Abstract
Objective
The study was undertaken to assess the impact of B cell depletion on humoral and cellular immune responses to severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) vaccination in patients with various neuroimmunologic disorders on anti‐CD20 therapy. This included an analysis of the T cell vaccine response to the SARS‐CoV‐2 Delta variant.
Methods
We investigated prospectively humoral and cellular responses to SARS‐CoV‐2 mRNA vaccination in 82 patients with neuroimmunologic disorders on anti‐CD20 therapy and 82 age‐ and sex‐matched healthy controls. For quantification of antibodies, the Elecsys anti‐SARS‐CoV‐2 viral spike (S) immunoassay against the receptor‐binding domain (RBD) was used. IFN‐gamma enzyme‐linked immunosorbent spot assays were performed to assess T cell responses against the SARS‐CoV‐2 Wuhan strain and the Delta variant.
Results
SARS‐CoV‐2‐specific antibodies were found less frequently in patients (70% [57/82]) compared with controls (82/82 [100%], p < 0.001). In patients without detectable B cells (<1 B cell/mcl), seroconversion rates and antibody levels were lower compared to nondepleted (≥1 B cell/mcl) patients (p < 0.001). B cell levels ≥1 cell/mcl were sufficient to induce seroconversion in our cohort of anti‐CD20 treated patients. In contrast to the antibody response, the T‐cell response against the Wuhan strain and the Delta variant was more pronounced in frequency (p < 0.05) and magnitude (p < 0.01) in B‐cell depleted compared to nondepleted patients.
Interpretation
Antibody responses to SARS‐CoV‐2 mRNA vaccinnation can be attained in patients on anti‐CD20 therapy by the onset of B cell repopulation. In the absence of B cells, a strong T cell response is generated which may help to protect against severe coronavirus disease 2019 (COVID‐19) in this high‐risk population. ANN NEUROL 2022;91:342–352
The severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) virus has caused a worldwide pandemic leading to significant morbidity and mortality particularly in people of higher age and people suffering from comorbidities. 1 , 2 The currently dominating Delta variant (B.1.617.2) is associated with even increased rates of transmission and disease severity. 3 In patients with immune‐mediated neurological disorders on B cell‐depleting therapy, the risk of severe coronavirus disease 2019 (COVID‐19) is increased due to impaired humoral immune responses. 4 , 5 , 6
Vaccination is the major key to control the COVID‐19 pandemic. However, recent data indicate that the immune response to SARS‐Cov‐2 mRNA vaccination 7 , 8 is attenuated or even abolished on anti‐CD20 therapy in neurologic 9 , 10 , 11 , 12 , 13 and rheumatic diseases. 14 , 15 , 16 Reconstitution of circulating B cells seems to play a critical role in promoting vaccine responses. 10 , 14
T cell responses are likely to contribute to vaccine efficacy 17 , 18 and may become even more important given the emergence of viral variants of concern that evade neutralizing antibodies. 19 In addition, T cells contribute to survival in patients infected with SARS‐CoV‐2 even in the context of B cell depleting therapies, 20 as shown recently.
An increasing number of studies in rheumatologic diseases 14 , 16 , 21 and in multiple sclerosis 10 , 13 , 21 , 22 , 23 , 24 , 25 indicate preserved but altered T cell responses to COVID‐19 vaccination in anti‐CD20 treated patients. Moreover, it was shown that patients on anti‐CD20 therapy who failed to develop antibodies following vaccination were able to generate robust T cell responses compared with patients with preserved humoral vaccine responses. 10
In this prospective cohort study, we studied humoral and T cell responses following SARS‐CoV‐2 mRNA vaccination in serial samples from 82 patients with neuroimmunologic disorders on anti‐CD20 therapy in order to determine the impact of B cell depletion on vaccine responses. This included an analysis of the cellular vaccine response to the Delta variant, which is of high relevance in the ongoing COVID‐19 pandemic.
Patients and Methods
Patients
This study is part of a prospective cohort study performed at the Medical University of Vienna, Vienna, Austria, “Characterization of the responsiveness after mRNA SARS‐CoV‐2 vaccination in patients with immunodeficiency or immunosuppressive therapy”; Eudra CT Nr. 2021–000291‐11. For this substudy, patients were recruited at the outpatient clinic of the Department of Neurology, Medical University of Vienna, Austria between March 12 and May 31, 2021. Adult patients (≥18 years of age), with a confirmed diagnosis of an immune‐mediated disease of the central nervous system, the peripheral nervous system, or the neuromuscular junction who were going to have a SARS‐CoV‐2 mRNA vaccination (Pfizer/BioNTech or Moderna) were included in the study. As the control group, we selected an equal number of age‐ and sex‐matching individuals from the original cohort of healthy controls. Participants gave written informed consent. The ethics committee of the Medical University of Vienna, Austria, approved the study (EK Nr. 1,073/2021).
Study Design
Blood samples for testing for SARS‐CoV‐2 antibodies, differential blood counts, and CD19+ B cell counts were taken 0 to 28 days before the first vaccination (V1). Samples for antibody testing were taken 14 to 21 days after the first vaccination (V2), and 21 to 28 days after the second vaccination (V3). Blood samples for peripheral blood mononuclear cell (PBMC) analysis were taken 1 to 2 weeks (T1) and 6 weeks (T2) after the second vaccination in a subgroup of patients (n = 38) and age‐ and sex‐matched healthy controls (n = 16). Serum samples for antibody tests were stored at the Biobank of the Medical University of Vienna (MedUni Wien Biobank), a centralized facility for the preparation and storage of biomaterial with certified quality management (ISO 9001:2015). 26 PBMCs were isolated by density gradient centrifugation and stored in liquid nitrogen until further use. Methods for quantification of CD19+ B cells, quantification of antibodies, and T cell assays were described previously. 14
Quantification of Peripheral CD19+ B Cells
Immunological phenotyping was performed by flow cytometry (FACS Canto II flow cytometer equipped with FACS Diva software – both from Becton Dickinson, San Jose, CA, USA) using the whole blood first stain and then the lyse and wash method (Becton Dickinson). A combination of monoclonal antibodies (FITC‐labeled anti‐CD3, PE‐labeled anti‐CD16 + 56, PerCP‐cy5.5‐labeled anti‐CD4, PE‐Cy7‐labeled anti‐CD19, APC‐Cy7‐labeled anti‐CD8, V450‐labeled anti‐HLA‐DR, V500‐labeled anti‐CD45, and APC‐labeled anti‐CD14, all provided by Becton Dickinson) was used for characterization of lymphocyte subsets. In order to recover a significant B‐cell population of at least 50 cells, 20,000 to 500,000 events were acquired. The LeucoGATE (CD45/CD14) fluorescent information with forward scatter (FSC) and side scatter (SSC) was used to set an electronic gate around the lymphoid population. Results were expressed as the proportion of CD19 positive B‐cells among total lymphocytes. 14
Anti‐SARS‐CoV‐2 Testing
For quantification of antibodies to the receptor‐binding domain (RBD) of the viral spike (S) protein the Elecsys Anti‐SARS‐CoV‐2 S immunoassay was used. 27 The quantitation range is between 0.4 and 2500.0 BAU/ml. Values >0.8 BAU/ml are considered as positive. Results below the lower level of quantification were defined as 0.2 BAU/ml to allow for calculations. Nucleocapsid‐specific antibodies were measured with the qualitative Elecsys Anti‐SARS‐CoV‐2 assay 28 at baseline. Cobas e801 analyzers (Roche Diagnostics, Rotkreuz, Switzerland) at the Department of Laboratory Medicine, Medical University of Vienna (certified acc. to ISO 9001:2015 and accredited acc. to ISO 15189:2012) were used for antibody testing. 14
Peptides
PepMix SARS‐CoV‐2 peptide pools were bought from JPT (Berlin, Germany) for T cell stimulation. The pools cover the entire sequences of the SARS‐CoV‐2 spike protein and comprise 15 mer peptides overlapping by 11 amino acids (aas). The spike peptides are split into 2 sub‐pools S1 (aa 1–643) and S2 (aa 633–1,273). Peptides were dissolved in dimethyl sulfoxide and diluted in AIM‐V medium for use in ELISpot assays. 14
IFN‐γ ELISpot Assay
PBMCs collected at 1 to 2 and 6 weeks after the second vaccine dose were stimulated with pools of peptides covering the spike (S)‐protein of the ancestral Wuhan strain or the B.1.167.2 (Delta) variant. The S‐specific T cells were identified using IFN‐γ ELISpot assays. For ex vivo ELISpot assays, PBMCs were thawed. A total of 1 to 2 × 105 cells per well were incubated with SARS‐CoV‐2 peptides (2 μg/ml; duplicates), AIM‐V medium (negative control; 3–4 wells), or PHA (L4144, Sigma; 0,5 μg/ml; positive control) in 96‐well plates coated with 1.5 μg anti‐IFN‐γ (1‐D1K, Mabtech) for 24 hours. After washing, spots were developed with 0.1 μg biotin‐conjugated anti‐IFN‐γ (7‐B6‐1, Mabtech), streptavidin‐coupled alkaline phosphatase (Mabtech, 1:1000), and 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitro blue tetrazolium (Sigma). For counting spots, a Bio‐Sys Bioreader 5,000 Pro‐S/BR177 and Bioreader software generation 10 was used. Data were calculated as spot forming cells (SFCs) per 106 PBMCs after subtraction of the spots from the negative control (mean spot number from 3 to 4 unstimulated wells). 14
Statistical Analysis
Missing values were imputed by multiple imputation using the package “mice” (multivariate imputation by chained equations). In total 1.2% (1/82) values were missing for anti‐SARS‐CoV‐2 IgG titers at visit 1 and visit 2 (not in the same participant). Imputation was based on the anti‐SARS‐CoV‐2 IgG levels at the other visits, lymphocyte counts, and B cell counts. For values below the limit of quantification, half of the limit of quantification was imputed. The control group was matched to the study data by age and sex using propensity score matching. This was implemented using the “MatchIt” package. According to the distribution, continuous variables are presented as median with interquartile range (IQR). Categorical variables of unpaired groups were compared using Fisher's exact test. Continuous variables of unpaired groups were compared by Wilcoxon rank sum test. Differences in paired groups were compared using McNemar's test for categorical variables and Wilcoxon matched pair rank sum test for continuous variables. Bonferroni correction for multiple testing was applied when indicated. Correlations between continuous variables were assessed via Kendall rank correlation coefficient (τ). Sensitivity analyses were performed by repeating the analyses after excluding patients with ocrelizumab treatment, mRNA‐1,273 (Moderna) vaccine type or both. To assess the factors that facilitate seroconversion, univariate logistic regression was applied. To assess whether the extent of B cell and T cell responses increased the odds of side effects, univariate and multivariate logistic regression analyses were performed. McFadden's R squared was used as a measure for the goodness of fit for the logistic regression models. Continuous variables (age, months since last therapy, months since therapy onset, disease duration, and lymphocyte counts) and categorical variables (sex and diagnosis) were selected based on their expected relevance. The diagnosis variable was grouped into 2 factors (diagnosis of multiple sclerosis and other). Statistical analysis was performed using R version 4.1.0 (R Core Team 2021, Vienna, Austria). The R packages “ggplot2” and “viridis” were used for graphical representation. A p value of <0.05 was set as statistically significant.
Results
Patient Characteristics
Eighty‐two patients (median age 40 years [IQR = 22], 72% women) and 82 age‐ and sex‐matched healthy controls were included (Table S1). Patients were diagnosed with multiple sclerosis (n = 64), followed by neuromyelitis optica spectrum disorders (n = 7), myasthenic syndromes (n = 7), autoimmune encephalitis (n = 2), or chronic inflammatory demyelinating polyneuropathy (n = 2). Among the patients, 82 were treated with rituximab (RTX; n = 76) or ocrelizumab (OCR; n = 6). Ten patients received comedication (azathioprine n = 3, tocilizumab n = 3, oral prednisone n = 2, subcutaneous or intravenous immunoglobulins n = 2, eculizumab n = 1, or mycophenolate mofetil n = 1). Timing and dosing of anti‐CD20 treatment were performed according to the discretion of the treating physician. The median time between the last anti‐CD20 infusion and the first vaccine dose was 6 months (IQR = 5). The time between the last infusion and baseline was 0 to 6 months in 43 patients (52%), >6 to 12 months in 27 patients (33%), and >12 months in 12 patients (15%), respectively. Seventy one of 82 (87%) patients and all healthy controls received the BNT162b2 (Pfizer/BioNTech) vaccine, whereas 11 of 82 (13%) patients were vaccinated with the mRNA‐1,273 (Moderna) vaccine.
Humoral Response to SARS‐CoV‐2 Vaccination
At baseline (V1), 3 patients and 1 healthy participant had detectable antibodies against the SARS‐CoV‐2 spike (S) RBD.
After the first vaccination (V2), fewer patients on anti‐CD20 therapy (28 of 82, 33%) generated an antibody response compared with healthy controls (80 of 82, 98%, p < 0.001, Fisher's exact test). Median SARS‐CoV‐2 S‐specific antibody levels were lower in patients on anti‐CD20 therapy (<0.4 BAU/ml) compared with healthy controls (43.7 BAU/ml [IQR = 61], p < 0.001, Wilcoxon rank sum test).
After the second vaccination (V3), all healthy controls developed humoral immune responses against the SARS‐CoV‐2 virus. The frequency of seroconverters among B cell‐treated patients increased from 33% to 70% (57 of 82 patients) after the second vaccination (p < 0.001, McNemar's test). Median antibody levels in patients (26.5 BAU/ml [IQR = 1,026]) were lower compared with healthy controls (1,711 BAU/ml [IQR = 1665], p < 0.001, Wilcoxon rank sum test; Fig 1A, B). Individual trajectories across the three visits are shown in Figure 1C.
FIGURE 1.
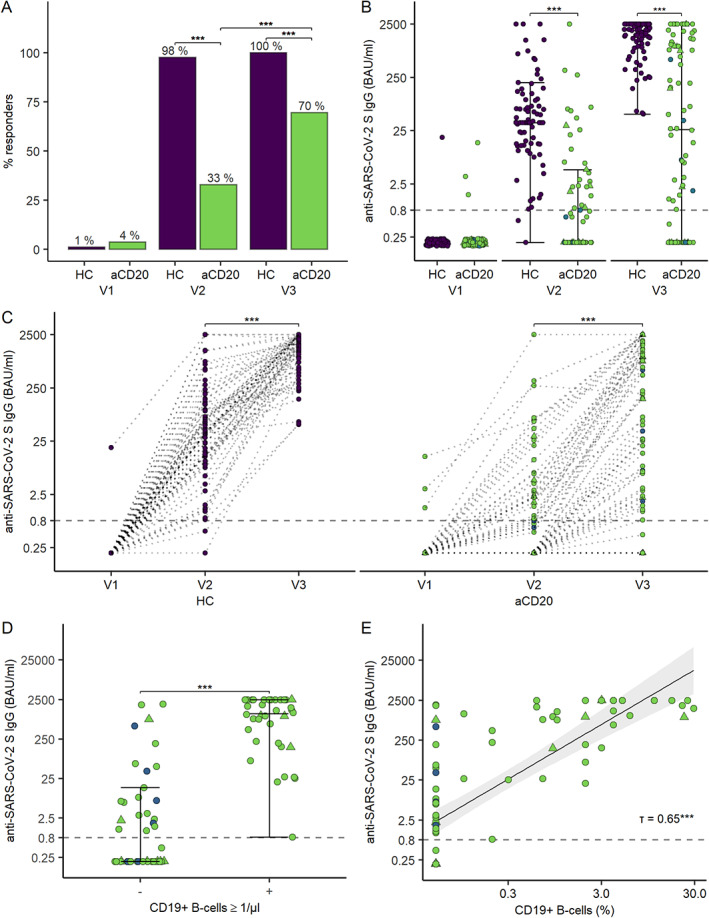
Humoral immune response to SARS‐CoV‐2 mRNA vaccination in patients on anti‐CD20 therapy and in healthy controls. (A) Seroconversion rates before vaccination (V1), after the first vaccination (V2), and after the second vaccination (V3). (B) Anti‐SARS‐CoV‐2 RBD S‐antibody levels before (V1), after the first (V2), and after the second vaccination (V3). (C) Individual trajectories of anti‐SARS‐CoV‐2 RBD S‐antibody levels across the 3 visits. (D) Anti‐SARS‐CoV‐2 RBD S‐antibody levels according to the presence or absence of peripheral B cells (≥ 1 cell/μl). (E) Scatter plot of antibody levels to the RBD of the S protein and the percentage of peripheral B cells with linear regression line including a 95% CI. Each data point is a participant and the solid horizontal lines represent the group medians. The dotted lines represent the cutoff (IgG titers <0.8 BAU/ml are considered negative). Participants are marked as follows: Rituximab, green; Ocrelizumab, blue; BNT162b2 vaccine, circle; mRNA‐1,273 vaccine, triangle. CI = confidence interval; HC = healthy control; S = viral spike; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2; RBD = receptor‐binding domain. [Color figure can be viewed at www.annalsofneurology.org]
In patients with detectable circulating B cells (≥1 cell/μl), the seroconversion rate was 100% (36/36) at V3 (see Fig 1D). In patients without detectable B cells (<1 cell/μl), the seroconversion rate was 46% (21/46). Comparing antibody levels in patients with different percentages of circulating B cells showed that all patients with more than 0% but less than 1% detectable B cells were able to generate an antibody response, suggesting that the presence of peripheral B cells per se allows seroconversion irrespective of the B cell level (p < 0.001, Fisher's Exact test; τ = 0.65, Kendall's tau; see Fig 1E). Sensitivity analyses, excluding patients with ocrelizumab treatment, mRNA‐1,273 (Moderna) vaccine type or both, confirmed the results (Table 1).
TABLE 1.
Sensitivity Analysis: SARS‐CoV‐2 Antibody Levels and Correlation with Percentage of Peripheral B cells After Excluding Patients with Ocrelizumab Treatment or mRNA‐1,273 (Moderna) Vaccine or Both
| SARS‐CoV‐2 S IgG levels (median BAU/ml, IQR) | Correlation between percentage of peripheral B cells and SARS‐CoV‐2 IgG S levels | ||||
|---|---|---|---|---|---|
| <1 B cell/μl | ≥1 B cell/μl | p | Kendall rank correlation coefficient (τ) | p | |
| Without ocrelizumab (n = 76) | 0.2 (3.84) | 1,101 (2286) | <0.001 | 0.66 | <0.001 |
| Without mRNA‐1,273 vaccine (n = 71) | 0.44 (6.70) | 1237.50 (2286) | <0.001 | 0.64 | <0.001 |
| Without ocrelizumab and mRNA‐1,273 vaccine (n = 65) | 0.2 (6.06) | 1237.50 (2286) | <0.001 | 0.65 | <0.001 |
IQR = interquartile range; S = viral spike; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
Logistic regression analysis was performed in order to evaluate a potential association among age, sex, months since the last infusion, months since the onset of therapy, disease duration, diagnosis, and lymphocyte counts with seroconversion. However, only the time since the last infusion increased the odds for seroconversion (odds ratio [OR] = 1.46, 95% confidence interval [CI] = 1.2–1.86, p < 0.001; Fig 2).
FIGURE 2.
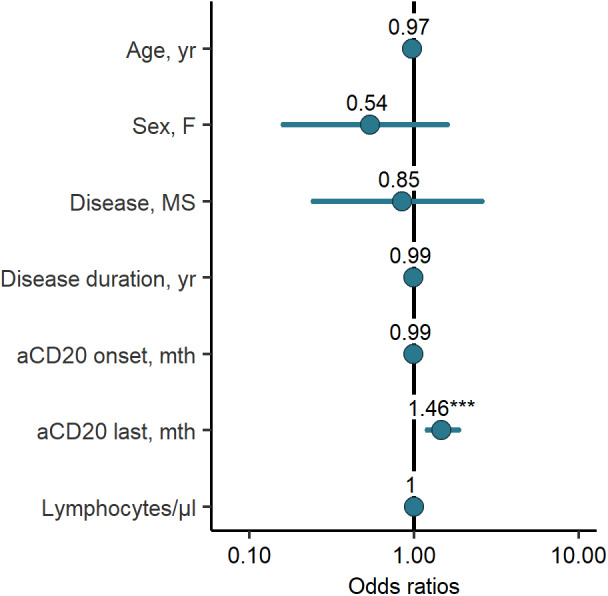
Odds ratios of univariate logistic regression assessing seroconversion. Each of the variables was tested individually against seroconversion in all patients on anti‐CD20 therapy in a univariate regression model. Due to quasi‐complete separation, the B cell‐variables were excluded. MS = multiple sclerosis. [Color figure can be viewed at www.annalsofneurology.org]
Cellular Response to SARS‐Cov‐2 Vaccination
To assess whether or not SARS‐CoV‐2 vaccination generated T cell responses in our patient cohort, PBMCs collected at 1 to 2 weeks and 6 weeks after the second vaccine dose were stimulated with pools of peptides covering the spike (S)‐protein of the ancestral Wuhan strain or the B.1.167.2 (Delta) variant. The S‐specific T cells were identified using IFN‐γ ELISpot assays. T cell responses were induced in 32 of 38 (84%) patients and 16 of 16 (100%) healthy controls with lower levels in patients (median SFCs/106 PBMC 183) compared with controls (median SFCs/106 PBMC 340; Fig 3A). There was no difference in the rate or magnitude of T cell response between those vaccinated with BNT/Pfizer (24/29 (83%), median SFCs/106 PBMC 179) or Moderna vaccine (8/9 (89%), median SFCs/106 PBMC 280; see Fig 3B, C). The response rate did not differ between the Wuhan strain (21/28 [75%] and the Delta variant (20/28 [71%]), but the magnitude of the individual responses was significantly lower against the Delta variant (p < 0.05, Wilcoxon matched‐pairs signed rank test; see Fig 3D). Longitudinal analysis of T cell responses with matched samples at 1 to 2 weeks and 6 weeks after the second vaccine dose showed that T cell responses were still detected at 6 weeks in 10 of 16 (63%) patients, and at levels that were similar compared to weeks 1 and 2 (see Fig 3E).
FIGURE 3.
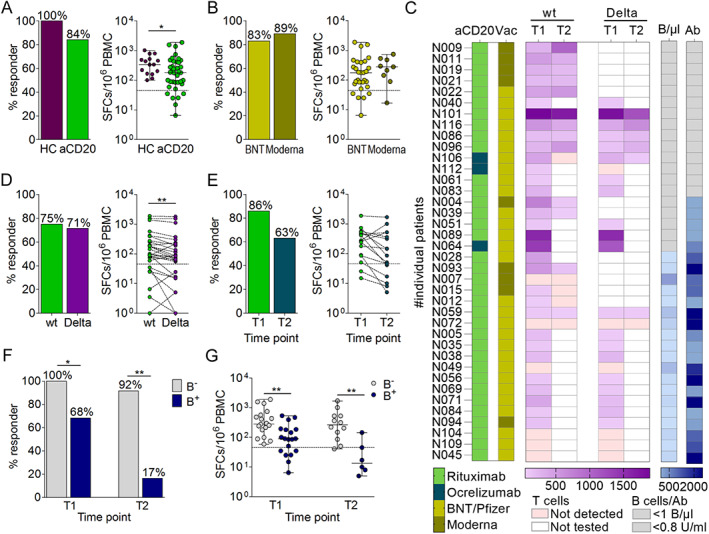
T cell response to SARS‐CoV‐2 vaccination. (A) SARS‐CoV‐2‐specific T cell response rates in patients and healthy controls (HCs), (B) by vaccine type (BNT = Biontec/Pfizer), and (C) in relation to treatment and B cell status. (D) T cell response against peptide pools derived from the Wuhan (wildtype [wt]) strain versus the B.1.617.2 (Delta) variant. (E) T cell response at 1 and 2 (T1) and 6 weeks (T2) after the second vaccine dose and (F) in patients with (B+) or without detectable B cells (B−). Bars indicate proportion of patients with a T cell response; each circle is a participant, and the solid horizontal lines represent group medians; dotted lines indicate the cutoff (T cells ≤46 spot forming cells [SFCs] per 106 PBMC are considered negative). SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2. [Color figure can be viewed at www.annalsofneurology.org]
There was a significantly higher frequency and magnitude of T cell response in B cell‐depleted patients (19/19 [100%], median SFCs/106 PBMC 280) as compared to nondepleted patients (13/19 [68%], median SFCs/106 PBMC 90) at 1 to 2 weeks after the second vaccine dose (p < 0.05, Fisher's exact test, p < 0·01, Wilcoxon rank sum test; see Fig 3F). This difference was even more pronounced at 6 weeks after the second vaccine dose with SARS‐CoV‐2 T cell responses detectable in 11 of 12 (92%) B cell‐depleted patients (median SFCs/106 PBMC 268) compared with 1 of 6 (17%) of nondepleted patients (median SFCs/106 PBMC 14, p < 0.01, Fisher's exact test, p < 0·01, Wilcoxon rank sum test; see Fig 3F). The correlation between the interval from the last treatment to vaccination and the SARS‐CoV‐2 antibody levels is presented in Figure 4A (τ=0.65, Kendall's tau, p < 0.001). Furthermore, an inverse correlation was observed between SARS‐CoV‐2 T cell levels and the time interval from last anti‐CD20 treatment to vaccination (τ=‐0.28, Kendall's tau, p < 0.05; see Fig 4B).
FIGURE 4.
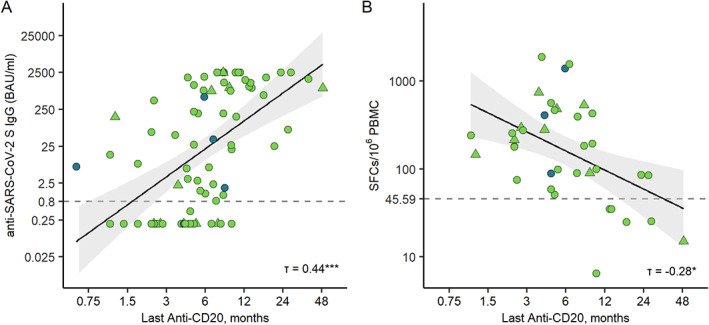
Correlation between the time since last anti‐CD20 treatment and vaccine responses. (A) Scatter plot of antibody levels to the RBD of the spike protein and time since last anti‐CD20 treatment with linear regression line including a 95% CI. (B) Scatter plot of SFCs/106 PBMC and time since last anti‐CD20 treatment dose, with linear regression line including a 95% CI. Participants are marked as follows: Rituximab, green; Ocrelizumab, blue; BNT162b2 vaccine, circle; mRNA‐1,273 vaccine; triangle. CI = confidence interval; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2; PBMC = peripheral blood mononuclear cell; RBD = receptor‐binding domain; SFC = spot forming cell. [Color figure can be viewed at www.annalsofneurology.org]
Adverse Events
In patients, data on adverse events were systematically recorded until visit 3. Local and systemic reactions after the first and after the second vaccine dose included fever (7/79 [9%] and 21/78 [27%]), local reaction (60/79 [76%] and 64/78 [82%]), nausea (8/79 [10%] and 8/78 [10%]), shivering (6/79 [8%] and 13/78 [17%]), fatigue (27/79 [34%] and 34/78 [44%]), headache (13/79 [16%] and 32/78 [41%]), sweating (5/79 [6%] and 13/78 [16%]), and myalgia (7/79 [9%] and 15/78 [19%]), respectively. Transient worsening of pre‐existing neurologic symptoms was reported in 6 of 79 (8%) patients after the first vaccination and in 8 of 78 (10%) patients after the second vaccination. Two infections (bacterial respiratory tract infection and urinary tract infection) and one serious adverse event (herpes zoster) occurred. Relapses requiring steroid therapy were reported in one patient after the first vaccination and in 2 patients after the second vaccination (Table 2).
TABLE 2.
Univariate Logistic Regression Model Assessing Seroconversion in Anti‐CD20 Treated Patients
| Variable | Univariate analysis | ||
|---|---|---|---|
| OR (95% CI) | p | R2 | |
| Age | 0.97 (0.94–1.00) | 0.081 | 0.031 |
| Sex, female | 0.54 (0.16–1.59) | 0.287 | 0.012 |
| Disease, multiple sclerosis | 0.85 (0.24–2.59) | 0.778 | 0.001 |
| Disease duration, years | 0.99 (0.92–1.07) | 0.803 | 0.001 |
| Months since anti‐CD20 onset | 0.99 (0.97–1.00) | 0.121 | 0.024 |
| Months since last anti‐CD20 | 1.46 (1.20–1.86) | 0.001 | 0.228 |
| Lymphocytes/mcl | 1.00 (1.00–1.00) | 0.569 | 0.003 |
CI = confidential interval; OR = odds ratio; R2 = McFadden's R squared.
There were no significant differences of anti‐SARS‐CoV‐2 antibody levels or T cell levels between patients with or without side effects (Table 3). Accordingly, univariate and multivariate logistic regression after adjusting for age, sex, and vaccine type did not show increased odds of side effects depending on antibody or T cell levels (Table 4).
TABLE 3.
Antibody and T Cell Levels in Patients With or Without Side Effects After the Second Vaccine Dose (Wilcoxon Rank Sum Test)
| Side effects | Anti‐SARS‐CoV‐2 S IgG levels (median, IQR), n = 78 | SFCs/106 PBMC (median, IQR), n = 38 | ||||
|---|---|---|---|---|---|---|
| In patients with side effects | In patients without side effects | p a | In patients with side effects | In patients without side effects | p a | |
| Fever | 794 (1,930.7) | 20.7 (886.3) | 0.769 | 345.0 (244.4) | 179.0 (205.0) | 0.775 |
| Local reaction | 20.7 (1,316.8) | 194 (701.7) | 1.000 | 248.5 (355.0) | 145.0 (110.0) | 1.000 |
| Nausea | 429 (2,499.8) | 23.2 (989.8) | 1.000 | 535.0 (745.0) | 179.5 (213.8) | 0.145 |
| Shivering | 969 (2,498.7) | 20.7 (886.3) | 0.690 | 420.5 (50.8) | 179.0 (205.0) | 0.240 |
| Fatigue | 8.51 (1,447.3) | 38.6 (971.8) | 1.000 | 265.0 (359.5) | 145.0 (205.0) | 0.820 |
| Headache | 27.5 (1,908.2) | 25.6 (971.8) | 1.000 | 248.5 (360.1) | 180.0 (200.0) | 1.000 |
| Sweating | 808 (1,576.8) | 25.6 (959.8) | 1.000 | 255.0 (340.0) | 182.5 (311.9) | 1.000 |
| Myalgia | 50.8 (1,142.5) | 25.6 (1043.8) | 1.000 | 240.0 (327.8) | 180.0 (325.0) | 1.000 |
IQR = interquartile range; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2.
After Bonferroni correction for multiple testing.
TABLE 4.
Logistic Regression Analyses Evaluating the Odds for Side Effects Depending on a Change in Anti‐SARS‐CoV‐2 S IgG Levels by 100 BAU/ml and Change in T Cell Levels by 10 SFCs/106 PBMC
| SARS‐CoV‐2 antibody levels and side effects | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis a | |||||
| Side effect (dependent variable) | OR (95% CI) | p | R2 | OR (95% CI) | p | R2 |
| Fever | 1.05 (0.99–1.10) | 0.082 | 0.032 | 1.05 (0.99–1.11) | 0.100 | 0.136 |
| Local reaction | 1.03 (0.96–1.11) | 0.453 | 0.008 | 1.02 (0.96–1.10) | 0.574 | 0.040 |
| Nausea | 1.05 (0.97–1.12) | 0.214 | 0.028 | 1.04 (0.97–1.12) | 0.276 | 0.085 |
| Shivering | 1.06 (1.00–1.12) | 0.049 | 0.053 | 1.06 (0.99–1.13) | 0.092 | 0.175 |
| Fatigue | 1.01 (0.97–1.06) | 0.564 | 0.003 | 1.01 (0.96–1.06) | 0.639 | 0.067 |
| Headache | 1.02 (0.98–1.08) | 0.322 | 0.009 | 1.02 (0.97–1.08) | 0.404 | 0.078 |
| Sweating | 1.04 (0.98–1.10) | 0.227 | 0.020 | 1.03 (0.97–1.10) | 0.317 | 0.056 |
| Myalgia | 1.01 (0.95–1.06) | 0.794 | 0.001 | 1.01 (0.95–1.07) | 0.803 | 0.054 |
| SFCs/106 PBMC and side effects | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis a | |||||
| Side effect (dependent variable) | OR (95% CI) | p | R2 | OR (95% CI) | p | R2 |
| Fever | 1.01 (0.99–1.02) | 0.380 | 0.624 | 1.00 (0.98–1.02) | 0.884 | 0.670 |
| Local reaction | 1.01 (0.99–1.04) | 0.585 | 0.596 | 1.01 (0.99–1.04) | 0.613 | 0.597 |
| Nausea | 1.02 (1.00–1.05) | 0.039 | 0.836 | 1.02 (1.00–1.05) | 0.138 | 0.862 |
| Shivering | 1.01 (0.98–1.02) | 0.579 | 0.751 | 1.00 (0.98–1.02) | 0.763 | 0.757 |
| Fatigue | 1.01 (1.00–1.03) | 0.207 | 0.517 | 1.01 (0.99–1.04) | 0.263 | 0.574 |
| Headache | 1.00 (0.98–1.02) | 0.921 | 0.513 | 1.00 (0.98–1.02) | 0.732 | 0.569 |
| Sweating | 0.99 (0.96–1.01) | 0.576 | 0.648 | 0.99 (0.96–1.01) | 0.499 | 0.669 |
| Myalgia | 0.99 (0.96–1.01) | 0.543 | 0.621 | 0.99 (0.95–1.01) | 0.407 | 0.633 |
CI = Confidence interval; OR = odds ratio; R2 = McFadden's R squared; SARS‐CoV‐2 = severe acute respiratory syndrome‐coronavirus 2; PBMC = peripheral blood mononuclear cell; SFC = spot forming cell.
The multivariate analysis was adjusted for age, sex, and vaccine type.
Discussion
Under the ongoing SARS‐CoV‐2 pandemic, patients with immune‐mediated neurologic disorders on anti‐CD20 therapy are at increased risk for severe COVID‐19. 4 , 5 , 6 Although the approved mRNA vaccines confer high levels of protection in immunocompetent individuals, 7 , 8 recent data indicate that vaccine efficacy is reduced in B cell‐depleted patients. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
In this prospective cohort study, we found that in patients with different neuroimmunologic disorders on anti‐CD20 therapy seroconversion rates and antibody levels were reduced after the first and the second SARS‐CoV‐2 vaccination compared with healthy individuals. Although we assessed seroconversion rates and antibody levels, our study design does not enable us to define a level of protection against (severe) COVID‐19 in anti‐CD20 treated patients. Antibody levels as correlates of protection have been suggested in nonimmunocompromised individuals after vaccination with the ChAdOx1n‐CoV‐19 vaccine. 29 However, in B cell‐depleted individuals, the immune response to vaccination differs on both a humoral and cellular level. 10 , 13 Therefore, the humoral correlates of protection found in immunocompetent individuals 29 may not apply to our cohort.
Our data are in line with recent studies, suggesting that B cell‐depletion may affect humoral immune responses to vaccination. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 However, in contrast to other reports, 9 , 14 higher seroconversion rates were observed in our cohort. Furthermore, we show that seroconversion strongly correlated with the onset of B cell reconstitution, with a cutoff level of 1 B cell/μl required for antibody induction following vaccination. Whereas our cohort mainly consisted of younger people on anti‐CD20 monotherapy, including also patients with longer intervals between the last anti‐CD20 treatment dose and vaccination, further differences in seroconversion rates may be due to small sample sizes, but also due to higher age, 9 , 15 , 16 the use of immunosuppressive comedication, 14 , 16 as well as the underlying disease. 14 , 15 , 16 Thus, the cutoff level of 1 B cell/μl is not an absolute feature, as there are cases of poor seroconversion despite the presence of B cells and seroconversion with no peripheral B cells. 10 , 13 , 14
Seroconversion rates may also be influenced by differences in the anti‐CD20 treatment regimen itself, because timing, dosing, and differences between anti‐CD20 antibodies may influence the kinetics of B cell reconstitution. 30 , 31 Accordingly, a longer time from the last infusion was associated with seroconversion in our cohort, whereas other parameters, such as the overall treatment period or disease duration, did not impact seroconversion.
Currently, it is not recommended to extend the dosing intervals due to a potential risk of disease reactivation. 32 However, limited data indicate that reducing the frequency of dosing does not compromise efficacy in patients with multiple sclerosis on anti‐CD20 therapy. 33 , 34 Although our study was not designed to draw conclusions on clinical implications of our data, the small number of patients experiencing relapses support these observations. In clinical practice, therefore, monitoring of B cell reconstitution may be justified in stable patients in order to facilitate humoral vaccine responses. Furthermore, in contrast to previous findings in immunocompetent individuals, 35 we could not find an association between side effects and antibody or T cell levels after vaccination in our cohort.
Although most vaccine efforts have focused on the generation of humoral immune responses, T cell‐mediated immunity also contributes to vaccine efficacy in healthy controls 17 , 18 and may be important for the protection against mutant SARS‐CoV‐2 variants. 19 Moreover, recent data indicate that T cell responses may reduce the disease burden in patients infected with COVID‐19 even in the context of B cell depleting therapies. 20
In this study, we showed that most of our anti‐CD20 treated patients developed robust SARS‐CoV‐2 T cell immunity in response to vaccination. These data are in line with preliminary reports, suggesting that patients on anti‐CD20 therapy can generate T cell responses against COVID‐19, even in the absence of humoral immunity. 10 , 13 , 14 , 21 , 22 , 23 , 24 , 25 However, here, we show that the T cell response was even more robust and sustained in B cell‐depleted (<1 cell/μl) compared to nondepleted (≥ 1cell/μl) patients. In line with this finding, we also observed that a longer interval between the last infusion and vaccination was associated with lower T cell responses. The mechanisms leading to these observations are incompletely understood. In accordance to our results, anti‐CD20 treated patients with multiple sclerosis were shown to generate robust CD4 and CD8 T cell responses following mRNA vaccination. 10 These findings suggest that patients on anti‐CD20 therapy can develop some protection against severe COVID‐19 on a cellular level, if the humoral vaccine response is compromised.
In our patients, the SARS‐CoV‐2 Wuhan strain as well as the Delta variant induced similar T cell responder rates to vaccination, but the quantity of the IFN‐γ SFC response to the Delta variant was lower. The Delta variant is associated with increased transmissibility, increased disease severity, 3 and reduced vaccine efficacy. 36 Because mutant variants may escape neutralizing antibodies, 19 preserved cellular immunity following vaccination may become even more important for vaccine‐induced protection against SARS‐CoV‐2‐induced disease.
In summary, our data show that following COVID‐19 vaccination, patients with various neuroimmunologic disorders on anti‐CD20 therapy are able to generate humoral responses with the onset of B cell repopulation or cellular responses in the absence of circulating B cells. Although our data need to be confirmed in clinical settings, they indicate that anti‐CD20 therapy does not preclude COVID‐19 vaccine efficacy in a population at risk for severe COVID‐19.
Author Contributions
Conception and design of the study: S.T. and S.W. Acquisition and analysis of data: B.K., F.L., P.R., M.K., L.S., H.H., R.T., F.Z., G.Z., G.B., A.D.B., W.R., K.Z., I.W., A.S., M.G., M.M., K.R., T.B., S.T., and J.A.H. Drafting the manuscript and figures: B.K., L.S., and J.A.H. All authors revised the manuscript for intellectual content and approved the final version of the manuscript to be published.
Potential Conflicts of Interest
Roche manufactures Ocrelizumab, which is studied in this paper. B.K., F.L., and P.R. have received honoraria for speaking and for consulting from Roche. P.R. has received research grants from Roche. G.Z. has participated in meetings sponsored by or received travel funding from Roche. G.B. has participated in meetings sponsored by, received speaker honoraria, honoraria for consulting or travel funding from Roche. A.D.B. has received speaker honoraria and participated in meetings sponsored by or travel funding from Roche. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from Roche. His institution has received financial support in the last 12 months for participation in clinical trials in multiple sclerosis sponsored by Roche. M.K., L.S., H.H., R.T., F.Z., W.R., K.Z., I.W., A.S., M.G., M.M., K.R., S.W., J.H.A., and S.T. report no conflicts of interest relevant to this study.
Supporting information
TABLE S1. Characteristics of Patients and Healthy Controls Who Have Been Vaccinated Against COVID‐19
Acknowledgments
Laboratory material was partly funded by a Medical‐Scientific fund of the Major of the federal capital of Vienna (grant Covid003).
References
- 1. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS‐CoV‐2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis 2022;22:35–42. 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sormani MP, De Rossi N, Schiavetti I, et al. Disease‐modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021;89:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louapre C, Maillart E, Papeix C, et al. Outcomes of Corona virus disease 2019 in patients with neuromyelitis optica and associated disorders. Eur J Neurol 2021;28:3461–3466. 10.1111/ene.14612. [DOI] [PubMed] [Google Scholar]
- 6. Jakubikova M, Tyblova M, Tesar A, et al. Predictive factors for a severe course of COVID‐19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur J Neurol 2021;28:3418–3425. 10.1111/ene.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐Cov‐2 vaccine. N Engl J Med 2020;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Achiron A, Mandel M, Dreyer‐Alster S, et al. Humoral immune response to Covid‐19 mRNA vaccine in patients with multiple sclerosis treated with high‐efficacy disease‐modifying therapies. Ther Adv Neurol Disord 2021;14:1–8. 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apostolidis SA, Kakara M, Painter MM, et al. Altered cellular and humoral responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med 2021;27:1990–2001. 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bigaut K, Kremer L, Lanotte L, et al. Impact of disease‐modifying treatments on humoral response after COVID‐19 vaccination: a mirror of the response after SARS‐CoV‐2 infection. Rev Neurol (Paris) 2021;177:1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali A, Dwyer D, Wu Q, et al. Characterization of humoral response to COVID‐19 mRNA vaccines in multiple sclerosis patients on disease‐modifying therapies. Vaccine 2021;39:6111–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Högelin KA, Ruffin N, Pin E. Development of humoral and cellular immunological memory despite SARS‐CoV‐2 despite B cell depleting treatment in multiple sclerosis. iScience 2021;24:103078. 10.1016/j.isci.2021.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mrak D, Tobudic S, Heinz LX, et al. SARS‐CoV‐2 vaccination in rituximab treated patients: B cells promote humoral immune response in the presence of T cell mediated immunity. Ann Rheum Dis 2021;80:1345–1350. [DOI] [PubMed] [Google Scholar]
- 15. Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1098–1099. 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moor MB, Stuer‐Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines in patients with a history of CD20 B‐cell‐depleting therapy (RituxiVac): an investigator‐initiated, single‐Centre, open‐label study. Lancet Rheumatol 2021;7:e789–e797. 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen‐specific CD4+ cells guide coordinated humoral and cellular immune responses to SARS‐CoV‐2 mRNA vaccination. Immunity 2021;54:2133–2142.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalimuddin S, Tham CYL, Qi M, et al. Early T cell and binding antibody responses are associated with COVID‐19 RNA vaccine efficacy onset. Medicine (N Y) 2021;2:682–688.e4. 10.1016/j.medj.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geers D, Shamier MC, Bogers S, et al. SARS‐CoV‐2 variants of concern partially escape humoral but not T‐cell responses in COVD‐19 convalescent donors and vaccinees. Sci Immunol 2021;6:eabj1750. 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bange EM, Han NA, Wileyto P, et al. CD8 (+) positive T cells contribute to survival in patients with COVID‐19 disease and hematologic cancer. Nat Med 2021;27:1280–1289. 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madelon N, Lauper K, Breville G, et al. Patients treated with anti‐CD20 therapy can mount robust T cell responses to mRNA‐based COVID‐19 vaccines. Clin Infect Dis 2021;2021:ciab954. 10.1093/cid/ciab954. [DOI] [Google Scholar]
- 22. Ferguson J, Murugesan K, Banaei N, Liu A. Interferon‐gamma release assay testing to assess Covid‐19 vaccination in a SARS‐CoV‐2 negative patient on rituximab: a case report. Int J Infect Dis 2021;110:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brill L, Rechtman A, Zveik O, et al. Humoral and T cell response to SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021;78:1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gadani SP, Reyes‐Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS‐CoV‐2 vaccination in people with multiple sclerosis on anti‐CD20 therapy. EBioMedicine 2021. Oct;16:103636. 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tallantyre EC, Vickaryous N, Anderson V, et al. Covid‐19 vaccine response in people with multiple sclerosis. Ann Neurol 2021;91:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haslacher H, Gerner M, Hofer P, et al. Usage data and scientific impact of the prospectively established fluid Bioressources at the hospital‐based MedUni Wien biobank. Biopreserv Biobank 2018;16:477–482. 10.1089/bio.2018.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins V, Fabros A, Kulasingham V. Quantitative measurement of anti‐SARS‐CoV‐2 antibodies: analytical and clinical evaluation. J Clin Microbiol 2021;59:e03149–20. 10.1128/jcm.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perkmann T, Perkmann‐Nagele N, Breyer MK, et al. Side by side comparison of three fully automated SARS‐CoV‐2 antibody assays with a focus on specifity. Clin Chem 2020;66:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat Med 2021;27:2032–2040. 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bar‐Or A, O'Brien SM, Sweeney ML, et al. Clinical perspectives on the molecular and pharmacological attributes of anti‐CD20 therapies for multiple sclerosis. CNS Drugs 2021;35:985–997. 10.1007/s40263-021-00843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellrichmann G, Bolz J, Peschke M, et al. Peripheral CD19+ B cell‐counts and infusion intervals as a surrogate for long‐term B cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spektrum disorders. J Neurol 2019;266:57–67. 10.1007/s00415-018-9092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giovannoni G, Hawkes CH, Lechner‐Scott J, et al. COVID‐19 vaccines and multiple sclerosis disease‐modifying therapies. Mult Scler Relat Disord 2021;53:103155. 10.1016/j.msard.2021.103155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker D, Pryce J, James LK, et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk:benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020;44:102279. [DOI] [PubMed] [Google Scholar]
- 34. Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of Covid‐19. Neurol Neuroimmunol Neuroinflamm 2021. Jul;8:e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT1626b vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur 2021;10:100208. 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seppälä E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta variant (B.1.617.2), Norway, April to august 2021. Euro Surveill 2021;26:2100793. 10.2807/1560-7917.ES.2021.26.35.2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Characteristics of Patients and Healthy Controls Who Have Been Vaccinated Against COVID‐19


