Abstract
Objectives
To systematically evaluate the efficacy and safety of arbidol and lopinavir/ritonavir (LPV/r) in the treatment of coronavirus disease 2019 (COVID‐19) using a meta‐analysis method.
Methods
The China Knowledge Network, VIP database, WanFang database PubMed database, Embase database, and Cochrane Library were searched for a collection of comparative studies on arbidol and lopinavir/ritonavir in the treatment of COVID‐19. Meta‐analysis was used to evaluate the efficacy and safety of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19.
Results
The results of the systematic review indicated that Arbidol had a higher positive‐to‐negative conversion rate of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleic acid on Day 7 (p = 0.03), a higher positive‐to‐negative conversion rate of SARS‐CoV‐2 nucleic acid on Day 14 (p = 0.006), a higher improvement rate of chest computed tomography on Day 14 (p = 0.02), a lower incidence of adverse reactions (p = 0.002) and lower rate of mortality (p = 0.007). There was no difference in the rate of cough disappearance on Day 14 (p = 0.24) or the rate of severe/critical illness (p = 0.07) between the two groups.
Conclusions
Arbidol may be superior to lopinavir/ritonavir in the treatment of COVID‐19. However, due to the small number of included studies and the number of patients, high‐quality multicenter large‐sample randomized double‐blind controlled trials are still needed for verification.
Keywords: arbidol, coronavirus disease 2019, lopinavir/ritonavir, meta‐analysis
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a clinical syndrome that predominantly affects the acute respiratory tract and is caused by a new type of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which has broken out globally. 1 , 2 The disease is characterized by pneumonia with fever, cough, shortness of breath, and fatigue as the main symptoms. Severe and critical cases of COVID‐19 may result in respiratory failure and renal failure and thus can be life endangering. 3 There is no effective and specific treatment plan for COVID‐19 infection, 4 , 5 and there is an urgent need to find an effective antiviral drug against SARS‐CoV‐2. At present, many choices of treatment drugs come from the clinical treatment experience of severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) infections. 6 , 7 , 8 Based on previous experience with the treatment of SARS and MERS and related clinical and basic research, it is speculated that lopinavir/ritonavir may have a certain effect on the treatment of COVID‐19. Lopinavir/Ritonavir is an aspartic protease inhibitor used to treat human immunodeficiency virus (HIV) infection and is currently a second‐line antiretroviral therapy drug. 9 The results of animal experiments show that lopinavir/ritonavir can inhibit the activity of β‐coronaviruses to a certain extent. 10 Arbidol, a drug for the prevention and treatment of influenza, is a synthetic broad‐spectrum antiviral compound mainly used to prevent and treat human influenza A and B (flu) and other acute respiratory viral infections. 11 , 12 In addition to having antiviral and anti‐inflammatory activities against various types of influenza viruses (especially H1N1), arbidol has broad‐spectrum antiviral activity in vitro and in vivo. 13 It is also recommended for COVID‐19. However, whether arbidol is an effective antiviral treatment for COVID‐19 compared with other antiviral treatments remains controversial. 14 Therefore, timely and systematic evaluation of the therapeutic effects of the above two drugs on COVID‐19 is of great significance. In this study, a systematic evaluation method was used to retrieve controlled clinical trials of Arbidol and lopinavir/ritonavir for COVID‐19 and to further evaluate the efficacy of arbidol and lopinavir/ritonavir for COVID‐19 patients to provide evidence‐based medical evidence for clinical treatment.
2. METHODS
We conducted this research according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, 15 and registered our review on the International Platform of Registered Systematic Review and Meta‐analysis Protocols (INPLASY). The registration number is INPLASY202190063.
2.1. Inclusion criteria and exclusion criteria
2.1.1. Inclusion criteria
(1) Patients with confirmed COVID‐19; (2) Age ≥18 years old; (3) Hospital stay longer than 14 days; (4) Intervention measures: use of Arbidol or lopinavir/ritonavir to treat COVID‐19 and compare the effects of the two groups; (5) Study types: randomized controlled trials, cohort studies, retrospective controlled trials; (6) Outcome indicators: rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 7, rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14, rate of improvement on chest computed tomography (CT) on Day 14, rate of cough disappearance on Day 14, rate of progression to severe/critically illness, rate of mortality and incidence of adverse reactions.
2.1.2. Exclusion criteria
(1) Literature types such as letters, comments, reviews, and duplicate studies; (2) Outcome indicators that were incomplete or unable to be extracted; (3) Low‐quality studies or studies with obvious bias; or (4) Studies without a control group.
2.2. Search strategy
The databases for the literature search include the China Knowledge Network Database (CNKI), WanFang Database (WanFang database), VIP Chinese Science and Technology Journal Database (VIP), PubMed, Embase, and Cochrane Library. The retrieval time was from December 1, 2019 to February 16, 2021. The search terms were Corona Virus Disease 2019, novel coronavirus‐infected pneumonia, 2019‐nCoV, COVID‐19, Arbidol, lopinavir, and lopinavir/ritonavir. We included the following items, #1: “Corona Virus Disease 2019” OR “novel coronavirus‐infected pneumonia” OR “2019‐nCoV” OR “COVID‐19”; #2: “Arbidol” OR “lopinavir” OR “lopinavir/ritonavir”; #3: #1 AND #2. The search references were combined in the literature retrieved, and as many relevant studies were obtained as possible.
2.3. Data extraction
Two researchers strictly followed the inclusion and exclusion criteria to independently screen the literature. If there was a disagreement, the full text of the literature was read, and then the two parties discussed the article; when necessary, a third researcher decided whether the study was included. Preliminary elimination was performed by reading the title and abstract, obtaining the full text of the preliminarily screened literature, and then screening further by reading the full text, extracting the information of the literature, including the first author, study type, study period, number of patients, sex, age, treatment time, case classification, drug usage, and outcome indicators.
2.4. Literature quality assessment
The literature quality evaluation was performed independently by two researchers, and a third party participated in the discussion and facilitated an agreement when they disagreed. The randomized trial used the modified Jadad et al. 16 scoring scale to evaluate the quality of the included studies from the following four aspects: (1) whether the random method was used (appropriate 2 points, unclear 1 point, inappropriate 0 points); (2) whether there was allocation hiding (2 points for proper, 1 point for unclear, 0 points for inappropriate or unused); (3) whether a blinding method was used (2 points for appropriate, 1 point for unclear, 0 points for inappropriate); and (4) whether there was loss to follow‐up or withdrawal (1 point if described, 0 points if not described). The total possible score is 7 points; scores from 1 to 3 are considered low‐quality research, and scores from 4 to 7 are categorized as high‐quality research. Four were case‐control studies, so the Newcastle‐Ottawa Scale (NOS) 17 was used for quality evaluation. According to the NOS scoring system, the selection (4 points), comparability (2 points) and outcome/exposure (3 points) of each study was determined. Studies with a score of >7 were considered to have a low risk of bias, studies with a score of 5–7 had a moderate risk of bias, and studies with a score of <5 had a high risk of bias. Articles with a high risk of bias were excluded. For randomized controlled trial (RCTs), in addition to using the modified Jadad scoring scale, we also used the Cochrane Collaboration tool to assess the risk of bias. 18
2.5. Statistical methods
RevMan 5.3 provided by the Cochrane Collaboration was used for data analysis. The rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 7, rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14, rate of cough disappearance on Day 14, rate of improvement of chest CT on Day 14, rate of becoming severely/critically ill, rate of mortality and incidence of adverse reactions were binary variables, and the odds ratio (OR) and 95% confidence interval (CI) were used to demonstrate an effect. For continuous variables, mean difference and its 95% CI were used as the effect value. If there was no significant difference by the Q test (p > 0.10, I 2 ≤ 50%), the fixed‐effects model was used for the meta‐analysis. 19 If there was a significant difference (p ≤ 0.10 and I 2 > 50%), If there was a significant difference (p ≤ 0.10 and I 2 > 50%), sensitivity analysis or subgroup analysis was conducted to determine the source of heterogeneity, and the source of heterogeneity was eliminated to check whether the results were the same. If the heterogeneity test could not be carried out and the source of heterogeneity could not be eliminated, then the statistical heterogeneity between studies was considered too large for a comparative analysis, and only a descriptive analysis was performed. When p < 0.05, there was a significant difference. If the number of studies was greater than 10, publication bias was evaluated by funnel plots. 20
3. RESULTS
3.1. Literature search results
Initially, 143 studies were retrieved through databases. By reading the titles and abstracts, 41 duplicate studies, 19 studies that were irrelevant to the research purpose, 62 reviews, 8 experience summaries, and 3 cases were excluded. The remaining 10 studies were rescreened after reading the full text, and 1 study without a control group and 2 studies with combination medication were excluded. After the above step‐by‐step screening process, 7 studies 21 , 22 , 23 , 24 , 25 , 26 , 27 were ultimately included. The screening process is shown in Figure 1. The basic information of the literature is shown in Table 1.
Figure 1.
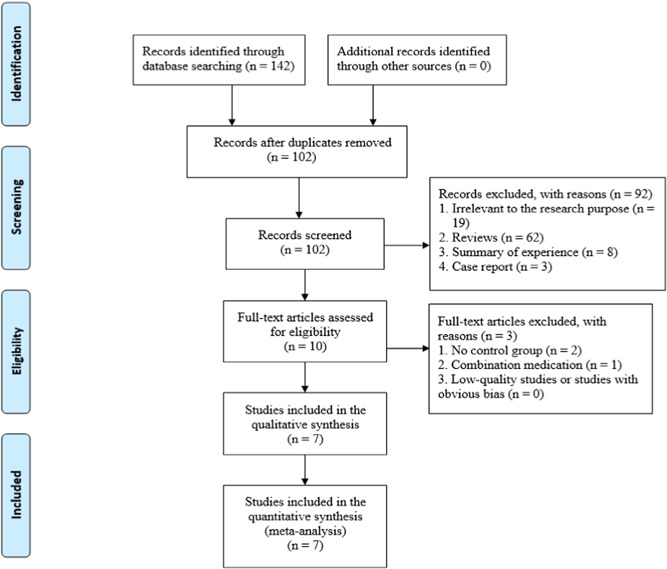
Literature screening process
Table 1.
Characteristics of the studies included in this meta‐analysis
| Study | Type of study | Range of time | Location of study | Group | Samples (M/F) | Age (years) | Treatment | Type of | Intervention | Score (Jadad/NOS) |
|---|---|---|---|---|---|---|---|---|---|---|
| time (day) | disease | |||||||||
| Chen et al. 21 | RCT | 2020.02.20 to 2020.03.01 | China | Arbidol | 120 (51/69) | NA | 10 | Moderate 111 | Arbidol (200 mg three times a day) | 4 |
| Severe 8 | ||||||||||
| Critical 1 | ||||||||||
| LPV/r | 116 (59/57) | NA | 10 | Moderate 98 | Favipiravir (1600 mg, twice first day, followed by 600 mg, twice daily, for the following days) | |||||
| Severe 16 | ||||||||||
| Critical 2 | ||||||||||
| Chen et al. 22 | Retro | 2020.01.20 to 2020.02.06 | China | Arbidol | 34 (18/16) | 44 (34,62) | 5 | Moderate 33 | Arbidol (200 mg, three times a day) | 8 |
| Severe 0 | ||||||||||
| Critical 1 | ||||||||||
| LPV/r | 52 (27/25) | 47 (35, 60) | 5 | Moderate 52 | 200 mg/50 mg of LPV/r, twice a day | |||||
| Severe 0 | ||||||||||
| Critical 0 | ||||||||||
| Li et al. 23 | RCT | 2020.02.01 to 2020.03.28 | China | Arbidol | 35 (16/19) | 50.5 ± 14.6 | 7–14 | Mild 2 | Arbidol (200 mg, three times a day) | 7 |
| Moderate 33 | ||||||||||
| LPV/r | 34 (17/17) | 50.7 ± 15.4 | 7–14 | Mild 6 | 200 mg/50 mg of LPV/r, twice a day | |||||
| Moderate 28 | ||||||||||
| Liu et al. 24 | Retro | 2019.12.13 to 2020.03.29 | China | Arbidol | 257 (132/125) | 59.5 ± 14.9 | 1.5 (0.5–31.5) | NA | NA | 9 |
| LPV/r | 259 (129/130) | 59.5 ± 14.9 | 0.50 (0.5–32.5) | NA | NA | |||||
| Wen et al. 25 | Retro | 2020.01.20 to 2020.02.10 | China | Arbidol | 36 (16/20) | 53.39 ± 15.37 | 7 | Mild 2 | Arbidol (200 mg, three times a day) | 8 |
| Moderate 34 | ||||||||||
| Severe 0 | ||||||||||
| LPV/r | 59 (27/32) | 51.66 ± 16.40 | 7 | Mild 11 | 200 mg/50 mg of LPV/r, twice a day | |||||
| Moderate 45 | ||||||||||
| Severe 3 | ||||||||||
| Zhu et al. 26 | Retro | 2020.01.23 to 2020.02.29 | China | Arbidol | 16 (10/6) | 26.5 (23.3–52.5) | 7 | NA | Arbidol (200 mg, three times a day) | 8 |
| LPV/r | 34 (20/14) | 40.5 (34.8–52.3) | 7 | NA | 400 mg/100 mg of LPV/r, twice a day | |||||
| Nojomi et al. 27 | 2020.04.20 to 2020.06.18 | Iran | Arbidol | 50 (33/17) | 56.6 ± 17.8 | 7–14 | Mild 5 | Arbidol (200 mg, three times a day) | 5 | |
| Moderate 33 | ||||||||||
| Severe 12 | ||||||||||
| LPV/r | 50 (27/23) | 56.2 ± 14.8 | 7–14 | Mild 6 | 400 mg of LPV/r, twice a day | |||||
| Moderate 33 | ||||||||||
| Severe 11 |
Abbreviations: F, female; LPV/r, lopinavir/ritonavir; M, male; NA, not available; NOS, Newcastle‐Ottawa Scale; RCT, randomized controlled trial; Retro, retrospective study.
3.2. Literature quality evaluation results
Three studies 21 , 23 , 27 were RCTs and were scored by modified Jadad et al. 16 one 21 with 4 points, one 27 with 5 points, and one 23 with 7 points. Four studies 22 , 24 , 25 , 26 were retrospective studies and scored by NOS: 3 studies 22 , 25 , 26 were 8 points, and 1 study 24 was 9 points. Assessment of the risk of bias using the Cochrane collaboration tool is presented in Figures 2 and 3.
Figure 2.
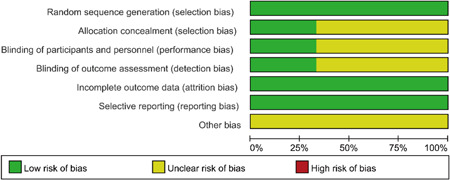
Risk of bias graph for the randomized controlled trials included in this study
Figure 3.
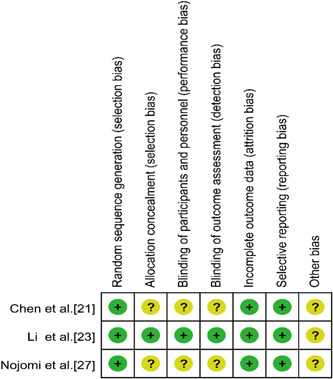
Risk of bias summary in of the randomized controlled trials included in this study
3.3. Meta‐analysis results
3.3.1. Rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 7
Four studies compared the rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 7 of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity among the studies (p = 0.53, I 2 = 0%) using a fixed‐effects model combined effect size for analysis. Through analysis, it was concluded that Arbidol has a higher nucleic acid conversion rate than lopinavir/ritonavir for the treatment of COVID‐19 in 7 days, and the difference was statistically significant (OR = 1.86, 95% CI: [1.05, 3.29], p = 0.03), as shown in Figure 4.
Figure 4.
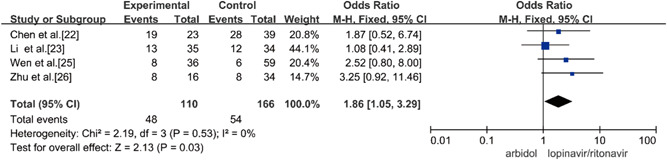
Rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 7 between the two groups. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3.2. Rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14 between the two groups
Three studies compared the rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14 of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity among the studies (p = 0.20, I 2 = 38%) using the fixed‐effects model to combine the effect size for analysis. Through the analysis, it was concluded that Arbidol has a higher rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14 than lopinavir/ritonavir for treatment of COVID‐19 in 14 days and that the difference was statistically significant (OR = 3.09, 95% CI: [1.38, 6.93], p = 0.006), as shown in Figure 5.
Figure 5.
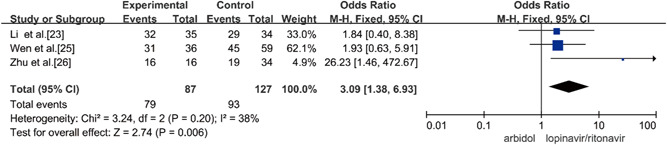
Rate of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Day 14 between the two groups. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3.3. Rate of cough disappearance on Day 14
Two studies compared the 14‐day cough disappearance rate of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity between the studies (p = 0.35, I 2 = 0%) using a fixed‐effects model to combine effect size for analysis. Through the analysis, it was concluded that there was no significant difference in the cough disappearance rate between Arbidol and lopinavir/ritonavir in the treatment of COVID‐19 in 14 days (OR = 0.65, 95% CI: [0.32, 1.33], p = 0.24], as shown in Figure 6.
Figure 6.
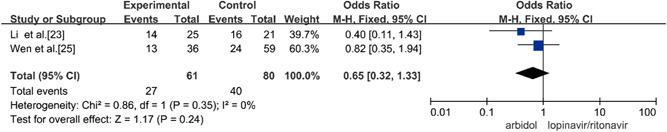
Rate of cough disappearance on Day 14 between the two groups
3.3.4. Rate of improvement of chest CT on Day 14
Four studies compared the rate of improvement of chest CT on Day 14 of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity between the studies (p = 0.14, I 2 = 46%) using a fixed‐effects model to combine effect size for analysis. Through the analysis, it was concluded that the rate of improvement of chest CT on Day 14 of A was higher than that of B in the treatment of COVID‐19 and that this difference was statistically significant (OR = 1.49, 95% CI: [1.06, 2.08], p = 0.02), as shown in Figure 7.
Figure 7.
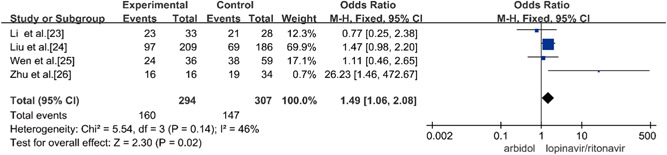
Rate of improvement of chest CT on Day 14 between the two groups. CT, computed tomography
3.3.5. Rate of becoming severely/critically ill
Three studies compared the rate of becoming severe/critically ill of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity among the studies (p = 0.88, I 2 = 0%) using the fixed‐effects model to combine effect size for analysis. Through the analysis, it was concluded that there was no significant difference between the conversion rates of Arbidol and lopinavir/ritonavir in the treatment of COVID‐19 (OR = 0.32, 95% CI: [0.10, 1.09], p = 0.07], as shown in Figure 8.
Figure 8.
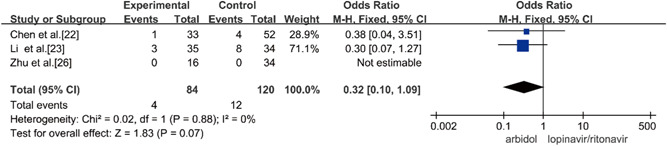
Rates of becoming severely/critically ill between the two groups
3.3.6. Rate of mortality
Three studies compared the rate of mortality between Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity between the studies (p = 0.95, I 2 = 0%). Fixed‐effect models were used to combine effect sizes for analysis. Through the analysis, it was concluded that the rate of mortality in the treatment of COVID‐19 by Arbidol was lower than that of lopinavir/ritonavir and that the difference was statistically significant (OR = 0.45, 95% CI: [0.26, 0.81], p = 0.007), as shown in Figure 9.
Figure 9.
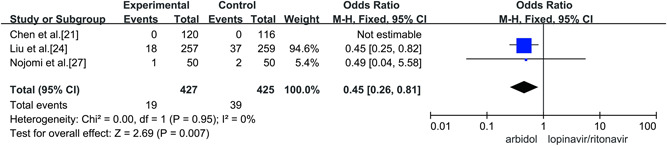
Rate of mortality between the two groups
3.3.7. Incidence of adverse reactions
Five studies compared the incidence of adverse reactions between Arbidol and lopinavir/ritonavir in the treatment of COVID‐19, and there was no statistical heterogeneity between the studies (p = 0.34, I 2 = 12%). Fixed‐effect models were used to combine effect sizes for analysis. Through the analysis, it was concluded that the incidence of adverse reactions in the treatment of COVID‐19 by Arbidol was lower than that of lopinavir/ritonavir and that the difference was statistically significant (OR = 0.55, 95% CI: [0.38, 0.81], p = 0.002), as shown in Figure 10.
Figure 10.
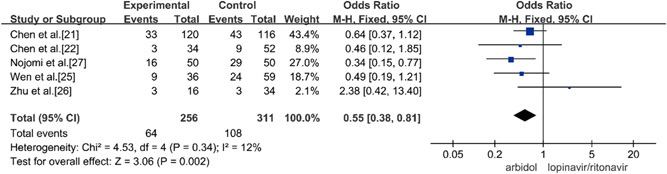
Incidence of adverse reactions between the two groups
3.4. Publication bias
Since the number of studies was less than 10, a funnel plot to demonstrate publication bias was not applied in this meta‐analysis.
4. DISCUSSION
The global situation of COVID‐19 is becoming increasingly severe, affecting hundreds of countries around the world. It is listed by the World Health Organization as a “public health emergency of international concern,” which seriously threatens the lives of people around the world and arouses global attention. 28 , 29 Since 2019, COVID‐19 has exhibited a high infection rate and high mortality rate; in addition to a lack of targeted antiviral drugs, 30 the number of confirmed cases and deaths is still increasing. Therefore, finding effective antiviral drugs is extremely important for the clinic.
LPV/r is mainly used to treat HIV and can also be used to treat acquired immunodeficiency syndrome (AIDS), SARS‐CoV, and MERS. Notably, LPV/r has been shown to inhibit SARS‐CoV in in vitro studies. 31 , 32 Studies have shown that SARS‐CoV‐2, like the SARS‐CoV, enters the cell through the angiotensin‐converting enzyme 2 receptor, 33 so LPV/r may inhibit the normal function of the coronavirus and then exert an antiviral effect. A South Korean study showed that after treatment with LPV/r, the patient's viral load decreased, and clinical symptoms were alleviated. 34 Arbidol is a broad‐spectrum antiviral over‐the‐counter drug developed in Russia with low toxicity. As an anti‐influenza drug, it has been used clinically in several countries for decades and has good efficacy and safety. 35 It blocks virus replication by inhibiting the fusion of the influenza virus lipid membrane with host cells 36 and can also be used to treat SARS and MERS coronavirus, 37 Zika virus, 35 Lhasa fever virus, and Ebola virus, 32 as well as many other viruses. Arbidol can prevent viruses from entering cells, 38 induce interferon, 39 and improve bodily immunity. 40 Li Lanjuan's team announced recent research results on the treatment of COVID‐19 in February 2020, confirming that Arbidol can effectively inhibit coronavirus in vitro. 41 Based on the results of in vitro experiments and the experience of clinical treatment of COVID‐19, on February 18, 2020, the “New Coronavirus Pneumonia Diagnosis and Treatment Plan (Sixth Edition)” issued by the National Health Commission of China recommended Arbidol for anti‐COVID‐19 treatment. 42
A total of 7 studies were included in this meta‐analysis, with 548 cases in the Arbidol group and 604 cases in the LPV/r group. Both groups were comparable at baseline. The results showed that compared with the LPV/r group, the Arbidol group had higher rates of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Days 7 and 14 and a higher rate of improvement of chest CT on Day 14. According to previous in vitro studies, LPV/r needs to reach 4 μg/ml to inhibit SARS‐CoV. 8 In the studies included in this meta‐analysis, blood concentration monitoring was not performed, so it was impossible to determine whether the results in the LPV/r group were due to insufficient blood concentrations leading to lower rates of positive‐to‐negative conversion of SARS‐CoV‐2 nucleic acid on Days 7 and 14 and a lower rate of improvement of chest CT on Day 14. Therefore, it is necessary to further study the human threshold concentration and monitor the blood concentration for confirmation. Four studies reported the incidence of adverse reactions. There were adverse reactions in both the Arbidol group and the LPV/r group. The overall adverse reactions of the Arbidol group were lower than those of the LPV/r group. Gastrointestinal symptoms were the most common adverse reactions, especially among patients using LPV/r, which may affect the overall recovery of patients in the LPV/r group. Therefore, it is necessary to pay close attention to adverse reactions and determine the threshold concentration of the drug during the treatment process, as it is expected to achieve the therapeutic effect while avoiding adverse reactions as much as possible.
This study has the following shortcomings: ① The number of included studies is small, and most of the research sites are in China. It is difficult to conduct subgroup analysis of other countries and races, and it is impossible to conduct bias testing; ② Most studies are nonrandomized double‐blind studies, and high‐quality multicenter large‐sample randomized double‐blind controlled trials are still needed to verify the results; ③ There is no mention of blinding in the randomized studies; and ④ The proportion of severe cases in the observed patients is relatively low.
5. CONCLUSIONS
Through a systematic review in this article, Arbidol may be superior to lopinavir/ritonavir in the treatment of COVID‐19, and the incidence of adverse reactions is low. However, given the small number of included studies and the number of patients, high‐quality multicenter large‐sample randomized double‐blind controlled trials are still needed for verification.
FUNDING INFORMATION
This work was not funded.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Miao Yu and Deng‐Chao Wang: designed the study. Sheng Li: ran the search strategy. Li‐Yan Huang: collected data. Deng‐Chao Wang and Miao Yu: re‐checked the data. Sheng Li and Jian Wei: performed analyses and Deng‐Chao Wang checked the analyses. Miao Yu and Li‐Yan Huang: assessed the quality of studies, and Deng‐Chao Wang confirmed the quality. Miao Yu: wrote the manuscript, and Yue‐Hua Lei edited the manuscript. All listed authors reviewed and revised the manuscript.
ACKNOWLEDGEMENTS
None.
Yu M, Wang D‐C, Li S, Lei Y‐H, Wei J, Huang L‐Y. Meta‐analysis of arbidol versus lopinavir/ritonavir in the treatment of coronavirus disease 2019. J Med Virol. 2022;94:1513‐1522. 10.1002/jmv.27481
DATA AVAILABILITY STATEMENT
All data relevant to this study are included in this article.
REFERENCES
- 1. Ralph R, Lew J, Zeng T, et al. 2019‐nCoV (Wuhan virus), a novel coronavirus: human‐to‐human transmission, travel‐related cases, and vaccine readiness. J Infect Dev Ctries. 2020;14(14):3‐17. [DOI] [PubMed] [Google Scholar]
- 2. Tobaiqy M, Qashqary M, Al‐Dahery S, et al. Therapeutic management of patients with COVID‐19: a systematic review. Infect Prev Pract. 2020;2:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS‐CoV‐2) based on current evidence. Int J Antimicrob Agents. 2020;55:105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vellingiri B, Jayaramayya K, Iyer M, et al. COVID‐19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meo SA, Alhowikan AM, Al‐Khlaiwi T, et al. Novel coronavirus 2019‐nCoV: prevalence, biological and clinical characteristics comparison with SARS‐CoV and MERS‐CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012‐2019. [DOI] [PubMed] [Google Scholar]
- 7. Cai H. Safety application of novel coronavirus pneumonia antiviral drugs. Adverse Drug React J. 2020;22:95‐102. [Google Scholar]
- 8. Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Croxtall JD, Perry CM. Lopinavir/Ritonavir: a review of its use in the management of HIV‐1 infection[J]. Drugs. 2010;7014:1885‐1915. [DOI] [PubMed] [Google Scholar]
- 10. Chan JF, Yao Y, Yeung ML, et al. Treatment with Lopinavir/Ritonavir or Interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset[J]. J Infect Dis. 2015;212(12):1904‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ. Arbidol: a broad‐spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997‐1005. [DOI] [PubMed] [Google Scholar]
- 12. Shi L, Xiong H, He J, et al. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152:1447‐1455. [DOI] [PubMed] [Google Scholar]
- 13. Zhong Q, Yang Z, Liu Y, et al. Antiviral activity of arbidol against Coxsackie virus B5 in vitro and in vivo. Arch Virol. 2009;154(4):601‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amani B, Amani B, Zareei S, Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID‐19: a systematic review and meta‐analysis[J]. Immun Inflamm Dis. 2021;9:1197‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 16. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials[J]. BMJ. 2011;343:343‐d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses[J]. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sedgwick P, Marston L. How to read a funnel plot in a meta‐analysis[J]. BMJ. 2015:351. [DOI] [PubMed] [Google Scholar]
- 21. Chen C, Huang J, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. 2020; medRxiv. 10.1101/2020.03.17.20037432 [DOI]
- 22. Hsieh SM, Pan SC, Chen SY, Huang PF, Chang SC. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020;38:86‐89. [Google Scholar]
- 23. Li Y, Xie Z, Lin W, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID‐19: an exploratory randomized controlled trial. Med (N Y). 2020;1:105‐113.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Fang X, Tian L, et al. The effect of arbidol hydrochloride on reducing mortality of Covid‐19 patients: a retrospective study of real world date from three hospitals in Wuhan. 2020;medRxiv. 10.1101/2020.04.11.20056523 [DOI]
- 25. Wen CY, Xie ZW, Li YP, et al. Real‐world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID‐19: an observational cohort study. Zhonghua Nei Ke Za Zhi. 2020;59:E012. [DOI] [PubMed] [Google Scholar]
- 26. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID‐19. J Infect. 2020;81:e21‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nojomi M, Yassin Z, Keyvani H, et al. Effect of arbidol (Umifenovir) on COVID‐19: a randomized controlled trial. BMC Infect Dis. 2020;20:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allam M, Cai S, Ganesh S, et al. COVID‐19 diagnostics, tools, and prevention. Diagnostics (Basel). 2020;10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Acter T, Uddin N, Das J, Akhter A, Choudhury TR, Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as coronavirus disease 2019 (COVID‐19) pandemic: a global health emergency. Sci Total Environ. 2020;730:138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee GA, Seneviratne T, Noor MA, et al. The metabolic effects of lopinavir/ritonavir in HIV‐negative men. AIDS. 2004;18:641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 3, 2006:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hulseberg CE, Fénéant L. Szymańska‐de Wijs KM, et al. Arbidol and other low‐molecular‐weight drugs that inhibit lassa and ebola viruses. J Virol. 2019;93:e02185‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of covid‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fink SL, Vojtech L, Wagoner J, et al. The antiviral drug arbidol inhibits zika virus. Sci Rep. 2018;8:8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teissier E, Zandomeneghi G, Loquet A, et al. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One. 2011;6:e15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID‐19: initial assessment. J Int AIDS Soc. 2020;23:e25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blaising J, Lévy PL, Polyak SJ, Stanifer M, Boulant S, Pécheur EI. Arbidol inhibits viral entry by interfering with clathrin‐dependent trafficking. Antivir Res. 2013;100:215‐219. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Ding Y, Yang C, et al. Inhibition of the infectivity and inflammatory response of influenza virus by arbidol hydrochloride in vitro and in vivo (mice and ferret). Biomed Pharmacother. 2017;91:393‐401. [DOI] [PubMed] [Google Scholar]
- 40. Arastoo M, Khorram Khorshid HR, Radmanesh R, Gharibdoust F. Combination of IMOD™ and arbidol to increase their immunomodulatory effects as a novel medicine to prevent and cure influenza and some other infectious diseases. J Med Hypotheses Ideas. 2014;8:53‐56. [Google Scholar]
- 41. Chen Y, Yang Z, Chen J, Li X, Shen Y. Status and research progress of clinical trials of COVID‐19 drugs. Pharm Today. 2020;30:160‐163. [Google Scholar]
- 42. National Health Commission, National Administration of Traditional Chinese Medicine. Guidelines for the diagnosis and treatment of novel coronavirus pneumonia (trial version sixth). Chin J Viral Dis. 2020;10:88‐92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this study are included in this article.


