Abstract
Background:
Generalization of conditioned-fear, a core feature of post-traumatic stress disorder (PTSD), has been the focus of several recent neuroimaging studies. A striking outcome of these studies is the frequency with which neural correlates of generalization fall within hubs of well-established functional networks including salience (SN), central executive (CEN), and default networks (DN). Neural substrates of generalization found to date may thus reflect traces of large-scale brain networks that form more expansive neural representations of generalization. The present study includes the first network-based analysis of generalization and PTSD-related abnormalities therein.
Methods:
fMRI responses in established intrinsic connectivity networks (ICNs) representing SN, CEN, and DN were assessed during a generalized conditioned-fear task in male combat veterans (N = 58) with wide-ranging PTSD symptom severity. The task included five rings of graded size. Extreme sizes served as conditioned danger-cues (CS+: paired with shock) and safety-cues (CS−), and the three intermediate sizes served as generalization stimuli (GSs) forming a continuum-of-size between CS+ and CS−. Generalization-gradients were assessed as behavioral and ICN response slopes from CS+, through GSs, to CS−. Increasing PTSD symptomatology was predicted to relate to less-steep slopes indicative of stronger generalization.
Results:
SN, CEN, and DN responses fell along generalization-gradients with levels of generalization within and between SN and CEN scaling with PTSD symptom severity.
Conclusions:
Neural substrates of generalized conditioned-fear include large-scale networks that adhere to the functional organization of the brain. Current findings implicate levels of generalization in SN and CEN as promising neural markers of PTSD.
Keywords: central executive network, conditioned-fear, default network, generalization, post-traumatic stress disorder, salience network
Introduction
Generalization of conditioned-fear is a basic, associative-learning process by which fear to a conditioned danger-cue (CS+) transfers to safe stimuli resembling the CS+ (Pavlov, 1927). Excessive fear-generalization is a core aspect of posttraumatic stress disorder (PTSD), reflected by the DSM-5’s inclusion of heightened distress to situations ‘resembling’ aspects of the trauma in its description of the disorder (American Psychiatric Association, 2013). Recent studies have documented overgeneralization in PTSD (Kaczkurkin et al., 2017; Lissek & Grillon, 2012; Morey et al., 2015), panic disorder (Lissek et al., 2010) and generalized anxiety disorder (Greenberg, Carlson, Cha, Hajcak, & Mujica-Parodi, 2013b; Lissek et al., 2014b) using lab-based generalization paradigms designed to assess fear-related responding across CS+, a conditioned safety-cue (CS−), and generalization stimuli (GSs) that form a continuum of perceptual similarity between CS+ and CS−. Most commonly, generalization is captured by generalization-gradients, or slopes, reflecting a gradual decline in fear responding (or increase in safety responding) to stimuli of decreasing similarity to CS+. The extent of generalization is indicated by the steepness of gradients from CS+ to GSs to CS−, with less-steep gradients indicating stronger levels of generalization.
Brain substrates of generalized conditioned-fear in humans
Healthy samples.
An emerging fMRI literature applying generalization-gradient methodology has identified discrete brain areas instantiating generalized conditioned-fear in healthy participants. As summarized in Table 1, a striking aspect of findings across such studies is the extent to which neural correlates of generalization map onto key hubs of established functional networks. For example, the majority of studies document positive gradients of generalization, defined by increasing fear-related responding as the presented stimulus becomes more similar to CS+, in both anterior insula (AI: seven studies) and dorsomedial prefrontal cortex (dmPFC: five studies), two hubs of the salience network (SN: e.g. Seeley et al., 2007). Such findings suggest robust salience detection of CS+ that gradually declines as stimuli differentiate from CS+. Findings of positive generalization-gradients in dorsal striatum (caudate: three studies) and mediodorsal thalamus (two studies) further implicate the involvement of SN in generalization, as striatum and thalamus have been identified as subcortical aspects of SN that connect prefrontal cortex to midbrain dopaminergic regions (Peters, Dunlop, & Downar, 2016). Additionally, six studies found negative generalization-gradients, defined by decreases in safety-related activity as presented stimuli become more similar to CS+, both in the ventromedial prefrontal cortex (vmPFC) and ventral precuneus (VPc), two important loci of the default network (DN). These findings suggest that DN-mediated internal mentation (e.g. Andrews-Hanna, Smallwood, & Spreng, 2014) is disrupted most by the genuine danger-cue (CS+) and is increasingly less disturbed as presented stimuli decrease in similarity to CS+. Finally, five of six studies showing negative generalization-gradients in vmPFC and VPc also found negative gradients in the hippocampus (HPC), a third constituent of the DN (e.g. Vincent et al., 2006).
Table 1.
Neuroimaging findings on generalized conditioned-fear in healthy controls.
| Study | Positive Generalization-Gradients | Negative Generalization-Gradients | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| AI | dmPFCa | Caud | MDN | dlPFCb | IPLc | vmPFCd | VPc | HPC | |
| 1. Dunsmoor et al., 2011 | X | X | |||||||
| 2. Greenberg et al., 2013 | X | X | X | X | X | ||||
| 3. Kaczkurkin et al., 2017 | X | X | X | X | X | X | X | X | X |
| 4. Lange et al., 2017 | X | X | X | X | X | X | X | X | |
| 5. Lissek et al., 2014 | X | X | X | X | X | X | X | ||
| 6. Onat & Büchel, 2015 | X | X | X | X | |||||
| 7. Tuominen et al., 2019 | X | X | X | X | X | ||||
|
| |||||||||
| Network | Salience | Salience (subcortical) | Central Executive | Default Mode | |||||
Results are organized by brain areas showing positive or negative generalization-gradients, with positive and negative gradients reflecting increasing and decreasing signal change, respectively, as presented stimuli become more similar to CS+. Results reflect generalization effects across healthy participants in a given study. AI = anterior insula; dmPFC = dorsomedial prefrontal cortex; Caud = caudate; MDN = mediodorsal nucleus of the thalamus; dlPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobule; vmPFC = ventromedial prefrontal cortex; VPc = ventral precuneus; HPC = hippocampus.
dmPFC includes Brodmann areas (BA) 6 and 8 and dorsal ACC;
BA 9 and 10;
BA 40;
BA 11.
In addition to nodes of SN and DN, functional hubs of the central executive network (CEN), linked to goal-directed cognitive control (e.g. Cole, Repovš, & Anticevic, 2014), are represented in three of seven studies, with positive generalization-gradients implemented in both dorsolateral prefrontal cortex (dlPFC) and inferior parietal lobule (IPL) nodes of the CEN. That constituents of CEN instantiate gradients of generalization in the same direction as those of SN is consistent with positively correlated activity found across these two networks (e.g. Chand & Dhamala, 2015). Furthermore, the opposite direction of findings in both CEN and SN relative to DN parallels previous findings of anti-correlated activity between DN and both CEN (e.g. Chen, Glover, Greicius, & Chang, 2017) and SN (e.g. Raichle, 2015).
PTSD patients.
Despite the centrality of fear learning and its generalization to PTSD, only two studies to date have investigated brain substrates of these processes in PTSD. One such study (Morey et al., 2015) identified neural correlates of the shift in peak responding from CS+ to safe stimuli with exaggerated threat-cue-like qualities, rendering this study less relevant to the present work targeting PTSD-related over-responding to safe stimuli with degraded threat-cue-like qualities. The other, a recent fMRI study from our group (Kaczkurkin et al., 2017), identified brain regions instantiating overgeneralization to safe stimuli with degraded threat-cue-like qualities in PTSD, with many such loci mapping onto functional hubs of the SN (AI, dmPFC, caudate), CEN (dlPFC, IPL), and DN (ventral hippocampus). These findings are consistent with the triple-network theory of psychopathology, according to which aberrant activity in, and connectivity among, SN, CEN, and DN characterize numerous psychiatric disorders (Menon, 2011) including PTSD (e.g. Akiki, Averill, & Abdallah, 2017).
The present study
The extent to which well-replicated neural correlates of generalization fall within key nodes of SN, CEN, and DN, together with evidence of overgeneralization in constituents of such networks in PTSD, implicates these brain networks as important contributors to the neurobiology and psychopathology of generalization. Importantly, the discrete neural substrates of generalization found to date may reflect traces of larger brain networks that form more extensive, spatially distributed neural representations of generalization that adhere to the large-scale functional organization of the brain. The current study is the first to apply network-based analyses to test this possibility.
Primary predictions.
fMRI activations in SN and CEN were expected to form positive generalization-gradients with overgeneralization in PTSD relative to trauma controls characterized by less-steep downward slopes as the presented stimulus differentiated from CS+. Additionally, DN activations were expected to fall along negative generalization-gradients, with overgeneralization in PTSD v. trauma controls (TC) characterized by less-steep upward slopes as presented stimuli differentiated from CS+.
Secondary predictions.
To further elucidate the degree to which generalization and PTSD-related overgeneralization relate to the triple-network model of psychopathology (Menon, 2011), we assessed relations between levels of generalization across networks and the moderation of such relations by PTSD. According to this model, SN-mediated salience detection reduces internally focused attention by disengaging the DN, and reorients cognitive resources toward the salient event by engaging the CEN. Based on this model, we predicted inverse relations between magnitudes of fear-generalization instantiated in SN and DN, and positive associations between such magnitudes displayed by SN and CEN. Further, because the model links abnormalities in SN-mediated salience detection, like those found in PTSD (e.g. Hayes, Hayes, & Mikedis, 2012), to altered patterns of connectivity between SN and both DN and CEN (Menon, 2011), we tested the influence of PTSD on SN-DN and SN-CEN relations during generalization. Specifically, we predicted weaker inverse relations between levels of generalization in SN and DN with increasing PTSD symptom severity, as those with PTSD have been found to show reduced anti-correlations between SN and DN activity (Brown et al., 2014; Sripada et al., 2012). Finally, we expected stronger generalization-related correspondence between SN and CEN with increasing PTSD symptomology based on previous work linking PTSD to enhanced SN-CEN functional connectivity during threat processing (Rabellino et al., 2015).
Methods and materials
Analyzed data derived from a previously-published fMRI study assessing generalization in 71 male U.S. military veterans of wars in Iraq and Afghanistan with PTSD, subthreshold PTSD (SubPTSD), or no PTSD (TC). PTSD status was established by the Clinician Administered PTSD Scale (CAPS: Blake et al., 1995). Participants in the SubPTSD and TC groups did not meet criteria for PTSD and were characterized by CAPS scores between 20–39 and 0–19 respectively, as recommended (Weathers, Keane, & Davidson, 2001). Participants who either failed to condition or displayed excessive head motion were excluded (see Supplementary material) leaving 20 PTSDs, 19 SubPTSDs, and 19 TCs available for final analyses. During fMRI runs, participants completed a validated, generalized conditioned-fear paradigm (Lissek et al., 2014a) described in Fig. 1.
Figure 1.
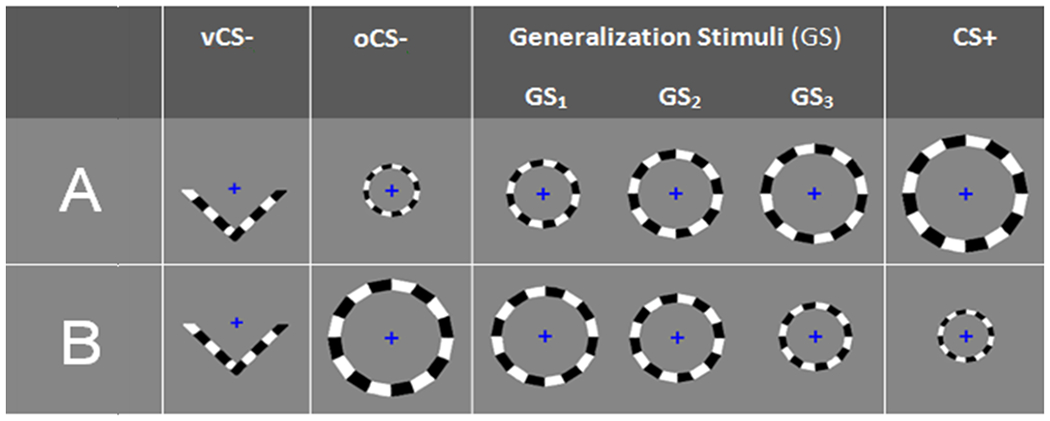
The applied generalization paradigm consists of five checkerboard textured rings that form a continuum-of-size across conditioned danger-cue (CS+), three classes of generalization stimuli (GS3, GS2, GS1), and conditioned safety-cue (oCS−). The CS+ is the largest ring for counterbalance (CB) Group A (50% of the sample) and the smallest ring for CB Group B. In addition to ringed stimuli, a checkerboard textured ‘V’ shaped stimulus was included as a non-circular conditioned safety-cue (i.e. vCS−). The vCS− served as a control condition to assess broader generalization to all things circular. The task was conducted in three phases: (1) pre-acquisition: 20 of each stimulus type (CS+, GS3, GS2, GS1, oCS−, vCS−) presented without electric shock, (2) acquisition: 15 CS+, oCS−, and vCS−, with 12 of 15 CS+ trials co-terminating with shock (100 ms, 3–5 mA, delivered to the right ankle), and (3) generalization: 20 of each stimulus type (CS+, GS3, GS2, GS1, oCS−, vCS−) with an additional 10 shock-reinforced CS + administered to prevent extinction of conditioned fear while leaving 20 unreinforced CS+ to index responses uninfluenced by the shock. Participants were not instructed of CS/US or GS/US contingencies. However, participants were told they may learn to predict shock if they attend to shapes on the screen during the task. On 50% of trials across phases, participants rated their perceived risk of shock using a three-button response pad (Lumina LP-404 by Cedrus).
Characterizing intrinsic connectivity networks (ICNs)
Networks were drawn from a set of 60 components identified via spatial independent component analysis (ICA) of resting-state data from a separate healthy sample (N = 218: Abram et al,. 2015). These components thus represent intrinsic connectivity networks (ICNs) of neural activity, and will be referred to as ICNs throughout. Twenty-seven of 60 ICNs were non-artifactual, with many showing substantial overlap with a variety of established, functional brain networks (Yeo et al., 2011) including visual-spatial, somatomotor, dorsal attention, fronto-parietal, cingulo-opercular, and anterior and posterior default networks. Additionally, networks centering on a diversity of brain regions including the striatum, striato-thalamus, cerebellum, frontal pole, and posterior insula were included among these 27 non-artifactual ICNs.
The initial stage of ICN classification followed Abram et al. (2015). Briefly, group-level ICN maps were normalized, thresholded (z/z max > 0.30: Poppe et al., 2013), and binarized (Yeo et al. 2011). Next, ICNs of interest were selected based on two criteria. The first was inclusion of brain areas found to underlie generalization or PTSD-related overgeneralization in studies listed in Table 1. Seventeen ICNs met this criterion. The second was instantiation of a generalization-gradient within the full ICN. To determine this, we measured BOLD activity within the 17 candidate ICNs to each stimulus-type and assessed the extent to which ICN activity fell along generalization-gradients reflecting gradual increases (positive gradients) or decreases (negative gradients) in responding from CS− through GSs to CS+. Eight ICNs met this criterion (see online Supplementary Table S3). These eight were then characterized by calculating their percent overlap (Dice, 1945) with established networks (Yeo et al., 2011), and visually inspecting for inclusion of the established networks’ functional hubs. Through these assessments, six of eight ICNs of interest were found to substantially align with the SN, CEN or DN.
Next, we assessed effects of group and symptom severity on gradients shape in the eight ICNs of interest and behavioral responding by testing: (1) Group × stimulus-type and CAPS × stimulus-type repeated-measures ANOVAs, (2) paired t tests comparing responses to each stimulus-type against oCS− for positive gradients or CS+ for negative gradients, separately for each group, (3) correlations between subject-level, single-value generalization indices and CAPS scores, and (4) effects of CAPS scores on inter-network dynamics using hierarchical regressions with CAPS as a moderator of between-network associations in the extent of generalization.
Additional details regarding sample characteristics (online Supplementary Tables S1 and S2), exclusion criteria, generalization-paradigm design, MRI acquisition-parameters, ICN selection-criteria, and analytic strategies for fMRI/behavioral data can be found in the Supplement.
Results
Behavioral findings
Group (TC, SubPTSD, PTSD) × stimulus-type and CAPS × stimulus-type linear trends emerged (Table 2) reflecting more gradual, linear decreases in perceived risk among PTSD and SubPTSD v. TC (Table 3) as stimulus similarity to the CS+ declined. As shown in Fig. 2, generalization-gradients in TC formed quadratic slopes that deviated substantially from a hypothetical linear-gradient (dotted line), whereas gradients in PTSD and SubPTSD fell along more gradual declines with less deviation from linearity, reflecting greater generalization of perceived risk in PTSD and SubPTSD v. TC. Further, as described in Fig. 2, generalization of risk extended to three degrees of CS+ differentiation (GS1, GS2, GS3) in PTSD and SubPTSD, but only one degree (GS3) in TC. Finally, the steepness of generalization-gradients calculated with linear deviation scores (LDS: Lissek et al., 2014a), reflecting the degree to which subject-level gradients departed from linearity (see Supplement), was not associated with PTSD symptom severity (r = −0.18, p = 0.19).
Table 2.
Results for Group by Stimulus-type interactions and CAPS by Stimulus-type interactions for risk ratings and ICN responses falling along positive and negative generalization-gradients.
| (A) | (B) | |||||
|---|---|---|---|---|---|---|
| Groupa x Stimulus-Type Interactions |
CAPS x Stimulus-Type Interactions |
|||||
| Wilks’ λ | Lin. | Quad. | Wilks’ λ | Lin. | Quad. | |
| Risk Ratings | 3.30** | 5.25** | 0.98 | 2.87* | 4.33* | 1.67 |
| Positive Gradients | ||||||
| Bil-SN | 1.65 | 1.23 | 4.81* | 2.74* | 0.84 | 7.30** |
| Bil-CEN | 2.16* | 0.89 | 6.52** | 2.73* | 0.53 | 5.36* |
| R-CEN | 2.02 | 2.38 | 5.53** | 2.37 | 0.99 | 6.73* |
| L-CEN | 1.73 | 5.55** | 0.71 | 2.18 | 8.07** | 0.03 |
| DS | 2.90** | 6.22** | 1.37 | 3.39* | 7.18* | 0.07 |
| S-T | 1.52 | 5.22** | 0.10 | 3.89** | 7.51** | <0.01 |
| Negative Gradients | ||||||
| P-DMN | 1.23 | 2.65 | 1.11 | 1.61 | 2.39 | 0.16 |
| A-DMN | 0.29 | 0.64 | 0.08 | 0.67 | 0.61 | 0.54 |
Group is defined as trauma controls (TC) versus subthreshold PTSD (SubPTSD) versus PTSD. Positive and negative gradients reflect increasing and decreasing signal change, respectively, as presented stimuli become more similar to CS+.
TC = trauma controls; SubPTSD = subthreshold PTSD; CAPS = Clinician Administered PTSD Scale; Wilks’ λ = Wilks’ lambda; Lin = linear trend; Quad = quadratic trend; Bil-SN = bilateral salience network; Bil-CEN = bilateral central executive network; R-CEN = right-sided central executive network; L-CEN = left-sided central executive network; DS = dorsal striatum, S-T = striatum and thalamus; P-DMN = posterior default mode network; A-DMN = anterior default mode network.
p<.05;
p<.01.
Table 3.
Group x Stimulus-type interactions across risk ratings and ICNs with group defined as PTSD versus Trauma Control (TC), Subthreshold PTSD (SubPTSD) versus TC, and PS-TD versus SubPTSD.
| Group by Stimulus-Type Interactions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| TC vs. PTSD |
TC vs. SubPTSD |
PTSD vs. SubPTSD |
|||||||
| Wilks’ λ | Lin. | Quad. | Wilks’ λ | Lin. | Quad. | Wilks’ λ | Lin. | Quad. | |
| Risk-Rating | 4.66** | 7.64** | 1.89 | 4.08** | 7.03* | 0.20 | 1.38 | 0.05 | 0.85 |
| Positive Gradients | |||||||||
| Bil-SN | 2.35 | 0.33 | 7.26* | 0.95 | 2.54 | 3.41† | 0.80 | 0.73 | 1.01 |
| Bil-CEN | 4.36** | 1.26 | 13.86** | 1.55 | 0.18 | 5.25* | 1.21 | 1.63 | 0.97 |
| R-CEN | 3.62* | 5.56 | 13.08*** | 0.99 | 3.13 | 0.33 | 1.33 | 0.12 | 5.27* |
| L-CEN | 2.32 | 7.85** | 1.67 | 0.23 | 0.37 | 0.02 | 2.62† | 9.30** | 0.42 |
| DS | 3.10* | 9.56** | 0.63 | 2.81* | 0.80 | 3.00 | 2.09 | 5.48* | 0.73 |
| S-T | 3.15 | 9.47** | 0.11 | 0.51 | 0.60 | 0.06 | 1.40 | 4.57* | 0.01 |
| Negative Gradients | |||||||||
| P-DMN | 1.17 | 1.13 | 1.37 | 0.86 | 1.63 | 1.75 | 1.59 | 6.22** | 0.07 |
| A-DMN | 0.17 | 0.03 | 0.13 | 0.37 | 1.28 | 0.01 | 0.44 | 1.25 | <0.01 |
Positive and negative gradients reflect increasing and decreasing signal change, respectively, as presented stimuli become more similar to CS+.
Wilks’ λ = Wilks’ lambda; Lin = linear trend; Quad = quadratic trend; Bil-SN = bilateral salience network; Bil-CEN = bilateral central executive network; R-CEN = right-sided central executive network; L-CEN = left-sided central executive network; DS = dorsal striatum; S-T = striatum and thalamus; P-DMN = posterior default mode network, A-DMN = anterior default mode network.
p<.05;
p<.01;
p<.001.
Figure 2.
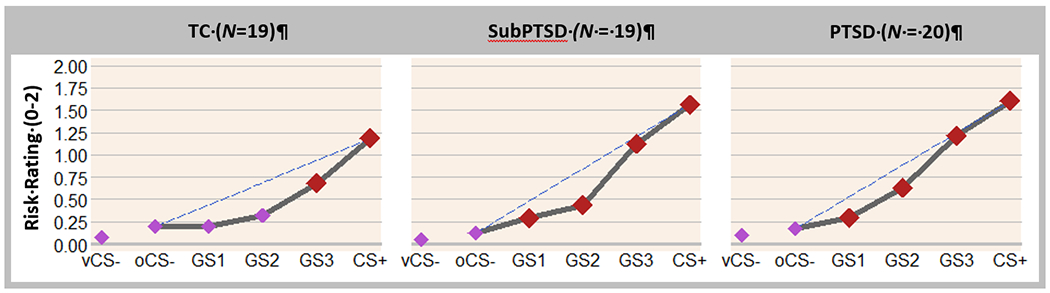
Averaged perceived risk of shock (0 = no risk, 1 = some risk, 2 = high risk) plotted across conditioned danger-cues (CS+), generalization stimuli (GS3, GS2, GS1), and conditioned safety-cues [ring-shaped (oCS−) and V-shaped (vCS−)] for each group. This reflects data for the current sample, which includes three fewer subjects than the prior analysis (Kaczkurkin et al., 2017) due to more stringent motion requirements. Main effects of stimulus-type were present for each group (ps < 0.0001). Dotted lines indicate hypothetical linear decreases in responding from CS+ to oCS− with which to visualize the deviation of gradients from linearity in each group. Such deviations reflect a significantly stronger linear component in the generalization-gradient of PTSD and SubPTSD relative to TC (ps < 0.05), indicating more gradual, linear declines indicative of overgeneralization in PTSD and SubPTSD. To identify the point on the continuum-of-similarity at which perceived risk ceased to generalize for each group, planned comparisons contrasting oCS− against CS+ and GS3, GS2, and GS1 were computed. Black data points signify stimulus types eliciting increased risk ratings relative to oCS− after applying Hochberg’s adjustment for multiple tests (Hochberg, 1988). In TC, perceived risk was elevated from oCS− to CS+ (p < 0.001) and GS3 (p < 0.001). By contrast, in PTSD and SubPTSD, perceived risk was elevated from oCS− to CS+ (all ps < 0.001), GS3 (all ps < 0.001), GS2 (all ps < 0.001), and GS1 (all ps < 0.016). Thus, while TC generalized perceived risk only to one degree of differentiation from CS+ (i.e. GS3), those in PTSD and SubPTSD groups generalized to three degrees of differentiation (i.e. GS3–GS1).
ICN findings
The eight ICNs meeting selection criteria are detailed in online Supplementary Table S3 and include: one bilateral cingulo-opercular SN (bilateral-SN), two ICNs centering on subcortical aspects of the SN (dorsal-striatum, striatum/thalamus), three CENs including a bilateral prefrontal (bilateral-CEN) and a predominantly right and left frontoparietal network (right-CEN, left-CEN), and two DNs (anterior-DN, posterior-DN).
Positive generalization-gradients.
Responses across stimuli in ICNs representing SN and CEN showed positive generalization-gradients, with strongest responses to CS+ and gradually declining responses as the presented stimulus differentiated from CS+ (Fig. 3a and b).
Figure 3.
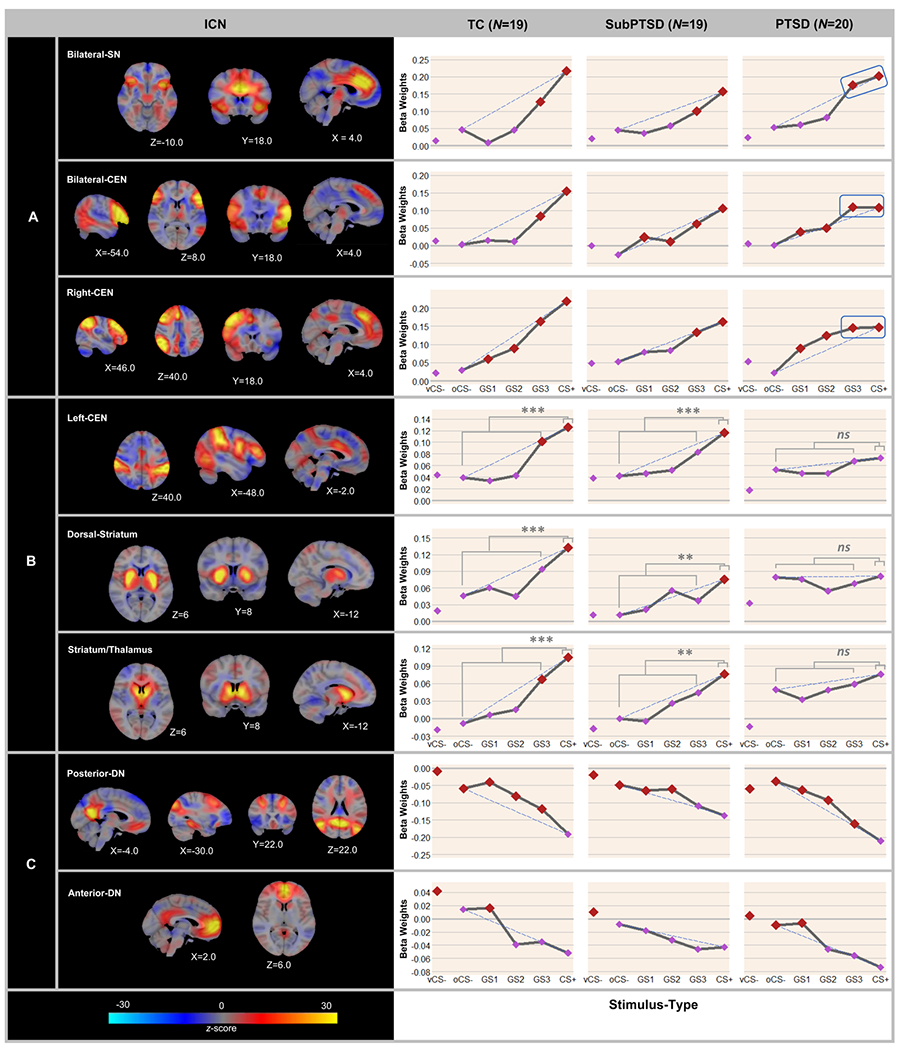
Averaged neural responses to conditioned and generalization stimuli across ICNs displaying positive (a and b) and negative (c) generalization-gradients across TC, SubPTSD and PTSD groups. Inset (a) demonstrates thresholding at z/zmax > 0.3. Thresholded maps were used to assess overlap with known neural substrates of generalization and known networks; full ICN maps were used in main analysis. Dotted lines indicate hypothetical linear increases (a and b) or decreases (c) in responding from the ring-shaped conditioned safety cue (oCS−) to CS+ with which to visualize the deviation of gradients from linearity in each group. To identify the point on the continuum of similarity at which ICN activations cease to generalize for positive gradients (a and b), beta weights for the ring-shaped conditioned safety cue (oCS−) were compared against CS+ and the three classes of generalization stimuli (GS1, GS2, and GS3). For negative gradients (c), beta weights for CS+ were compared against vCS−, oCS−, GS1, GS2, and GS3. Red data points signify stimulus-types to which responses generalized after applying Hochberg’s adjustment for multiple comparisons (Hochberg, 1988) at the group level for each network. As can be seen, responses in bilateral-CEN were elevated from oCS− to CS+ and all three GSs in PTSD and SubPTSD, but were only elevated to CS + and GS3 in TC reflecting overgeneralization of bilateral-CEN responses in PTSD and SubPTSD. Generalization did not extend farther in PTSD or SubPTSD v. TC for any other network. One of the more pronounced group effects on gradient shape across bilateral-SN, bilateral-CEN, and right-CEN (A) is less steep response-curve from CS+ to GS3 in PTSD v. TC [bilateral-SN:F(1,35) = 5.909, p = p.020; bilateral-CEN: F(1,35) = 10.843, p = 0.002, and right-CEN: F(1,35) = 5.614, p = 0.023]. Such effects indicate nearly complete generalization of responding in these three networks from CS+ to the GS3 among those with, but not without, PTSD. Graphs for left-SN, dorsal-striatum, and striatum/thalamus (b) include brackets and significance levels to indicate group differences in broad generalization to all safe circular stimuli [(oCS−, GS1, GS2, GS3)/4] – (CS+). Coordinates are based on the right-anterior-inferior (RAI) system. ns, not significant. **p < 0.01; ***p < 0.001.
Bilateral-SN, bilateral-CEN, and right-CEN.
Group (PTSD, SubPTSD, TC) × Stimulus-type and CAPS × Stimulus-type quadratic trends were found for each of these three ICNs (Table 2A and B). In bilateral-SN and bilateral-CEN such interactions were driven by steeper quadratic declines in generalization-gradients in TC v. PTSD and, in bilateral-CEN, steeper quadratic declines in TC v. SubPTSD (Table 3). As displayed in Fig. 3a, generalization-gradients in bilateral-SN and bilateral-CEN among TC formed steep, quadratic slopes that deviate substantially from a hypothetical linear-gradient (dotted line). By contrast, bilateral-SN gradients in PTSD, and bilateral-CEN gradients in PTSD and SubPTSD, fell along more gradual declines with less deviation from linearity, indicating greater generalization of bilateral-SN and bilateral-CEN responses. In right-CEN, the Group × Stimulus-type interaction was driven by less-steep gradients of generalization in PTSD v. both SubPTSD and TC (Table 3), with gradients being shallower and more convex than linear in PTSD and steeper and more concave than linear in both TC and SubPTSD (Fig. 3a). This less-steep gradient in PTSD demonstrates greater generalization of right-CEN responses in PTSD v. TC and SubPTSD.
Gradient shape and PTSD symptom severity.
We next assessed the degree to which gradient steepness (i.e. LDS) in bilateral-SN, bilateral-CEN, and right-CEN corresponded to PTSD symptom severity across all participants (N = 58). Results revealed significant correlations between CAPS scores and LDS for bilateral-SN (r = 0.35, p = 0.007), bilateral-CEN (r = 0.32, p = 0.014), and right-CEN (r = 0.34, p = 0.010) (online Supplementary Fig. S1A), demonstrating less-steep gradients (i.e. stronger generalization) in these three networks as a function of increasing PTSD symptom severity.
Left-CEN, dorsal-striatum, and striatum/thalamus.
A different pattern of group effects on positive generalization-gradients emerged in left-CEN, dorsal-striatum, and striatum/thalamus (Fig. 3b). Specifically, the Group (PTSD, SubPTSD, TC) × Stimulus-type linear trend was significant for each of these three ICNs, and such effects were the result of less-linear declines in PTSD relative to the other two groups. As displayed in Fig. 3b, responses in left-CEN, dorsal-striatum, and striatum/thalamus fell along expected positive generalization-gradients in TC and SubPTSD, but were all relatively flat across CS+, GSs, and oCS− in PTSD. Consistent with this observation, response in all three of these ICNs were significantly reduced to safe ring-shaped stimuli (GS3, GS2, GS1, oCS−) compared to CS+ among TC and SubPTSD (ps < 0.001), whereas responses to safe ring-shaped stimuli were not significantly different from CS+ in PTSD (ps > 0.12). That the ring-shaped CS+ elicited comparable responses to all safe rings in PTSD, but not TC or SubPTSD, suggests a broader form of PTSD-related overgeneralization in these three networks to all safe stimuli sharing the circular form of CS+. Furthermore, as shown in Fig. 3b, responses in these three ICNs among those with PTSD appear to sharply decline from the ring-shaped safety-cue (oCS−) to the one non-circular, safe stimulus (vCS), while such responses in TC and SubPTSD appear to bottom out at oCS− with little additional decline from oCS− to vCS−. In support of this visual assessment, oCS− v. vCS− elicited stronger left-CEN, dorsal-striatum, and striatum/thalamus responses in PTSD (ps < 0.02), but not TC or SubPTSD (ps > 0.39), with the one exception that dorsal-striatum responses to oCS− v. vCS− were elevated at the trend level in TC (p = 0.09). Taken together, such findings suggest broad overgeneralization to safe stimuli sharing the circular form of the CS+ in left-CEN, dorsal-striatum, and striatum/thalamus among those with PTSD.
Gradient shape and PTSD symptom severity.
We next assessed correlations between CAPS scores and broad generalization defined as difference scores between averaged ICN responses to all safe ring-shaped stimuli minus CS+. Results revealed positive correlations between CAPS scores and broad generalization in left-CEN (r = 0.32, p = 0.013), dorsal-striatum (r = 0.38, p = 0.003), and striatum/thalamus (r = 0.35, p = 0.046) (online Supplementary Fig. S1B), implicating a more expansive form of overgeneralization to all ring-shaped stimuli in these ICNs as brain markers of PTSD symptom severity.
Negative generalization-gradients
Responses across stimuli in posterior-DN and anterior-DN formed negative generalization-gradients, with strongest responses to vCS− and oCS− and gradually declining responses as stimuli became more similar to CS+ (Fig. 3c). All Group × Stimulus-type interactions in these DNs were non-significant (Table 2).
Between-network associations
To assess the extent to which SN works in tandem with CEN and DN during generalization, we correlated the magnitude of generalization within SN to such magnitudes in CEN and DN. As shown in online Supplementary Table S4, positive correlations were found between measures of generalization in bilateral-SN and bilateral-CEN (r = 0.52, p < 0.0001); striatum/thalamus and right-CEN (r = 0.35, p = 0.008); and left-CEN and bilateral-SN (r = 0.29, p = 0.027), dorsal-striatum (r = 0.40, p = 0.002), and striatum/thalamus (r = 0.36, p = 0.006). Additionally, an inverse correlation was found between measures of generalization in bilateral-SN and anterior-DN (r = 0.43, p = 0.001) (online Supplementary Table S5). All other inter-network associations were non-significant.
Moderating effects of CAPS.
CAPS scores positively moderated relations between generalization in bilateral-SN and both bilateral-CEN (p = 0.045) and right-CEN (p = 0.021) (online Supplementary Table S4). Online Supplementary Fig. S2 illustrates this finding further, showing effects for each group separately. No moderating effects of CAPS on SN-DN relations were found (online Supplementary Table S5).
Discussion
The present study is the first to assess the role of large-scale brain networks in either generalization of conditioned-fear or abnormalities in generalization associated with PTSD. This network-based approach aimed to expand on previously found neural correlates of both generalization and PTSD-related overgeneralization, which largely fall within central hubs of the SN, CEN, and DN.
The contribution of ICNs to generalized conditioned-fear
As predicted, activity in ICNs representing SN and CEN all fell along positive generalization-gradients reflecting peak activations to the conditioned danger-cue (CS+) and gradual declines as stimuli perceptually differentiated from CS+. In line with the known role of the SN in salience detection (Seeley et al., 2007; Uddin, 2015), the contribution of SN to generalization may lie in graded levels of threat-related salience, with highest levels to CS+ and diminishing levels to stimuli with decreasing CS+ resemblance. Generalization effects in the CEN may be understood in the context of previous work linking increases in cognitive load to heightened activity and connectivity (e.g. Tomasi, Chang, Caparelli, & Ernst, 2007) in this network. According to attentional control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) cognitive correlates of anxiety including worry, attentional bias toward threat, and top-down efforts to manage anxiety consume limited working-memory resources, thereby increasing the cognitive load of goal-oriented tasks. In the current study, participants were tasked with monitoring the color of crosshairs, rating perceptions of risk when the crosshair turned red, and maintaining a still body posture despite receiving shocks. Increases in anxiety to stimuli bearing increasing resemblance to CS+ may proportionately augment the cognitive load of study-related tasks, leading to a corresponding increase in CEN engagement. This perspective aligns with previous interpretations imputing CEN over-engagement in PTSD during threat to increased, top-down attempts to regulate fear reactivity (Rabellino et al., 2015), a response to threat thought to deplete cognitive resources (Eysenck et al., 2007). Furthermore, the positive association between levels of generalization in bilateral-SN and bilateral-CEN is consistent with the above-described interpretation linking heightened SN-mediated threat detection during GSs to increases in CEN-mediated cognitive-load/fear-regulation.
Within the DN, activations fell along negative generalization-gradients characterized by peak deactivations to CS+ and increasing activations as presented stimuli differentiated from CS+. This expected pattern of DN findings suggests that internal mentation, the primary function attributed to the DN (e.g. Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010), was disrupted most by CS+, with gradually less disruption by stimuli with less CS+ similarity. Additionally, levels of generalization in anterior-DN were inversely related to levels displayed by bilateral-SN, implying that increased generalization of threat detection by the SN was accompanied by reduced generalization of disruptions to internally-focused thought subserved by the DN.
Network-Based neural substrates of PTSD-related overgeneralization
While effects of PTSD on levels of generalization in the DN were not found, PTSD was associated with heightened generalization in bilateral-SN, bilateral-CEN, and right-CEN as reflected by less-steep declines in neural gradients of generalization from CS+ to GSs to CS−. Moreover, levels of generalization in these three ICNs, captured by gradient steepness, were positively correlated with PTSD symptom severity, strengthening conclusions that generalization-related activity in SN and CEN reflect promising brain-based markers of PTSD. Additionally, relations between levels of generalization instantiated in bilateral-SN and both bilateral-CEN and right-CEN strengthened with increasing PTSD symptom severity. These between-network findings may indicate that heightened generalization of threat-related salience detection to safe GSs (via SN) was accompanied by an amplified impact of such threat detection on cognitive load (via CEN), among those with PTSD.
Networks implementing broad overgeneralization in PTSD.
A second pattern of PTSD-related overgeneralization was found in left-CEN and subcortical aspects of SN centered on dorsal-striatum and striatum/thalamus. In these three networks, PTSD was associated with broader generalization to all safe stimuli sharing the circular form of the CS+. Furthermore, PTSD symptom severity was positively related to broad generalization to all safe circular stimuli instantiated in left-CEN, dorsal-striatum, and striatum/thalamus, implicating broad generalization to safe stimuli resembling danger-cues within these three ICNs as promising neural signatures of PTSD.
Such findings in dorsal-striatum and striatum/thalamus may reflect the cortico-striatal-thalamic loop (CSTL: Peters et al., 2016), a bidirectional regulatory circuit thought to flag motivationally-relevant stimuli and deploy cognitive control via cortical nodes in the SN and CEN, respectively, and facilitate control of behavioral responses to such stimuli. Dorsal-striatum and striatum/thalamus may represent the subcortical aspects of this circuit, as both include thalamic nodes and display sizeable generalization-related correlations with both bilateral-SN and left-CEN. Within the CSTL, striatal nuclei are proposed to exert modulatory control over behavior (Peters et al., 2016), a view consistent with past findings implicating dorsal/ventral striatum as an emotion-action interface (e.g. Cain & LeDoux, 2008). Although behavioral avoidance of shock was not possible in this study, subjects may have felt the impulse to avoid when exposed to CS+ or resembling GSs, reflecting an initial preparatory stage of action-selection putatively subserved by the striatum (Kimchi & Laubach, 2009). The found link between PTSD and broad overgeneralization in dorsal-striatum and striatum/thalamus may thus reflect more persistent readiness to avoid as presented circles become less similar to CS+ in PTSD. In other words, less threat information may be required to initiate striatally-mediated, protective behaviors among those with PTSD.
Similar patterns of broad generalization in left-CEN may implicate left-CEN in cognitive control processes that regulate preparation to act at the level of the striatum, as core nodes of left-CEN include dlPFC areas implicated in inhibitory control (e.g. Henderson, Pine, & Fox, 2015) that have extensive projections to the dorsal striatum (Haber, 2016).
CEN lateralization.
The lateralization of PTSD-related abnormalities in CEN presently found are broadly consistent with previous findings that CEN is more functionally lateralized than other networks (e.g. Smith et al. 2009; Son et al. 2017). Specifically, right-CEN has been found to display coupling with attentional networks at rest (Wang, Buckner, & Liu, 2014) and has been associated with implicit attention toward perceptual and interoceptive cues (Heine et al., 2012; Laird et al., 2011; Smith et al., 2009). In the present study, group effects in the right-CEN may therefore link PTSD to excessive attentional bias toward safe cues resembling danger-cues. Left-CEN, on the other hand, has been associated with control of cognition and language (Laird et al., 2011; Smith et al., 2009), which may support a role in many explicit emotion-regulation strategies (Kim & Hamann, 2007; Morawetz, Bode, Derntl, & Heekeren, 2017; Nicholson et al., 2018). Thus, to the extent that left-CEN activation represents explicit fear-regulation in the present task, the found pattern of broad over-generalization in PTSD suggests that less danger information is needed to prompt emotion regulation among those with PTSD.
Treatment implications
Current findings support therapeutic approaches to PTSD aimed at reducing the threat-related salience of benign events resembling aspects of the trauma. According to the triple-network model of psychopathology (Menon, 2011), the SN serves as the gateway to the SN-CEN-DN system and coordinates downstream activity in CEN and DN. Thus, therapeutically targeting the SN-mediated salience of safe stimulus events resembling the trauma should have cascading effects on CEN and DN, including reductions in PTSD-related overgeneralization within the CEN. Such decreases in threat-related salience could be achieved through in vivo exposure therapy using a fear hierarchy of trauma-related stimuli, with exposures to stimuli resembling the trauma (i.e. GSs) added at each level of the hierarchy. These exposures to GSs delivered in the absence of aversive outcomes would serve to attenuate the threat-related salience of GSs by both disconfirming erroneous threat beliefs and habituating distress responses, two key therapeutic mechanisms underlying exposure treatments (e.g. Craske, Hermans, & Vervliet, 2018).
Network-based substrates of overgeneralization may also be treated through interventions more directly targeting CEN and SN. For example, low frequency, repetitive transcranial magnetic stimulation (rTMS) could be applied to lateral nodes of the CEN to inhibit overgeneralization of CEN responses among those with PTSD during therapeutic exposures to safe situations resembling the trauma. The potential efficacy of this intervention is consistent with meta-analytic findings of reduced PTSD symptom severity following inhibitory rTMS stimulation of dlPFC (Boggio et al., 2010). Additionally, using rTMS to modulate the SN during generalization could be achieved by stimulating the inferior-frontal-gyrus, an aspect of the SN (Yeo et al., 2011) previously targeted to alter SN function (Li et al., 2019).
The added value of network-derived neural correlates of generalization
Though network findings reported herein are consistent with past findings localizing neural correlates of generalization in discrete nodes of the SN, CEN, and DN (see Table 1), current results provide a number of novel insights. First, well documented psychological correlates of neural dynamics within and between SN, CEN, and DN (e.g. Menon, 2011) sharpen psychological interpretations of brain activations instantiating generalization. Specifically, as described in more detail above, current network-based results bring interactive effects between salience detection (via SN: e.g. Seeley et al., 2007), cognitive control (CEN: e.g. Cole et al., 2014), and internally-focused thought (DN: e.g. Andrews-Hanna et al., 2014) into the fold to better understand the cognitive processes engaged during generalization. A second novel insight derives from the found moderating effect of PTSD symptoms on relations between levels of generalization instantiated in SN and CEN, providing the first evidence that PTSD may increase the extent to which generalized salience-detection by SN leads to greater attempts at CEN-mediated cognitive control (e.g. emotion regulation). Third, results of present analyses are the first to identify neural markers of broad over-generalization in PTSD. Namely, as PTSD symptoms increased across our sample, left-CEN and striato-thalamic networks showed corresponding increases in responses to all stimuli bearing the circular form of CS+. Accordingly, generalization-related abnormalities in these networks among those with PTSD may facilitate the transfer of fear from CS+ to stimuli with only minimal CS+ resemblance, conferring risk for a more pathogenic form of over-generalization in PTSD. Finally, current network-based analyses reveal untapped, candidate neural-targets for interventions aimed at reducing generalization. For example, findings substantiating SN and DN as neural substrates of generalization implicate the lateral extensions of SN into inferior frontal gyri (Yeo et al., 2011), or lateral aspects of DN in the angular gyrus or temporal cortices (Yeo et al., 2011) as more accessible targets for noninvasive neuromodulation of generalization than deeper structures of the SN (e.g. AI) or DN (e.g. vmPFC).
Conclusions
SN, CEN, and DN were all found to instantiate the clinically relevant process of generalized conditioned-fear. Additionally, PTSD patients displayed overgeneralization in SN and CEN, but not DN, and the extent of SN and CEN generalization scaled with PTSD symptom severity. These findings implicate overgeneralization in SN and CEN as promising network-based markers of PTSD, and suggest both elevated threat detection by the SN and increases in CEN-mediated cognitive load and/or emotion regulation during exposure to safe experiences resembling the traumatic encounter among combat veterans with PTSD.
Supplementary Material
Financial support
This research was made possible through an NIMH R00 grant (MH080130) to Dr Lissek, and by the Congressionally Directed Medical Research Program and the Department of Defense grant (PT074550) to Dr Sponheim.
Footnotes
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Disclosures
All authors contributed substantially to the content of this manuscript. Additionally, the corresponding author of this paper (Shmuel Lissek) assumes responsibility for the integrity of data and the accuracy of data analyses, and that all authors had full access to all the data in the study.
References
- Abram SV, Wisner KM, Grazioplene RG, Krueger RF, MacDonald AW III, & DeYoung CG (2015). Functional coherence of insula networks is associated with externalizing behavior. Journal of Abnormal Psychology, 124(4), 1079–1091. doi: 10.1037/abn0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, & Abdallah CG (2017). A network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports, 19(11), 81. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, … Fregni F (2010). Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 71(8), 992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Workgroup M-AM, Beall SK, … Morey RA (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology, 39(2), 361–369. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, & LeDoux JE (2008). Brain mechanisms of Pavlovian and instrumental aversive conditioning. In Blanchard RJ, Blanchard DC, Griebel G, & Nutt D (Eds.), Handbook of Anxiety and Fear (Vol. 17, pp. 103–124). Handbook of Behavioral Neuroscience.. doi: 10.1016/S1569-7339(07)00007-0 [DOI] [Google Scholar]
- Chand GB, & Dhamala M (2015). Interactions among the brain default-mode, salience, and central-executive networks during perceptual decision-making of moving dots. Brain Connectivity, 6(3), 249–254. doi: 10.1089/brain.2015.0379. [DOI] [PubMed] [Google Scholar]
- Chen JE, Glover GH, Greicius MD, & Chang C (2017). Dissociated patterns of anti-correlations with dorsal and ventral default-mode networks at rest. Human Brain Mapping, 38(5), 2454–2465. doi: 10.1002/hbm.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repovš G, & Anticevic A (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist, 20(6), 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Hermans D, & Vervliet B (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373(1742), 20170025. doi: 10.1098/rstb.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice LR (1945). Measures of the amount of ecologic association between species. Ecology, 26(3), 297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, & LaBar KS (2011). Neurobehavioral mechanisms of human fear generalization. NeuroImage, 55(4), 1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion (Washington, D.C.), 7(2), 336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, & Mujica-Parodi LR (2013a). Neural reactivity tracks fear generalization gradients. Biological Psychology, 92(1), 2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, & Mujica-Parodi LR (2013b). Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depression and Anxiety, 30(3), 242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2, 9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L, Soddu A, Gómez F, Vanhaudenhuyse A, Tshibanda L, Thonnard M, … Demertzi A (2012). Resting state networks and consciousness: Alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness States. Frontiers in Psychology, 3, 295. doi: 10.3389/fpsyg.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: A dual-processing perspective. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(1), 207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y (1988). A sharper Bonferroni procedure for multiple tests of significance. Biometrika, 75(4), 800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, … Lissek S (2017). Neural substrates of overgeneralized conditioned fear in PTSD. American Journal of Psychiatry, 174(2), 125–134. doi: 10.1176/appi.ajp.2016.15121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, & Hamann S (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience, 19(5), 776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kimchi EY, & Laubach M (2009). Dynamic encoding of action selection by the medial striatum. Journal of Neuroscience, 29(10), 3148–3159. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Fox PT (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Goossens L, Michielse S, Bakker J, Lissek S, Papalini S, … Schruers K (2017). Behavioral pattern separation and its link to the neural mechanisms of fear generalization. Social Cognitive and Affective Neuroscience, 12(11), 1720–1729. doi: 10.1093/scan/nsx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LM, Violante IR, Leech R, Hampshire A, Opitz A, McArthur D, Sharp DJ (2019). Cognitive enhancement with Salience Network electrical stimulation is influenced by network structural connectivity. NeuroImage, 185, 425–433. doi: 10.1016/j.neuroimage.2018.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, & Grillon C (2014a). Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Social Cognitive and Affective Neuroscience, 9(8), 1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, & Grillon C (2012). Learning models of PTSD. In Beck JG & Sloan DM (Eds.), The oxford handbook of traumatic stress disorders. New York: Oxford University Press; 10.1093/oxfordhb/9780195399066.013.0013. [DOI] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, & Grillon C (2014b). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75(11), 909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Luckenbaugh D, Geraci M, Pine DS, & Grillon C (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167(1), 47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, & Heekeren HR (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 72, 111–128. doi: 10.1016/j.neubiorev.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, … LaBar KS (2015). Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Translational Psychiatry, 5, e700. doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Rabellino D, Densmore M, Frewen P, Paret C, Kluetsch R, … Lanius R (2018). Intrinsic connectivity network dynamics in PTSD during amygdala downregulation. Human Brain Mapping, 39. doi: 10.1002/hbm.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat S, & Büchel C (2015). The neuronal basis of fear generalization in humans. Nature Neuroscience, 18(12), 1811–1818. doi: 10.1038/nn.4166. [DOI] [PubMed] [Google Scholar]
- Pavlov I (1927). Conditioned reflexes. New York: Oxford University Press. [Google Scholar]
- Peters SK, Dunlop K, & Downar J (2016). Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Frontiers in Systems Neuroscience, 10, 104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe AB, Wisner K, Atluri G, Lim KO, Kumar V, & MacDonald A W III. (2013). Toward a neurometric foundation for probabilistic independent component analysis of fMRI data. Cognitive, Affective, & Behavioral Neuroscience, 13(3), 641–659. doi: 10.3758/s13415-013-0180-8. [DOI] [PubMed] [Google Scholar]
- Rabellino D, Tursich M, Frewen PA, Daniels JK, Densmore M, Théberge J, & Lanius RA (2015). Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatrica Scandinavica, 132(5), 365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S, Miyata J, Mori Y, Isobe M, Urayama S, Aso T, … Takahashi H (2017). Lateralization of intrinsic frontoparietal network connectivity and symptoms in schizophrenia. Psychiatry Research: Neuroimaging, 260, 23–28. doi: 10.1016/j.pscychresns.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, & Liberzon I (2012). Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74(9), 904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, & Ernst T (2007). Different activation patterns for working memory load and visual attention load. Brain Research, 1132(1), 158–165. doi: 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen L, Boeke E, DeCross S, Wolthusen RPF, Nasr S, Milad M, … Holt D (2019). The relationship of perceptual discrimination to neural mechanisms of fear generalization. NeuroImage, 188, 445–455. doi: 10.1016/j.neuroimage.2018.12.034. [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, & Buckner RL (2006). Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology, 96(6), 3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, & Liu H (2014). Functional specialization in the human brain estimated by intrinsic hemispheric interaction. Journal of Neuroscience, 34(37), 12341–12352. doi: 10.1523/JNEUROSCI.0787-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JRT (2001). Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


