Abstract
Aim
The study aimed to describe clinical characteristics and outcomes of pregnant women with COVID‐19 undergoing cesarean section, and evaluated the association of blood values at admission with severe COVID‐19 disease in this group of patients.
Method
We retrospectively analyzed the clinical data of 110 patients infected with COVID‐19 who underwent cesarean section at Adana City Education and Research Hospital in Turkey. The COVID‐19 severity of the patients was classified as either severe or nonsevere disease according to World Health Organization of COVID‐19 clinical management guidance. We compared blood values, clinical characteristics, and outcomes between severe and nonsevere patients. Receiver operating characteristics (ROC) curves analyses and area under the ROC curve (AUC) value was calculated to evaluate the predictive value of blood parameters on the COVID‐19 severity.
Results
Of the 110 women, 12 were severe cases. Severe patients had higher ferritin, neutrophil‐to‐lymphocyte ratio (NLR), lactate dehydrogenase (LDH), alanine transaminase (ALT), aspartate transaminase (AST), and procalcitonin levels on admission (p < 0.05). The ROC analysis demonstrated AUC of NLR, LDH, AST, ALT, ferritin, and procalcitonin was 0.757, 0.856, 0.840, 0.771, 0.821, and 0.698, respectively. The LDH had a maximum specificity (90.8%), with the cutoff value of 365. The O‐blood group was more likely to have severe illness than the non‐O‐blood group (relative risk: 3.6; 95% confidence interval; 1.2–10.4).
Conclusion
This study shows that LDH values at admission are an early and powerful predictor of severe infection for pregnant women with COVID‐19 who will undergo a cesarean section.
Keywords: COVID‐19, L‐lactate dehydrogenase, maternal outcome, pregnancy, SARS‐CoV‐2, Turkey
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is affecting many women during pregnancy and postpartum worldwide. Pregnant women and their fetuses are a high‐risk group during infectious disease epidemics. Pregnancy‐related physiological changes and immunological adaptations increase susceptibility to infections and have serious effects on the severity and outcome of viral diseases in pregnant women. 1 , 2 Previous studies on the 2009 H1N1 influenza virus found that pregnancy increased the risk of severe pneumonia, acute respiratory distress syndrome (ARDS), mechanical ventilation, and mortality when compared to nonpregnant women of reproductive age. 3 , 4 Similar findings were observed during the severe acute respiratory syndrome and Middle East respiratory distress syndrome outbreaks, in which pregnant women were more likely to suffer organ failure and die. 2 , 5
Several studies have reported the perioperative outcome of SARS‐CoV‐2‐infected patients. 6 , 7 A recent study found that surgical mortality was 23.8% in patients infected with COVID‐19, and more than half of patients developed postoperative pulmonary complications. 6 In a similar study, mortality in surgical patients was shown to be two times higher in COVID‐19‐infected patients. 7 In a recently published meta‐analysis, Francesca et al reported 8% of the pregnant women with COVID‐19 needed intensive care unit (ICU) admission. 8 The authors reported maternal mortality in 5 out of 1100 pregnant women. The finding of that meta‐analysis showed the most frequent laboratory findings in pregnant women with COVID‐19 were elevated C‐reactive protein (CRP) and lymphocytopenia. Other studies showed that increased lactate dehydrogenase (LDH), procalcitonin, RDW, and liver enzymes are associated with COVID‐19 severity. 9 , 10 , 11 It is also well known that patients with severe disease were more likely to have any comorbid disease. 12 Assuming that surgery and COVID‐19 disease association increase adverse maternal outcomes, information on specific risk factors for severe COVID‐19 infection among pregnant women may become important in the early recognition of this vulnerable population. To date, there are limited data examining the role of blood markers in COVID‐19 infected pregnant women undergoing cesarean delivery.
To address the above issues, we conducted a retrospective cohort study focusing only on COVID‐19‐infected pregnant women who underwent cesarean section at our tertiary referral center, Adana City Education and Research Hospital, Turkey. Our primary objective was to describe the perioperative characteristics of pregnant women infected with COVID‐19 who underwent cesarean section and their maternal and neonatal outcomes. As a secondary objective, we assessed the baseline values of laboratory indices, inflammatory biomarkers, and hemocytometer variables of pregnant women and their association with the severity of COVID‐19 infection.
Materials and Methods
This single‐center retrospective cohort analysis was conducted on pregnant women with confirmed SARS‐CoV‐2 infection who were admitted to Adana City Education and Research Hospital and underwent cesarean section between April 1, 2020, and June 1, 2021. Turkey has the fourth‐highest number of cases in Europe, 13 and Adana is the most populous city in Turkey's south. Annually, Adana City Education and Research Hospital receives approximately 12 000 births. 14 With the COVID‐19 pandemic, the Turkish Ministry of Health released a guideline including testing, treatment, hospitalization, and ICU admission of COVID‐19 patients. 15 We follow this guideline in our clinical practice. Due to the city's high COVID‐19 prevalence, COVID‐19 reverse transcription‐polymerase chain reaction (RT‐PCR) testing has been routinely performed on all patients undergoing surgery since April 1, 2020. The study was approved by the Turkish Ministry of Health and Institutional Review Board (May‐2021, Ref 1392‐80) and conducted in accordance with the principles of the Helsinki Declaration.
The inclusion criteria were parturients who underwent cesarean section and had a positive RT‐PCR test result for COVID‐19 with nasopharyngeal swabs. The COVID‐19 diagnosis was made according to the World Health Organization's interim guidance. 16 We excluded pregnant women with incomplete data. COVID‐positive pregnant women who had vaginal birth were also excluded, as our specific aim in this study was to examine risk factors and outcomes after cesarean section.
Our blood panel investigation for the cesarean section includes complete blood count, blood glucose, blood type, aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, urea, creatine, and coagulation tests. If the patient is hospitalized with COVID‐19, procalcitonin, CRP, D‐Dimer, fibrinogen, LDH, and ferritin levels are also checked.
The patients' written and electronic medical charts were reviewed. The following data were recorded: maternal age, gestational age, gravida, parity, comorbid disease, clinical symptoms, a laboratory at admission, therapy for COVID‐19, radiographic findings of patients, indication for cesarean section, American Society of Anesthesiologists score, type of anesthesia, hypotension during surgery, need for blood transfusion, need for ICU admission, need for ventilator support, hospital mortality, neonatal outcomes, and maternal outcomes. Hypotension is defined as a fall in systolic blood pressure of more than 20% below the baseline and to less than 100 mmHg. 17
The patients were divided into two groups: severe and nonsevere COVID‐19 disease. Patients with severe disease were defined as having a fever or any signs of respiratory tract infection and at least one of the following: respiratory rate ≥30/min, severe respiratory distress (dyspnea, use of accessory respiratory muscles), or room oxygen saturation in air ≤90% (PaO2/FiO2 <300 in patients receiving oxygen). 18
Our obstetric management was as follows. For women with severe COVID‐19 disease, delivery was considered for new or worsening oxygen requirement if they were at or near term. COVID‐19 was not considered an indication for cesarean delivery. However, patients who needed intubation or whose oxygen saturation did not rise above 92% despite high oxygen therapy, regardless of gestational age, underwent emergency cesarean section. Asymptomatic and nonsevere COVID‐19 patients were followed up for developing severe disease and self‐isolation was recommended. The timing of delivery for those patients was decided according to obstetric/medical indications. If a pregnant woman with asymptomatic or nonsevere COVID‐19 disease developed severe COVID‐19 disease, she was treated as described for severe COVID‐19 disease.
Statistical analysis
The Shapiro–Wilk test was used to verify the normality of the distribution of continuous variables. Continuous variables are presented as mean ± SD or median (interquartile range: 25%–75%) in the presence of abnormal distribution, and categorical variables are presented as frequencies (%). Comparisons between the groups were made using Fisher's exact test for categorical variables, the independent samples t‐test for normally distributed continuous variables, and the Mann–Whitney U test for abnormal distributions. Receiver‐operating characteristic (ROC) curves were used to analyze the power of laboratory indices at admission in predicting the severity of COVID‐19. The optimal cutoff points for each parameter were determined using Youden's index (sensitivity + specificity −1) and the maximized area under the curve (AUC). Sensitivity and specificity were calculated for these cutoff values. All statistical procedures were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL). A p value of <0.05 was considered significant.
Results
General characteristics, maternal, and fetal outcomes
There were 11 303 deliveries performed at Adana City Education and Research Hospital during the study period. Of these, 6508 (57.6%) were cesarean sections; 177 (1.6%) COVID‐19 RT‐PCR positive pregnant women delivered. Sixty‐seven patients were excluded from the study because of vaginal delivery (n = 61) and incomplete data (n = 6). The maternal baseline, clinical characteristics, and pregnancy outcomes of 110 women included in the study are presented in Tables 1 and 2. Their mean age was 31 ± 6 years. Of the 110 women, 106 (96.4%) were in their third trimester and four (3.6%) were in their second trimester of gestation. There were three stillbirths. Of the 110 cesarean sections, 26 were emergency procedures related to fetal indications (11/26), maternal obstetric indications (7/26), and severe COVID‐19 disease in the mother (8/26). Four women delivered twins. Preterm delivery occurred in 37 of 110 women who gave birth to 38 of 111 live neonates. There was 17 early preterm deliveries (gestation at less than 34 weeks). Maternal COVID‐19 severity was associated with lower gestational age at birth and an increased risk of preterm birth (p < 0.05). Of the 110 women, 89.1% (98/110) were operated on under spinal anesthesia. Hypotension was observed in 33.7% (33/98) of the patients under spinal anesthesia. Intraoperatively, only one patient required blood transfusion due to placenta previa.
TABLE 1.
Demographic and characteristics of COVID‐19 patient
| Variables | Severe patients (n = 12) | Non‐severe patients (n = 98) | All patients (n = 110) | p‐Value |
|---|---|---|---|---|
| Age, median (min.–max.), years | 31 (21–36) | 30 (19–42) | 30 (19–42) | 0.758 a |
| Gestational age, median (min.–max.), weeks | 35 (22–38) | 37 (28–41) | 37 (22–41) | 0.005 a |
| Maternal country of birth | ||||
| Turkey | 10 (83.3%) | 92 (93.9%) | 102 (92.7%) | 0.21 b |
| Syria | 2 (16.7%) | 6 (5.1%) | 8 (7.3%) | |
| Coexisting disorders | ||||
| Pneumonia | 12 (100%) | 45 (45.6%) | 57 (51.8%) | 0.003 c |
| Preeclampsia/hypertensive disease | 3 (25%) | 8 (8.2%) | 11 (10%) | 0.09 c |
| Diabetes (pregestational and gestational) | 2 (16.7%) | 7 (7.1%) | 9 (8.1%) | 0.25 c |
| Asthma | 2 (16.7%) | 3 (3.1%) | 5 (4.5%) | 0.091 c |
| ASA score | ||||
| 2 | 3 (25%) | 79 (80.1%) | 82 (74.5%) | 0.001 c |
| 3 | 4 (33.3) | 16 (16.3%) | 20 (18.2%) | 0.22 c |
| 4 | 5 (41.7%) | 3 (3.1%) | 8 (7.3%) | <0.001 c |
| Emergency operation | 8 (66.7%) | 18 (18.4) | 26 (23.6%) | <0.001 c |
| Nulliparity | 1 (8.3%) | 17 (17.3%) | 18 (16.4%) | 0.68 c |
| Twin pregnancies | 1 (8.3%) | 3 (3.1%) | 4 (3.6%) | 0.37 c |
Note: Significant values are in bold and italics.
Abbreviation: ASA, American Society of Anesthesiology.
Mann–Whitney U test.
Fisher exact test for two race groups.
Fisher exact test.
TABLE 2.
Characteristics and outcomes of newborn babies whose mothers tested positive for COVID‐19
| Variables | Severe patients (n = 12) | Non‐severe patients (n = 98) | All patients (n = 110) | p‐Value |
|---|---|---|---|---|
| Gestational age <37 weeks at delivery | 8 (66.7%) | 29 (29.6%) | 37 (33.6%) | 0.019 b |
| Gestational age <34 weeks at delivery | 5 (41.7%) | 9 (9.2%) | 14 (12.7) | 0.007 b |
| Stillbirth | 1 (8.3%) | 2 (2%) | 3 (2.7%) | 0.295 b |
| Neonates, No a | 12 | 99 | 111 | |
| Weight, median (min.–max.), g | 2600 (1340–3150) | 3200 (1630–4470) | 3150 (1340–4470) | 0.01 c |
| 1‐min Apgar score 7 ≤ | 4 (33.3%) | 13 (13.1%) | 17 (15.3%) | 0.08 b |
| 5‐min Apgar score 7 ≤ | 2 (16.6%) | 2 (2%) | 4 (3.6%) | 0.057 b |
Note: Significant values are in bold and italics.
Includes multiple births but excludes stillbirths.
Fisher exact test.
Mann–Whitney U test.
Initial blood values and COVID‐19 severity
According to the respiratory system parameters of 110 pregnant patients, 12 (10.9%) were severe COVID‐19 and 98 (89.1%) were nonsevere COVID‐19 cases. The first presentation of 11 of 12 severe patients was mild disease. These patients were followed in obstetric service designed for COVID‐19 pregnant women. Eight of them developed the severe COVID‐19 disease and underwent an emergency cesarean section. The other three patients and one asymptomatic patient (four patients) also developed severe COVID‐19 disease when they were postpartum period.
The blood values of severe and nonsevere COVID‐19 patients upon first admission to the hospital are compared in Table 3. Compared to the nonsevere patients, ferritin (p < 0.001), neutrophil‐to‐lymphocyte ratio (NLR) (p = 0.009), lactate dehydrogenase (LDH) (p < 0.001), alanine transaminase (ALT) (p = 0.003), aspartate transaminase (AST) (p < 0.001), and procalcitonin (p = 0.022) levels in severe patients were significantly higher at admission. The ROC curves analysis was used to compare the performance of these variables to predict severe disease, and the analytical results of these curves are shown in Table 4.
TABLE 3.
Comparison of admission laboratory values of pregnant patients with severe versus nonsevere COVID‐19
| Variable | All patients | Nonsevere patients | Severe patients | p‐Value |
|---|---|---|---|---|
| Age (median, IQR) | 30 (25–36) | 30 (25–36) | 31 (25–33) | 0.758 |
| White blood cell (×103/mm3) | 9.9 (7–13) | 9.7 (7–12.5) | 11.6 (17.3–15.6) | 0.151 |
| NLR | 7 (5.1–10.3) | 6.2 (5–9.2) | 10 (7.2–15.7) | 0.009 |
| LDH (U/L) | 259 (212–329.5) | 255 (204–295.5) | 447 (270–526) | <0.001 |
| ALT (U/L) | 15 (9–19) | 14 (9–18) | 20 (16.2–36) | 0.003 |
| AST (U/L) | 25 (19–35) | 22 (19–31) | 50.5 (32.7–75.5) | <0.001 |
| Ferritin (ng/mL) | 20 (11–56) | 14 (10–38) | 78 (41.2–129) | <0.001 |
| Hemoglobin (g/dL) | 11.2 (19.9–12.1) | 11.3 (9.9–12.5) | 11 (9.9–12.3) | 0.875 |
| RDW (%) | 15 (14–16.1) | 15 (13.9–15.9) | 15.2 (14.4–16.9) | 0.271 |
| D‐dimer (μg/L) | 1160 (789–2145) | 1260 (810–2450) | 922 (530–1310) | 0.128 |
| C‐reactive protein (mg/L) | 43 (10–91.4) | 27.9 (8.5–27.9) | 76.2 (33–112.5) | 0.064 |
| Procalcitonin (ng/L) | 0.07 (0.04–0.11) | 0.05 (0.03–0.1) | 0.13 (0.06–0.55) | 0.022 |
Note: Significant values are in bold and italics. p‐Values represent Mann–Whitney U test results.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; IQR, interquartile range; LDH, lactate dehydrogenase; NLR, neutrophil‐to‐lymphocyte ratio; RDW, red cell distribution width.
TABLE 4.
ROC curve analysis of NLR, LDH, ferritin, AST, ALT, and procalcitonin for severe COVID‐19
| Variables | AUC | SD | 95% CI | Cut‐off | Sensitivity (%) | Specificity (%) | p‐Value |
|---|---|---|---|---|---|---|---|
| NLR | 0.757 | 0.074 | 0.708–0.972 | 8.8 | 72.7 | 75.4 | 0.007 |
| LDH (U/L) | 0.856 | 0.050 | 0.759–0.953 | 365 | 72.7 | 90.8 | <0.001 |
| ALT (U/L) | 0.771 | 0.070 | 0.632–0.909 | 16.5 | 72.7 | 70.8 | 0.004 |
| AST (U/L) | 0.840 | 0.067 | 0.759–0.953 | 34 | 72.7 | 81.5 | <0.001 |
| Ferritin (ng/ml) | 0.821 | 0.060 | 0.704–0.938 | 41.5 | 72.7 | 78.5 | 0.001 |
| Procalcitonin (ng/L) | 0.698 | 0.078 | 0.545–0.850 | 0.07 | 63.6 | 60 | 0.038 |
Note: Significant values are in bold and italics.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; AUC, area under the curve; CI, confidence interval; LDH, lactate dehydrogenase; NLR, neutrophil‐to‐lymphocyte ratio.
The AUC for NLR, LDH, AST, ALT, ferritin, and procalcitonin were 0.757, 0.856, 0.840, 0.771, 0.821, and 0.698, respectively (Figure 1). Among laboratory indices, the AUC of LDH is the largest. As determined at admission, the best cut‐off value of LDH for predicting COVID‐19 severity was 365, with a sensitivity of 0.727 and specificity of 0.908. These results with LDH were greater than all other indices.
FIGURE 1.
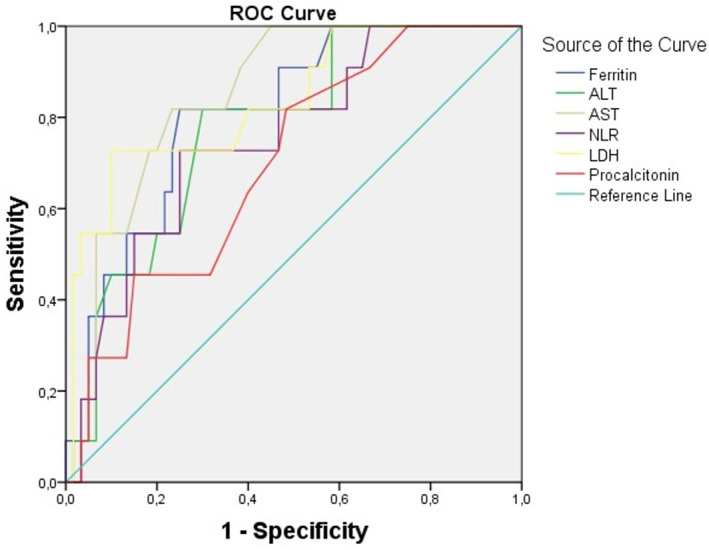
Receiver‐operating characteristic (ROC) curve for predicting the severity of COVID‐19 using NLR, LDH, ferritin, AST, ALT, and procalcitonin at admission. ALT, alanine transaminase; AST, aspartate transaminase; LDH, lactate dehydrogenase; NLR, neutrophil‐to‐lymphocyte ratio
The ABO‐blood groups of the 110 pregnant women displayed a frequency distribution of 44.5%, 15.5%, 11.8%, and 28.2% for A, B, AB, and O, respectively. Among 110 pregnant women, those in the O‐blood group were more likely to have a severe illness (7/31) than the non‐O‐blood group (5/79) (relative risk: 3.6; 95% confidence interval, 1.2–10.4).
Intensive care follow‐up
Among the 110 pregnant women, 14 (12.7%) required ICU admission, 5 (4.5%) required mechanical ventilation, and 4 (3.6%) died from COVID‐19. The reasons for ICU admission were respiratory failure due to severe COVID infection in 12 patients, obstetric hemorrhage in 1 patient, and liver toxicity in another. The median length of stay in the ICU was 7.5 days (interquartile range: 4.75–12 days). Pneumothorax developed in three (2.7%) patients and pneumomediastinum with pneumothorax developed in one patient (0.9%). One patient who developed pneumothorax was being followed up with using high‐flow nasal cannula in spontaneous respiration, and there was no known risk factor for pneumothorax. Another patient developed bilateral pneumothorax while intubated (Figure 2). The ICU duration of stay (83 days) and mechanical ventilation (41 days) was prolonged. Despite having two CPRs (less than 10 minutes), she was discharged from the hospital without support after 120 days. Deep vein thrombosis was also diagnosed in one patient and brachial artery thrombosis in another. The medical records of four patients who died were analyzed for risk factors. One patient had diabetes mellitus, obesity, and hypertension, another had autoimmune thyroid disease, another had macrophage activation syndrome with COVID‐19, and the fourth had no known risk factors.
FIGURE 2.
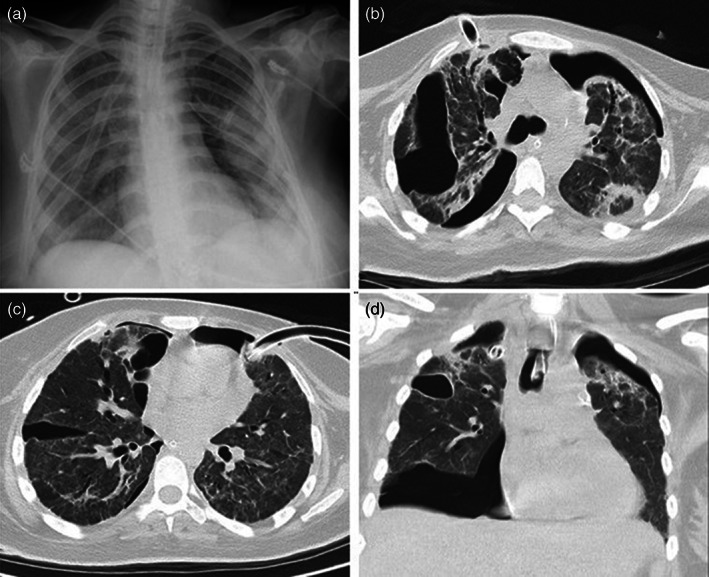
Chest CT and X‐ray imaging of the patient. (a) In the posterior–anterior chest X‐ray imaging, drainage catheters in the upper lobes of both lungs and pneumothorax in the left lung are observed. (b) In the axial chest CT image parenchymal window, pneumothorax in both lungs and consolidated areas in the lung parenchyma, interlobular thickening, bronchiectasis changes, and a drain catheter in the anterior upper lobe of the right lung are observed. (c) In the axial chest CT image parenchymal window, pneumothorax in both lungs, and a drain catheter in the left lung upper lobe anterior are observed. (d) In the axial chest CT image parenchymal window, an intubation tube is observed in the trachea with pneumothorax in both lungs and consolidations in both upper lobes
COVID‐19‐related clinical findings and treatment
The most commonly reported symptoms among the 110 pregnant patients were cough (31.8%), shortness of breath (13.6%), tiredness (11.8%), fever (8.2%), and muscle aches (7.2%); 53.6% (59/110) were asymptomatic. Of the 95 pregnant women who underwent chest computed tomography, 50 (52.6%) had abnormalities suggestive of pneumonia or ARDS. Among the patients with available data, 89.1% received antiviral treatment (favipiravir or a lopinavir and ritonavir combination). Eleven patients (10%) received hydroxychloroquine in addition to antiviral and antibiotic regimens. One patient (0.9%) treated with levofloxacin and hydroquinone developed long QT and bradycardia.
Discussion
This retrospective cohort study includes 110 patients with confirmed COVID‐19 positivity and who were undergoing cesarean section. Approximately half of them were asymptomatic, and cough was the most common symptom. Of the 110 patients, 96.4% were in their third trimester. An invasive ventilator was required in 4.5% of cases. The mortality rate was 3.6% (4/110) in all patients, 28.6% (4/14) in patients needing ICU admission, and 80% (4/5) in patients with invasive mechanical ventilation. There were three stillbirths, and the preterm birth rate was 33.6%.
An increased risk of preterm delivery and fetal compromise has been described in pregnancies complicated with COVID‐19. 1 , 19 , 20 , 21 The majority of pregnant women in the study were in their third trimester, and approximately one‐third of them delivered preterm. Importantly, our study supports that preterm delivery and low birth weight are associated with maternal COVID‐19 severity.
Past studies showed a high preference for regional anesthesia in COVID‐19 patients. 20 , 21 In this study, 89.1% of the patients were operated on under spinal anesthesia. Before the COVID‐19 pandemic, we reported that only 35.8% of pregnant women were operated on under neuraxial anesthesia. 22 Compared to our previous report, this shows an increased tendency to use neuraxial techniques in pregnant women with COVID‐19. In an earlier study, Chen et al. 23 found the rate of hypotension to be 86% in pregnant women infected with COVID‐19 who underwent regional anesthesia. Several later studies addressed this concern and reported that COVID‐19 positivity did not increase susceptibility to hypotension following neuraxial blocks. 24 , 25 We observed hypotension in 33.7% of the patients under spinal anesthesia, which is comparable to other studies.
Analyzing our patients' first‐admission laboratory values revealed that NLR, LDH, AST, ALT, ferritin, and procalcitonin were all significantly elevated in the group of pregnant women with severe COVID‐19 versus the nonsevere group (p < 0.05). Several studies also had noted the importance of LDH as an indicator of lung diseases. Poggiali et al. 10 reported an inverse correlation between LDH and oxygen index (arterial partial pressure of oxygen/fraction of inspired oxygen) in 123 patients infected with COVID‐19, indicating that LDH elevation was associated with lung injury. A systematic review revealed that elevated LDH levels were related to a 5.33‐fold increased risk of poor outcomes. 11 According to our ROC curve analysis, LDH (AUC: 0.856) had the best performance in predicting the severity of COVID‐19. Combining LDH with other clinical prognostic parameters could provide an incremental predictive value for the disease severity.
Recent studies have demonstrated an association between ABO‐blood types and COVID‐19 risk. Zhao et al. 26 claimed that the A‐blood group was associated with a higher risk of acquiring COVID‐19 and that the O‐blood group was associated with a lower risk of infection. Similarly, Ahmed et al. 27 reported that A‐blood‐group women had a higher risk of developing COVID‐19 infection. However, Alina et al. 28 investigated the association between ABO‐blood‐group type and ARDS in 168 pregnant patients, finding that the O‐blood group's risk of developing ARDS was 2.96 times higher. Similarly, in the present study, the risk of severe COVID‐19 infection in the O‐blood group was 3.6 times higher than in the non‐O‐blood group. The possible mechanism of the increased risk in the O‐blood group may be due to the differences in von Willebrand factor (vWF) and plasma factor VIII (FVIII) levels among the blood groups. ABO blood types are the main determinant of plasma levels of vWF and FVIII. Individuals with the O‐blood group have lower plasma levels of both glycoproteins (approximately 25%). 29 This association is of clinical significance. It was found that the O‐blood group was associated with higher mortality, more bleeding, and larger transfusion volumes in severe abdominal trauma patients. 30 Lior et al. reported that the O‐blood group was associated with an increased risk of postpartum hemorrhage and hemoglobin drop. 31 The discrepancies between previous studies and our study might be explained by the structure of the study group and the severity definition of COVID‐19. Our study includes a young, pregnant, and operated patient group.
The studies have reported that COVID‐19 infection may confer adverse effects on pregnant women, including maternal death, admission to ICUs, and needing advanced oxygen support. The respiratory management of critically ill pregnant women with COVID‐19 has been limited to a small case series. Our series describes 14 women requiring ICU admission with a median duration of 7.5 days. Twelve of them had severe COVID‐19 pneumonia. Pneumothorax developed in 21.4% (3/14) of the ICU patients. A recent multicenter study showed a high incidence of pneumothorax (13%) in mechanically ventilated COVID‐19 patients and its association with increased mortality. 32 Although the mechanism is not exactly known, it is partially explained by the alveolar wall rupture caused by the rising pressure differential between the alveolus and the pulmonary interstitium. Thus, it is critical to monitor these complications because early detection and intervention can significantly minimize associated morbidity and mortality.
There are also several limitations in this report. The first is that we did not include pregnant women who delivered via vaginal birth; we also did not follow up in the outpatient department, which may restrict our findings' generalizability to all pregnant women. Cesarean section is a risk factor for maternal mortality without a diagnosis of COVID‐19. The combination of surgery and COVID‐19 infection may further increase the risk. The second limitation is the study's retrospective design and the relatively small number of patients. The results must be interpreted under the constraints of a limited sample size, which must be considered.
In summary, these data suggest that LDH could be identified as a powerful predictive factor for early recognition of severe COVID‐19 cases in pregnant women undergoing cesarean section. Pregnant women with COVID‐19 who undergo cesarean surgery are at an increased risk of morbidity and mortality. The severity of the maternal COVID‐19 disease appears to impact neonatal outcomes.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Umit Kara from Adana City Education and Research Hospital for his help with the literature search. His contribution was invaluable.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions
References
- 1. Abedzadeh‐Kalahroudi M, Sehat M, Vahedpour Z, Talebian P. Maternal and neonatal outcomes of pregnant patients with COVID‐19: a prospective cohort study. Int J Gynecol Obstet. 2021;153(3):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Special report and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205(1):10–8. [DOI] [PubMed] [Google Scholar]
- 4. Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. Obstet Gynecol Surv. 2010;65(4):227–8. [DOI] [PubMed] [Google Scholar]
- 5. Wong SF, Chow KM, De Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG. 2003;110(7):641–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhangu A, Nepogodiev D, Glasbey JC, Li E, Omar OM, Gujjuri RR, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐COV‐2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haffner MR, Le HV, Saiz AM, Han G, Fine J, Wolinsky P, et al. Postoperative in‐hospital morbidity and mortality of patients with COVID‐19 infection compared with patients without COVID‐19 infection. JAMA Netw Open. 2021;4(4):10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Toro F, Gjoka M, Di Lorenzo G, De Santo D, De Seta F, Maso G, et al. Impact of COVID‐19 on maternal and neonatal outcomes: a systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lombardi A, Duiella S, Li Piani L, Comelli A, Ceriotti F, Oggioni M, et al. Inflammatory biomarkers in pregnant women with COVID‐19: a retrospective cohort study. Sci Rep. 2021;11(1):13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, et al. Lactate dehydrogenase and C‐reactive protein as predictors of respiratory failure in CoVID‐19 patients. Clin Chim Acta. 2020;509:135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID‐19: a systematic review and meta‐analysis. Postgrad Med J. 2021. 10.1136/postgradmedj-2020-139542 [DOI] [PubMed] [Google Scholar]
- 12. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020. May 14;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. COVID live update: 213,733,585 Cases and 4,460,783 Deaths from the Coronavirus ‐ Worldometer. https://www.worldometers.info/coronavirus/?%22%5Cl%22countries#countries. Accessed 24 Aug 2021
- 14. Kukrer S, Kukrer AP. Delivery method of the placenta in cesarean deliveries and the effect of uterine incision repair area on morbidity: a randomized controlled study. Turk J Obstet Gynecol. 2021;18(2):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Republic of Turkey, Ministry of Health COVID‐19 (SARS‐CoV‐2 Infection) Guide. https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID‐19_Rehberi20200414_eng_v4_002_14.05.2020.pdf. Accessed 18 Sep 2021.
- 16. World Health Organization Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. https://www.who.int/publications/i/item/laboratory‐testing‐of‐2019‐novel‐coronavirus‐(‐2019‐ncov)‐in‐suspected‐human‐cases‐interim‐guidance‐17‐january‐2020. Accessed 4 June 2021
- 17. Ngan Kee W, Khaw K, Lee B, Lau T, Gin T. A dose‐response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2000;90(6):1390–5. [DOI] [PubMed] [Google Scholar]
- 18. WHO headquarters Clinical management living guidance COVID‐19. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐clinical‐2021‐1. Accessed 4 June 2021
- 19. Angelidou A, Sullivan K, Melvin PR, Shui JE, Goldfarb IT, Bartolome R, et al. Association of Maternal Perinatal SARS‐CoV‐2 infection with neonatal outcomes during the COVID‐19 pandemic in Massachusetts. JAMA Netw Open. 2021;4(4):e217523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karasu D, Kilicarslan N, Ozgunay SE, Gurbuz H. Our anesthesia experiences in COVID‐19 positive patients delivering by cesarean section: a retrospective single‐center cohort study. J Obstet Gynaecol Res. 2021;47(8):2659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahin D, Tanacan A, Erol SA, Anuk AT, Eyi EGY, Ozgu‐ Erdinc AS, et al. A pandemic center's experience of managing pregnant women with COVID‐19 infection in Turkey: a prospective cohort study. Int J Gynecol Obstet. 2020;151(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arslan B, Ozturk N, Onuk Z, Karsli B. Factors affecting selection of anesthesia type in elective cesarean operations and pregnant preferences for anesthesia outcome. Med Sci. 2018;8(1):113–6. [Google Scholar]
- 23. Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing cesarean delivery: a case series of 17 patients. Can J Anesth. 2020;67(6):655–63. 10.1007/s12630-020-01630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ASRA/ESRA . COVID‐19 guidance for regional anesthesia neuraxial anesthesia and peripheral nerve blocks. https://esraeurope.org/wp-content/uploads/2020/04/ESRAASRA-COVID-19-Guidelines-.pdf.
- 25. Jan M, Bhat WM, Rashid M, Ahad B. Elective cesarean section in obstetric COVID‐19 patients under spinal anesthesia: a prospective study. Anesth Essays Res. 2020;14(4):611–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. Clin Infect Dis. 2021;73(2):328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed I, Quinn L, Tan BK. COVID‐19 and the ABO blood group in pregnancy: a tale of two multiethnic cities. Int J Lab Hematol. 2021;43(1):e45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blazer A, Mitsakakis N, Lapinsky S. ABO blood type association with respiratory failure in pregnancy. Chest. 2017;152(4):A374. 10.1016/j.chest.2017.08.400 [DOI] [Google Scholar]
- 29. Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46(10):1836–44. [DOI] [PubMed] [Google Scholar]
- 30. Takayama W, Endo A, Murata K, Hoshino K, Kim S, Shinozaki H, et al. The impact of blood type on the mortality of patients with severe abdominal trauma: a multicenter observational study. Sci Rep. 2021;11(1):16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drukker L, Srebnik N, Elstein D, Levitt L, Samueloff A, Farkash R, et al. The association between ABO blood group and obstetric hemorrhage. J Thromb Thrombolysis. 2016;42(3):340–5. [DOI] [PubMed] [Google Scholar]
- 32. Chopra A, Al‐Tarbsheh AH, Shah NJ, Yaqoob H, Hu K, Feustel PJ, et al. Pneumothorax in critically ill patients with COVID‐19 infection: incidence, clinical characteristics and outcomes in a case control multicenter study. Respir Med. 2021;184:106464. 10.1016/j.rmed.2021.106464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions


