outcomes (hospitalization, oxygen supplementation, intensive care unit [ICU] admission, mechanical ventilation, or death).
Data collection process
Two reviewers independently extracted data using Qualtrics software version Oct–May 2020–2021. A third reviewer assessed the forms to resolve any conflicts. Extracted data included study characteristics (first author, year of publication, country of origin, study design, sample size, and study sponsor), baseline demographic and clinical characteristics of the participants (age, sex, race or ethnicity, and comorbidities [each comorbidity individually reported as well as grouped into categories of diabetes, respiratory, cardiovascular, smoking, or other]), SARS–CoV‐2 infection status, and COVID‐19 outcomes (hospitalization, oxygen supplementation, ICU admission, mechanical ventilation, or death).
Risk of bias in individual studies
Two reviewers independently assessed the risk of bias using the Joanna Briggs Institute (JBI) checklists for prevalence and analytical cross‐sectional studies (3, 4, 5) and the Newcastle‐Ottawa scale for case–control and cohort studies (6, 7, 8). The JBI checklists for prevalence and cross‐sectional analytical studies are divided into 3 categories for assessment of bias (for prevalence studies, scores 0–3 = high risk of bias, scores 4–6 = some concerns, and scores 7–9 = low risk of bias; for cross‐sectional analytical studies, scores 0–3 = high risk of bias, scores 4–6 = some concerns, and scores 7–8 = low risk of bias). In this context, the comparability domain of the Newcastle‐Ottawa scale was primarily used to differentiate risk of bias and was used to determine the global risk of bias (global risk of bias on a scale of 0–2, where score 0 = high risk of bias, score 1 = some concerns, and score 2 = low risk of bias) (9, 10). Disagreements were resolved by a third reviewer.
Data analysis
Studies included in the systematic review were further evaluated for their suitability in the meta‐analyses if comparative data were reported for patients with and those without RMDs. Regarding COVID‐19 prevalence, studies were included if the number of COVID‐19 cases among patients in the RMD group was reported, and if the number of COVID‐19 cases within the overall regional populations was reported. Regarding hospitalization, ICU admission, mechanical ventilation, and death, studies were included if they provided raw data demonstrating the rate of each outcome among patients with RMDs who were clinically diagnosed as having COVID‐19 as well as the rate of each outcome among a comparator group of patients without RMDs who were clinically diagnosed as having COVID‐19. Data were insufficient to meta‐analyze the risk of oxygen supplementation. Studies were excluded if the non‐RMD comparator group was selected from a nonrepresentative sample, such as those with similar diseases (e.g., inflammatory bowel disease patients) or family members.
Meta‐analyses were performed using R version 4.1.0 and the meta package version 4.18‐1. Risk estimates were calculated using the Mantel‐Haenszel formula with random effects models. Risk estimates with 95% confidence intervals (95% CIs) are reported as risk ratios (RRs) for prevalence of COVID‐19, and as odds ratios (ORs) for risk of hospitalization, oxygen supplementation, ICU admission, mechanical ventilation, or death. Heterogeneity among studies was assessed using I2 and Cochran's chi‐square tests. We performed sensitivity analyses to evaluate the effect of the study design (limited to cohort studies or cross‐sectional studies), risk of bias (limited to studies with a low risk of bias), country of origin, and study size (limited to studies with >20 RMD patients). Funnel plots and Egger's regression tests were used to evaluate publication bias. P values less than 0.05 were considered significant.
RESULTS
The literature search resulted in identification of 13,076 articles; after duplicates were removed, 5,799 articles remained for title/abstract screening. We undertook a full text review of 534 articles (Figure 1). Of these, 98 articles met the inclusion criteria. Two additional studies were identified via review of the bibliographies of included articles, resulting in the inclusion of 100 studies (see Supplementary Table 3 and Supplementary References 1–100, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Studies were excluded due to having an incorrect study design, not having original data, having irrelevant outcomes, examining an incorrect study population, being duplicates, including irrelevant exposures, being conference abstracts, and containing duplicate study data (Supplementary Table 4, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). The majority of single region/country studies were from Europe (63%), with the rest from Asia (14%), North America (13%), worldwide (6%), or South America (1%). The majority (75%) focused on adult populations; however, 14% of studies included pediatric and adult populations, and 1% included only pediatric populations. In 10% of studies, the age range was unspecified.
Figure 1.
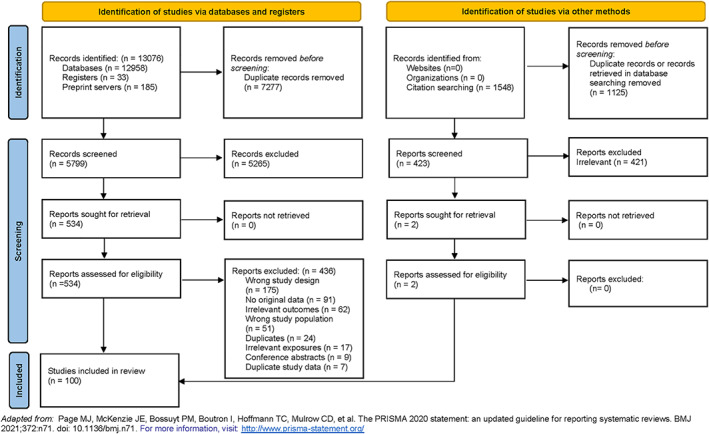
Flow chart of the methods used for identification of studies in patients with rheumatic and musculoskeletal diseases in which comparative rates of SARS–CoV‐2 infection, hospitalization, oxygen supplementation, intensive care unit admission, mechanical ventilation, and death attributed to COVID‐19 are reported. The flow chart is designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42030/abstract.
Risk‐of‐bias assessment
Overall, the majority of studies had a low risk of bias. Of the 4 studies assessed using the Newcastle‐Ottawa scale for case–control studies, 2 had a low risk of bias, 1 had some concerns, and 1 had a high risk of bias (Supplementary Table 5, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Of the 46 studies assessed using the Newcastle‐Ottawa scale for cohort studies, 33 had a low risk of bias, 6 had some concerns, and 7 had a high risk of bias (Supplementary Table 6, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Of the 37 studies assessed using the JBI checklist for prevalence studies, 25 had a low risk of bias, 8 had some concerns, and 4 had a high risk of bias (Supplementary Table 7, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Of the 13 studies assessed using the JBI checklist for analytical cross‐sectional studies, 8 had a low risk of bias, 2 had some concerns, and 3 had a high risk of bias (Supplementary Table 7).
SARS–CoV‐2 infection
In 46 studies, comparative rates of SARS–CoV‐2 infection in patients with RMDs were reported (Supplementary Table 8, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). A total of 15 studies showed increased rates of SARS–CoV‐2 infection, 27 showed no difference, and 4 showed decreased rates. A total of 23 studies met the inclusion criteria for the meta‐analysis (Supplementary Table 9, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). The pooled relative risk based on unadjusted data demonstrated an increased risk of COVID‐19 infection among patients with RMDs (RR 1.53 [95% CI 1.16–2.01]) (Figure 2). We observed moderately high heterogeneity (I2 = 73% [95% CI 59–82%]; P < 0.01) but did not detect evidence of publication bias (Supplementary Figure 1, http://onlinelibrary.wiley.com/doi/10.1002/art.42030).
Figure 2.
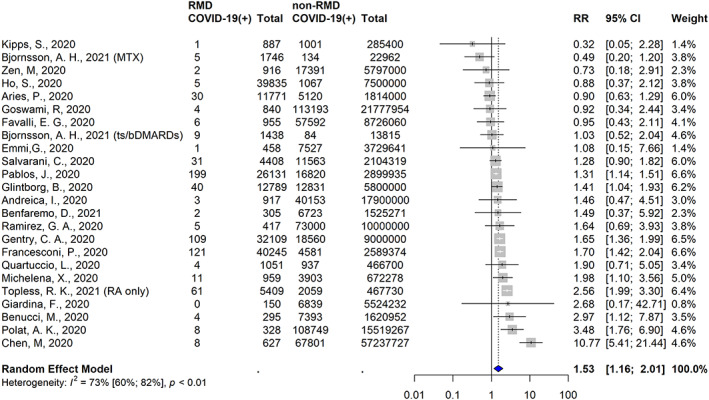
Studies identified in the systematic literature review and meta‐analysis in which the risk of COVID‐19 among populations of patients with rheumatic and musculoskeletal diseases (RMDs) compared to those without RMDs was reported. The risk of COVID‐19 is assessed as risk ratios (RRs) with 95% confidence intervals (95% CIs). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42030/abstract.
In 8 studies, adjusted comparative risk measures were reported, with 2 outcomes reported in 2 of these studies. The 5 studies that demonstrated an increased risk of COVID‐19 among patients with RMDs were the study by Pablos et al (OR 1.3 [95% CI 1.15–1.52]) (14), the study by Zhong et al (OR 2.68 [95% CI 1.14–6.27]) (13), the study by Francesconi et al in patients with rheumatoid arthritis (RA) (OR 1.64 [95% CI 1.32–2.05]) (12), the study by Topless et al in patients with RA (OR 1.34 [95% CI 1.02–1.77]) (18), and the study by Chen et al in patients with RA (OR 10.90 [95% CI 5.43–21.89]) (11). The 5 studies that showed no difference in risk of COVID‐19 were the study by Topless et al in patients with gout (OR 1.01 [95% CI 0.83–1.23]) (18), the study by Jung et al (OR 1.13 [95% CI 0.57–2.24]) (15), the study by Francesconi et al in patients with connective tissue disease (CTD) (OR 1.09 [95% CI 0.72–1.66]) (12), the study by Salvarani et al (OR 0.94 [95% CI 0.66–1.34]) (17), and the study by Kipps et al (RR 0.32 [95% CI 0.05–2.28]) (16). Unless specified otherwise, risk in patients with multiple RMDs was reported as a combined group.
Hospitalization
In 70 studies, hospitalization rates were reported among patients with RMD who were clinically diagnosed as having COVID‐19 and/or who were diagnosed as having COVID‐19 by polymerase chain reaction (PCR) testing (Supplementary Table 10, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Among these, 11 studies compared hospitalization rates among patients with RMDs to those among the general population (n = 2) or those among other non‐RMD comparator populations (n = 9) (Supplementary Table 11 http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Three studies showed an increased risk of hospitalization among patients with RMDs and 7 showed no significant effect. None of the 70 studies demonstrated a decreased risk of hospitalization among patients with RMDs. In a meta‐analysis of 10 comparative studies that included unadjusted hospitalization rates, the risk of hospitalization was not increased among patients with RMDs when compared to non‐RMD comparators (OR 1.25 [95% CI 0.68–2.31]) (Figure 3A).
Figure 3.
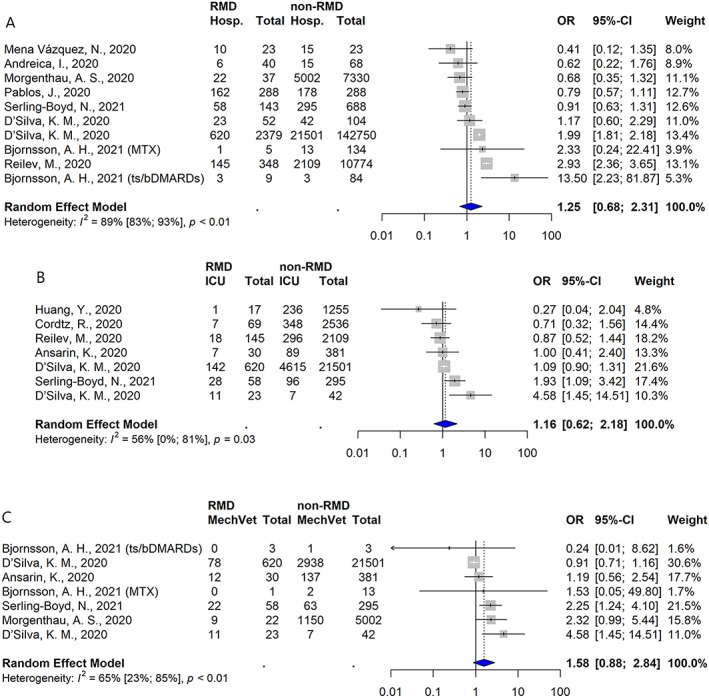
Studies showing the likelihood of hospitalization (Hosp.) (A), intensive care unit (ICU) admission (B), and mechanical ventilation (MechVet) (C) following the development of COVID‐19 among populations of patients with RMDs relative to those without RMDs. Values are the odds ratios (ORs) with 95% CIs. See Figure 2 for other definitions. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42030/abstract.
Among the 5 studies in which adjusted risk estimates were reported, 3 of the studies included patients with clinical symptom–based or PCR‐confirmed COVID‐19 diagnoses, whereas 2 included patients whose COVID‐19 diagnosis was confirmed by PCR only. Data from 2 studies demonstrated an increased risk of hospitalization: the study by Cordtz et al (adjusted HR 1.46 [95% CI 1.15–1.86]) (19) and the study by Reilev et al (adjusted OR 1.5 [95% CI 1.1–1.9]) (20). D'Silva et al reported an increased risk of hospitalization in 1 matched model (RR 1.14 [95% CI 1.03–1.26]) and a neutral risk in an extended matched model (RR 1.06 [0.96–1.17]) (21). Finally, in 2 studies, no significant difference in risk of hospitalization was reported: in the study by Serling‐Boyd et al, adjusted HR 0.87 (95% CI 0.68–1.11) (23); in and the study by D'Silva et al, adjusted OR 1.27 (95% CI 0.61–2.64) in model 1, adjusted OR 1.22 (95% CI 0.56–2.63) in model 2, and adjusted OR 1.10 (95% CI 0.51–2.38) in model 3 (22).
Oxygen supplementation, ICU, and mechanical ventilation
Sixty‐two studies included the proportion of patients requiring new oxygen supplementation (n = 28), ICU admission (n = 52), and mechanical ventilation (n = 42) during hospitalization for COVID‐19. In these studies, the diagnosis of COVID‐19 was based on clinical symptoms and/or based on the results of PCR testing (for the list of studies, see Supplementary Table 12, http://onlinelibrary.wiley.com/doi/10.1002/art.42030).
In terms of the risk of oxygen supplementation, in 3 studies, comparative findings in patients with and those without an RMD were reported (Supplementary Table 11, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). One of these studies showed an increased risk of oxygen supplementation among patients with RMDs, and the other 2 studies showed no significant difference between the groups. No studies included adjusted analyses.
Regarding ICU admission, 11 studies compared the rates between patients with and those without RMDs (Supplementary Table 11, http://onlinelibrary.wiley.com/doi/10.1002/art.42030), with 2 studies showing evidence of an increased risk among patients with RMDs and the rest showing no statistically significant difference. Adjusted risk estimates were reported in 2 studies, including the study by Serling‐Boyd et al (adjusted HR 1.27 [95% CI 0.86–1.86]) (23), which demonstrated no effect, and the study by D'Silva et al (22), in which 3 adjusted models were evaluated, all demonstrating a positive association between RMD status and ICU admission/mechanical ventilation rates (in model 1, adjusted OR 3.26 [95% CI 1.17–9.09]; in model 2, adjusted OR 3.11 [95% CI 1.07–9.05]; in model 3, adjusted OR 2.92 [95% CI 1.002–8.49]).
Finally, 8 studies compared the rates of mechanical ventilation between patients with and those without RMDs (Supplementary Table 11, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Seven studies showed no significant difference based on RMD status, including the study by Serling‐Boyd et al, in which an adjusted HR of 1.51 (95% CI 0.93–2.44) was reported (23). However, D'Silva et al reported that the risk of ICU admission/mechanical ventilation was significantly increased in patients with RMDs, as shown in unadjusted models (OR 3.22 [95% CI 1.16–8.92]) and in adjusted models (as described above) (22).
Pooled risk estimates included those for the reported comparative ICU admission rates in 7 studies, and those for the mechanical ventilation rates in another 7 studies (all determined in unadjusted models), as shown in Figures 3B and C. Overall, the risks of ICU admission or mechanical ventilation were not significantly different between patients with and those without RMDs.
Mortality rate
Mortality rates were reported in 71 studies (Supplementary Table 13, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Of these, in 16 studies, mortality rates were reported in RMD patents in comparison to the general population (n = 7) or non‐RMD comparator populations (n = 9) (Supplementary Table 14, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). For RMD patients, in 5 studies an increased risk of death was reported, in 9 a neutral effect of death was reported, and in 2 a decreased risk of death was reported. A meta‐analysis of 13 studies that included comparative mortality rates showed an unadjusted OR of 1.74 (95% CI 1.08–2.80) for the risk of death in those with RMDs (Figure 4A). Moderately high heterogeneity was observed (I2 = 83% [95% CI 71–89%]), but we did not detect evidence of publication bias (Supplementary Figure 2, http://onlinelibrary.wiley.com/doi/10.1002/art.42030). Among 6 studies focused solely on hospitalized patients, the unadjusted OR for the risk of death among hospitalized RMD patients was 1.26 (95% CI 1.03–1.53) (Figure 4B).
Figure 4.
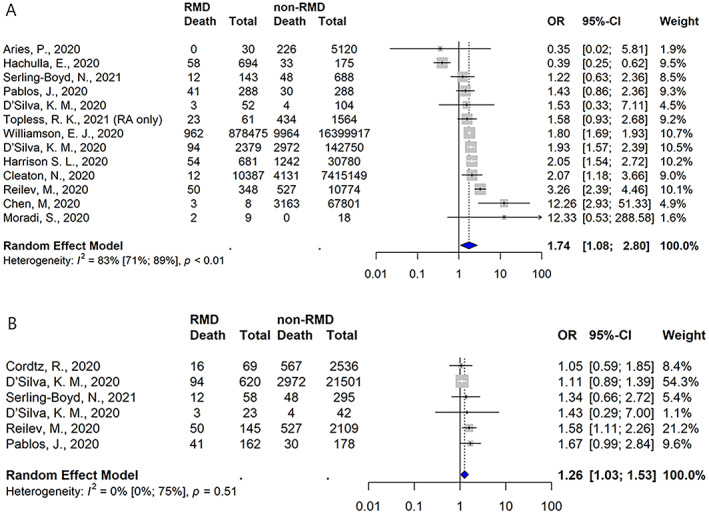
Studies showing the likelihood of death occurring following the development of COVID‐19 among populations of patients with RMDs relative to those without RMDs overall (A) and among populations limited to hospitalized patients only (B). Values are the odds ratios (ORs) with 95% CIs. See Figure 2 for other definitions. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42030/abstract.
Adjusted risk estimates for the risk of death were reported in 7 studies. Compared to the general population, the adjusted estimates for the risk death in different studies were as follows: in the study by Williamson et al, HR 1.19 (95% CI 1.11–1.27) in patients with RA/systemic lupus erythematosus/psoriasis (24); in the study by Topless et al, OR 1.9 (95% CI 1.2–3.0) in patients with RA, and OR 1.2 (95% CI 0.9–1.7) in patients with gout (18); and in the study by Reilev et al, OR 0.9 (95% CI 0.6–1.3) in patients with RA/CTD (20). In studies assessing the risk of death in RMD patients compared to non‐RMD comparators, D'Silva et al reported an RR of 1.08 (95% CI 0.81–1.44) (21), Harrison et al reported an OR of 1.17 (95% CI 0.85–1.60) (25), and Serling‐Boyd et al reported an HR of 1.02 (95% CI 0.53–1.95) (23), while in the French RMD COVID‐19 cohort (FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE Consortium), an OR of 1.45 (95% CI 0.87–2.42) was reported (26).
Sensitivity analyses
Our sensitivity analyses demonstrated overall stability in terms of the pooled estimates for each of the outcomes when we limited the samples based on study design (limited to cohort or cross‐sectional studies), risk of bias (limited to studies with a low risk of bias), country of study (excluding Italy, which had a disproportionate number of studies in which the prevalence of SARS–CoV2 infection was reported), or study size (limited to studies with >20 patients diagnosed as having an RMD only). Not unexpectedly, we observed that 95% CIs widened as sample sizes decreased (Supplementary Figures 3–10, http://onlinelibrary.wiley.com/doi/10.1002/art.42030).
DISCUSSION
In this study, we conducted a systematic review and meta‐analysis to quantify the risk of COVID‐19 and COVID‐19 outcomes in patients with RMDs. In an unadjusted meta‐analysis, the relative risk of developing SARS–CoV‐2 infection was 52% higher in patients with RMDs compared to the general population. Compared to patients without an RMD, those with RMDs were also at a higher risk of having a poor outcome following COVID‐19 infection, with a 74% increase in risk of death. Other measures of severity, including rates of hospitalization, rates of oxygen supplementation, rates of ICU admission, and rates of mechanical ventilation, were not significantly higher among patients with RMDs versus non‐RMD comparators.
Our study focused on RMDs as a combined group, which limits our ability to extrapolate our findings to any individual patient with an RMD. This group was composed of patients with many different diseases that have different organ manifestations, severity, and treatments. Prior studies have shown differences in COVID‐19 outcomes in specific rheumatic diseases (27, 28). Some RMDs (e.g., gout) may be associated with increased prevalence of general COVID‐19 risk factors, such as cardiovascular disease, but none of the studies included in our meta‐analysis included patients with gout (29). Findings from previous studies have also suggested a differential effect of baseline use of rheumatic disease medications on COVID‐19 outcomes (30, 31). Other factors, such as age, sex, comorbidities, and disease activity, have also been shown to influence COVID‐19 outcomes in patients with RMDs (31, 32). Due to the heterogeneity of the study designs, it was not possible to statistically combine the results of the included studies to generate additional pooled estimates of the overall influence of these risk factors on COVID‐19 outcomes.
The discrepancy between the observed increased risk of COVID‐19 infection and associated mortality rate without a corresponding increased risk of hospitalization, ICU admission, and mechanical ventilation may appear surprising. However, these findings may be related to the overall power to detect differences given the smaller number of studies in which these outcomes were reported, which may be more difficult to systematically assess. Our pooled analysis, focusing only on studies of hospitalized patients (Figure 4B), allowed for comparison between RMD and non‐RMD groups of subjects whose characteristics may be more similar in terms of risk factor profile (e.g., age, presence of multiple comorbidities) than might be observed between an RMD population and a general population comparator group, and we still found a significantly increased risk of death. However, it is important to take the smaller number of studies and smaller effect size into consideration.
This is not the first systematic literature review and meta‐analysis assessing outcomes in patients with immune‐mediated diseases. Wang et al performed a meta‐analysis of 14 studies assessing RMD patients diagnosed as having COVID‐19 that were published through October 2020; their findings showed that RMD patients had a 53% increased risk of SARS–CoV‐2 infection with no increased risk of death or other markers of poor outcomes (33). Xu et al conducted a meta‐analysis of 31 studies of COVID‐19 in rheumatic disease patients published through August 2020 to evaluate the comparative RRs across regions of the world, but comparison to a non‐RMD group was not included (34). Akiyama et al performed a meta‐analysis of 62 studies in patients with autoimmune diseases and COVID‐19 published through July 2020; however, this study included a more heterogeneous group of autoimmune diseases, such as inflammatory bowel disease and multiple sclerosis, which may have different outcomes compared to RMDs (35). Their findings demonstrated an increased risk of SARS–CoV‐2 infection in patients with RMDs but no increase in the frequency of severe outcomes in those with autoimmune diseases. Interpretation of the results of these previously conducted meta‐analyses is limited by the low number of included studies and patients, as evidenced by the generally wide confidence intervals for the reported risk estimates.
Applying these results in clinical care is complex, but these findings suggest that patients with RMDs are at an increased risk of developing SARS–CoV‐2 infection and severe COVID‐19 compared to the general population. The reasons for this lie outside the scope of the present study, but 3 plausible explanations should be considered. First, bias resulting from greater baseline contact with the health care system or a lower threshold for seeking care when a patient becomes symptomatic could falsely inflate the rate of COVID‐19 among patients with RMDs. Second, patients with RMDs may have a greater burden of comorbidities that are typically associated with worse outcomes. Finally, it may be that immune dysregulation related to RMD treatments or to the RMDs themselves may result in higher rates of symptomatic infections and severe outcomes (21, 30, 36). All 3 of these explanations may account for prior observations that higher RMD disease activity is associated with worse outcomes in COVID‐19, since these patients are more likely to be identified, are more likely to have comorbidities, and are more likely to have immune dysregulation or to be receiving immunosuppressive therapies. Regardless of the cause, patients with RMDs should be encouraged to be vaccinated against SARS–CoV‐2 and should be encouraged to employ risk mitigation strategies as much as possible (37, 38).
Our study has considerable strengths. We comprehensively identified potential studies from 14 databases through February 2021, making it the most current literature review and meta‐analysis of COVID‐19 in RMDs. We assembled a geographically diverse study team, enabling the inclusion of studies in all available languages. This is particularly relevant in COVID‐19 because it exhibits wide regional variation in outcomes (39). To ensure reliability of the literature search and data extraction process, these tasks were performed manually; machine learning methods are being developed to streamline this process, and these approaches have potential strengths but remain exploratory at this time (40). Despite these strengths, our study had several limitations. The studies we included are significantly heterogenous in design and reporting, as evidenced by the formal testing of heterogeneity performed in the meta‐analysis. The study protocol was created a priori; the increased volume of relevant articles rapidly published during the COVID‐19 pandemic resulted in an amendment to the protocol to exclude case reports and case series. COVID‐19 outcomes have changed and generally improved over time, which may limit comparability between cohorts assembled at different periods during the pandemic (41, 42). Due to the small number of studies that include adjusted RRs, our meta‐analysis was limited to the analysis of unadjusted numbers. However, we have presented the complementary adjusted RRs from those individual studies in which adjusted RRs were included. Interpretation of the unadjusted RRs is complicated by the potential imbalance of other risk factors between RMD patients and general populations.
In conclusion, we performed the most comprehensive systematic literature review and meta‐analysis assessing COVID‐19 outcomes in patients with RMDs to date. Our findings show that patients with RMDs have higher rates of SARS–CoV‐2 infection and death from COVID‐19 in unadjusted analyses. This may be mediated by factors other than the RMD itself.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Conway had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Conway, Grimshaw, Konig, Putman, Duarte‐García, Liew, Grainger, Wallace, Hsieh.
Acquisition of data
Conway, Grimshaw, Konig, Putman, Duarte‐García, Tseng, Cabrera, Chock, Degirmenci, Duff, Egeli, Graef, Gupta, Harkins, Hoyer, Jayatilleke, Jin, Kasia, Khilnani, Kilian, Kim, Lin, Low, Proulx, Sattui, Singh, Sparks, Tam, Ugarte‐Gil, Ung, Wise, Yang, Young, Liew, Grainger, Wallace, Hsieh.
Analysis and interpretation of data
Conway, Grimshaw, Konig, Putman, Duarte‐García, Wang, Liew, Grainger, Wallace, Hsieh.
Supporting information
Disclosure Form
Appendix S1: Supplementary Information
ACKNOWLEDGMENT
We would like to thank all members of the COVID‐19 Global Rheumatology Alliance. Open access funding was provided by IReL.
PROSPERO registry no.: CRD42020205668.
The views expressed here are those of the authors and participating members of the COVID‐19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology (ACR), the European Alliance of Associations for Rheumatology, the UK NHS, the NIHR, the UK Department of Health, or any other organization.
Supported by the ACR. The COVID‐19 Global Rheumatology Alliance is supported by Amgen, Janssen, AbbVie, Gilead, Novartis, UCB Pharma, Pfizer, GlaxoSmithKline, and Bristol‐Myers Squibb.
Dr. Conway and Ms. Grimshaw contributed equally to this work.
Request for access to data should be made to the Data Access and Sharing Committee of the COVID‐19 Global Rheumatology Alliance.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42030&file=art42030‐sup‐0001‐Disclosureform.pdf.
Contributor Information
Richard Conway, Email: drrichardconway@gmail.com.
Evelyn Hsieh, Email: evelyn.hsieh@yale.edu.
REFERENCES
- 1. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 4. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 6. Wells G, Shea B, O'Connell D, Robertson J, Peterson J, Welch V. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. URL: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf.
- 7. Moola S. Systematic reviews of etiology and risk. In: Joanna Briggs institute reviewer's manual. Adelaide: The Joanna Briggs Institute; 2017. URL: https://jbi‐global‐wiki.refined.site/space/MANUAL/3283910762/Chapter+7%3A+Systematic+reviews+of+etiology+and+risk. [Google Scholar]
- 8. Monteiro F, Canavarro MC, Pereira M. Prevalence and correlates of psychological distress of middle‐aged and older women living with HIV. Psychol Health Med 2017;22:1105–17. [DOI] [PubMed] [Google Scholar]
- 9. Viswanathan M, Patnode CD, Berkman ND, Bass EB, Chang S, Hartling L, et al. Recommendations for assessing the risk of bias in systematic reviews of health‐care interventions. J Clin Epidemiol 2018;97:26–34. [DOI] [PubMed] [Google Scholar]
- 10. Putman M, Chock YP, Tam H, Kim AH, Sattui SE, Berenbaum F, et al. Antirheumatic disease therapies for the treatment of COVID‐19: a systematic review and meta‐analysis. Arthritis Rheumatol 2021;73:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen M, Wei Y, Zhang Q, Wan Q, Chen X. Epidemiology and clinical characteristics of COVID‐19 in rheumatic diseases at a tertiary care hospital in Wuhan, China. Clin Exp Rheumatol 2021;39:442–3. [PubMed] [Google Scholar]
- 12. Francesconi P, Cantini F, Profili F, Mannoni A, Bellini B, Benucci M. COVID‐19 epidemiology in rheumatic diseases in Tuscany: a case‐control study. Joint Bone Spine 2021;88:105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong J, Shen G, Yang H, Huang A, Chen X, Dong L, et al. COVID‐19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol 2020;2:e557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pablos JL, Abasolo L, Alvaro‐Gracia JM, Blanco FJ, Blanco R, Castrejon I, et al. Prevalence of hospital PCR‐confirmed COVID‐19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 2020;79:1170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung SY, Kim MS, Kim MC, Choi SH, Chung JW, Choi ST. Effect of hydroxychloroquine pre‐exposure on infection with SARS‐CoV‐2 in rheumatic disease patients: a population‐based cohort study. Clin Microbiol Infect 2020;27:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kipps S, Paul A, Vasireddy S. Incidence of COVID‐19 in patients with rheumatic disease: is prior health education more important than shielding advice during the pandemic? Clinical Rheumatol 2021;40:1575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salvarani C, Mancuso P, Gradellini F, Viani N, Pandolfi P, Reta M, et al. Susceptibility to COVID‐19 in patients treated with antimalarials: a population based study in Emilia‐Romagna, Northern Italy. Arthritis Rheumatol 2021;73:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topless RK, Phipps‐Green A, Leask M, Dalbeth N, Stamp LK, Robinson PC, et al. Gout, rheumatoid arthritis, and the risk of death related to coronavirus disease 2019: an analysis of the UK Biobank. ACR Open Rheumatol 2021;3:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cordtz R, Lindhardsen J, Soussi BG, Vela J, Uhrenholt L, Westermann R, et al. Incidence and severeness of COVID‐19 hospitalisation in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2020;28:SI159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reilev M, Kristensen KB, Pottegård A, Lund LC, Hallas J, Ernst MT, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT‐PCR test for SARS‐CoV‐2 in Denmark: a nationwide cohort. Int J Epidemiol 2020;49:1468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Silva KM, Jorge A, Cohen A, McCormick N, Zhang Y, Wallace ZS, et al. COVID‐19 outcomes in patients with Systemic Autoimmune Rheumatic Diseases (SARDs) compared to the general population: a US multi‐center comparative cohort study. Arthritis Rheumatol 2021;73:914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Silva KM, Serling‐Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID‐19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis 2020;79:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serling‐Boyd N, D'Silva KM, Hsu TYT, Wallwork R, Fu X, Gravallese EM, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2020;80:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrison SL, Fazio‐Eynullayeva E, Lane DA, Underhill P, Lip GY. Comorbidities associated with mortality in 31,461 adults with COVID‐19 in the United States: a federated electronic medical record analysis. PLoS Medicine 2020;17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Florence A, Nassim AA, Jean‐David A, Didier A, Yannick A, Blanca AB, et al. Severity of COVID‐19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID‐19 cohort of 694 patients. Ann Rheum Dis 2020;80:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conway R, Nikiphorou E, Demetriou C, Low C, Leamy K, Ryan J, et al. POS1162 predictors of hospitalisation in patients with rheumatic disease and COVID‐19 in Ireland: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80 suppl:859–60.33568387 [Google Scholar]
- 28. Pablos JL, Galindo M, Carmona L, Lledo A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID‐19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020;79:1544–9. [DOI] [PubMed] [Google Scholar]
- 29. Dalbeth N, Robinson PC. Patients with gout: an under‐recognised group at high risk of COVID‐19. Lancet Rheumatol 2021;3:e317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sparks JA, Wallace ZS, Seet AM, Gianfrancesco MA, Izadi Z, Hyrich KL, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID‐19 severity in rheumatoid arthritis: results from the COVID‐19 Global Rheumatology Alliance physician registry. Ann Rheum Dis 2021;80:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson‐Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hasseli R, Mueller‐Ladner U, Hoyer BF, Krause A, Lorenz HM, Pfeil A, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID‐19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open 2021;7:e001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Liu J, Shao R, Han X, Su C, Lu W. Risk and clinical outcomes of COVID‐19 in patients with rheumatic diseases compared with the general population: a systematic review and meta‐analysis. Rheumatol Int 2021;41:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu C, Yi Z, Cai R, Chen R, Thong BY, Mu R. Clinical outcomes of COVID‐19 in patients with rheumatic diseases: a systematic review and meta‐analysis of global data. Autoimmun Rev 2021;20:102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID‐19 in patients with autoimmune diseases: a systematic review and meta‐analysis. Ann Rheum Dis 2020. DOI: 10.1136/annrheumdis-2020-218946. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N Engl J Med 2021;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smolen JS, Landewé RB, Bijlsma JW, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 38. Fraenkel L, Bathon JM, England BR, St.Clair EW, Arayssi T, Carandang K, et al. 2021. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. [DOI] [PubMed] [Google Scholar]
- 39. Mazumder A, Arora M, Sra MS, Gupta A, Behera P, Gupta M, et al. Geographical variation in case fatality rate and doubling time during the COVID‐19 pandemic. Epidemiol Infect 2020;148:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van de Schoot R, de Bruin J, Schram R, Zahedi P, de Boer J, Weijdema F, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat Mach Intell 2021;3:125–33. [Google Scholar]
- 41. Anesi GL, Jablonski J, Harhay MO, Atkins JH, Bajaj J, Baston C, et al. Characteristics, outcomes, and trends of patients with COVID‐19‐related critical illness at a learning health system in the United States. Ann Intern Med 2021;174:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roth GA, Emmons‐Bell S, Alger HM, Bradley SM, Das SR, de Lemos JA, et al. Trends in patient characteristics and COVID‐19 in‐hospital mortality in the United States during the COVID‐19 pandemic. JAMA Netw Open 2021;4:e218828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1: Supplementary Information


