Abstract
Case series
Patients: Female, 64-year-old • Female, 74-year-old • Female, 49-year-old
Final Diagnosis: Breast adenomyoepithelioma
Symptoms: Breast tumor
Medication: —
Clinical Procedure: —
Specialty: Oncology • Pathology • Radiology • Surgery
Objective:
Rare disease
Background:
Breast adenomyoepithelioma is a rare benign breast tumor characterized by a biphasic proliferation of epithelial and myoepithelial cells with variable clinical and diagnostic features. Establishing the diagnosis, determining optimal therapy, and predicting outcome are problematic because of the rarity of this entity. There have been only 2 large series of adenomyoepitheliomas of the breast, reported by Tavassoli and Rosen, which included 27 and 18 patients, respectively. In this report, we present 3 cases of breast adenomyoepithelioma.
Case Reports:
Herein, we report 3 cases of breast adenomyoepithelioma. The first case is of a 64-year-old woman who was found to have right breast microcalcification on a screening mammogram. The second case is of a 74-year-old woman who had a right breast mass. These 2 patients were managed by wide local excision. Postoperative microscopic examination revealed adenomyoepithelioma. The third case is of a 49-year-old woman with bilateral saline breast implants who presented with a left breast mass. A core needle biopsy was done and revealed adenomyoepithelioma associated with usual ductal hyperplasia and ductal carcinoma in situ.
Conclusions:
Breast adenomyoepithelioma is a rare condition that can pose diagnostic challenges due to variable imaging presentations, necessitating percutaneous core biopsy for initial diagnosis. Correct diagnosis is usually possible only on excisional biopsy and confirmed by demonstrating the biphasic nature of the tumor by IHC. Clinical suspicion coupled with utilizing both radiological and histopathological facilities can aid in the accurate diagnosis and management. For the most part, they are considered to be benign, but they can locally recur.
Keywords: Adenomyoepithelioma, Breast Neoplasms, Case Reports
Background
Adenomyoepithelioma (AME) of the breast is an uncommon tumor characterized by dual proliferation of myoepithelial and luminal cells [1]. There have been only 2 large series of adenomyoepitheliomas of the breast, reported by Tavassoli and Rosen, which included 27 and 18 patients, respectively [1,2]. Breast AME often presents as a palpable, painless, well-defined, and centrally-located mass, but some are asymptomatic and detected incidentally on imaging [1]. Malignant transformation is a rare event that can occur in 1 or both cellular components [3]. A spectrum of histological patterns is observed in these tumors, which poses radiological challenges in preoperative diagnosis. Surgical excision is the suggested treatment for most AME tumors [4]. We present 3 cases of AME that occurred in different age groups with similarities and variations in both clinical presentations and outcomes.
Case Reports
Case 1
A 64-year-old asymptomatic woman, who was known to have hypertension and dyslipidemia, was discovered to show an interval development of suspicious grouped microcalcification in the right breast on annual breast screening. The patient had no significant family history of breast cancer. Clinical examination did not reveal palpable masses or enlarged axillary lymph nodes. A mammogram was performed and reported as breast imaging-reporting and data system category 4 (BI-RADS 4). Complementary ultrasound and magnetic resonance imaging (MRI) confirmed the BI-RADS 4 report corresponding to the area of concern (Figure 1). Histology was obtained using ultra-sound-guided biopsy. It was reported as focal adenosis with microcalcification, stromal fibrosis, with no evidence of malignancy. Taking into account the new development and age of the patient, a wide local excision (WLE) of the lesion with safety margins was performed. The report was of a rarer type of AME with mucoepidermoid/divergent differentiation, without evidence of malignancy. No evidence of recurrence was discovered in the subsequent 2 years of annual follow-up imaging.
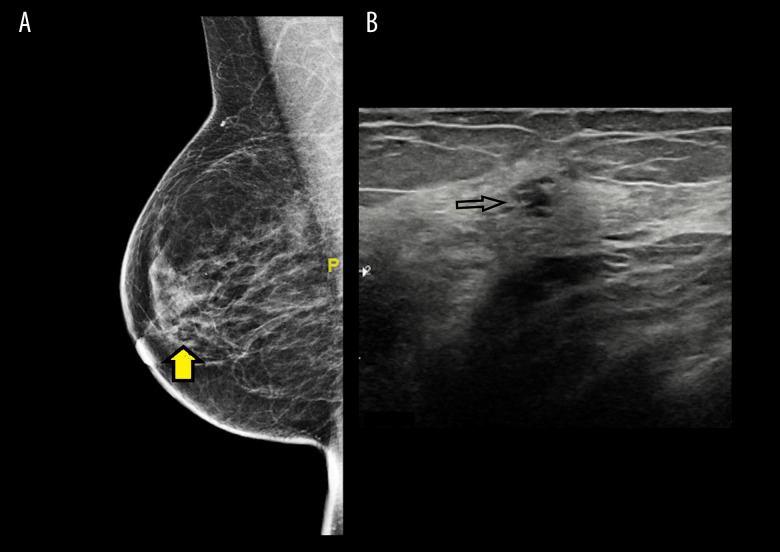
Figure 1.
(A) Mammogram with mediolateral oblique view (MLO) that shows the partially obscured breast mass, denoted by the arrow. (B) Ultrasound confirms the findings of the mammogram in Figure 1A.
Case 2
A 74-year-old woman, who was known to have multiple comorbidities, presented with a right breast mass of 1-month duration. There were no local or systemic symptoms related to the breasts. She had no family history of breast diseases. Local breast examination revealed a clinically suspicious firm lobulated breast mass with limited mobility. No palpable axillary lymph nodes were appreciated. Both mammogram and ultra-sound confirmed the presence of a lesion measuring 2×1.5 cm in the right upper outer quadrant and were reported as BIRADS 4 (Figure 2). An ultrasound-guided core needle biopsy was obtained and reported a ductal adenoma with no malignancy. WLE was performed with the final diagnosis of AME associated with areas of sclerosis, fibrocystic changes, apocrine metaplasia, and peripheral intraductal papilloma, without evidence of malignancy (Figure 3). CK 5/6 highlighted myoepithelial cells (Figure 4). Five years of annual mammogram and clinical follow-up showed no evidence of recurrence.
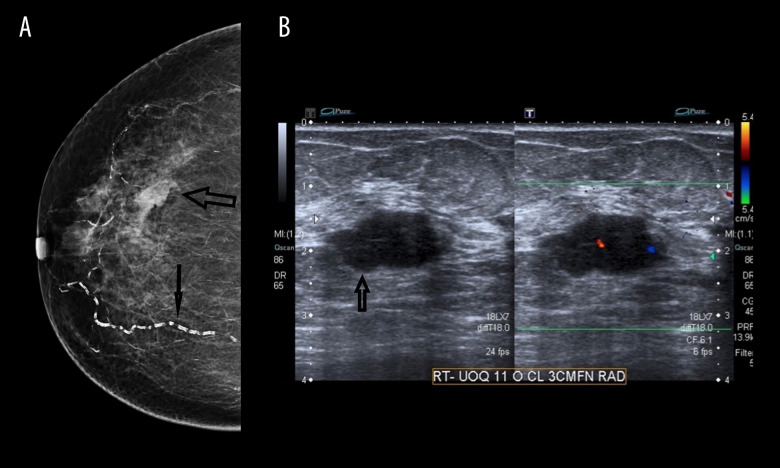
Figure 2.
(A) Mammogram with craniocaudal (CC) view demonstrates the poorly defined mass on a background of fatty breast tissue (large arrow) and vascular calcification (small arrow). (B) Ultrasound confirms the findings of the mammogram in Figure 2A.
Figure 3.
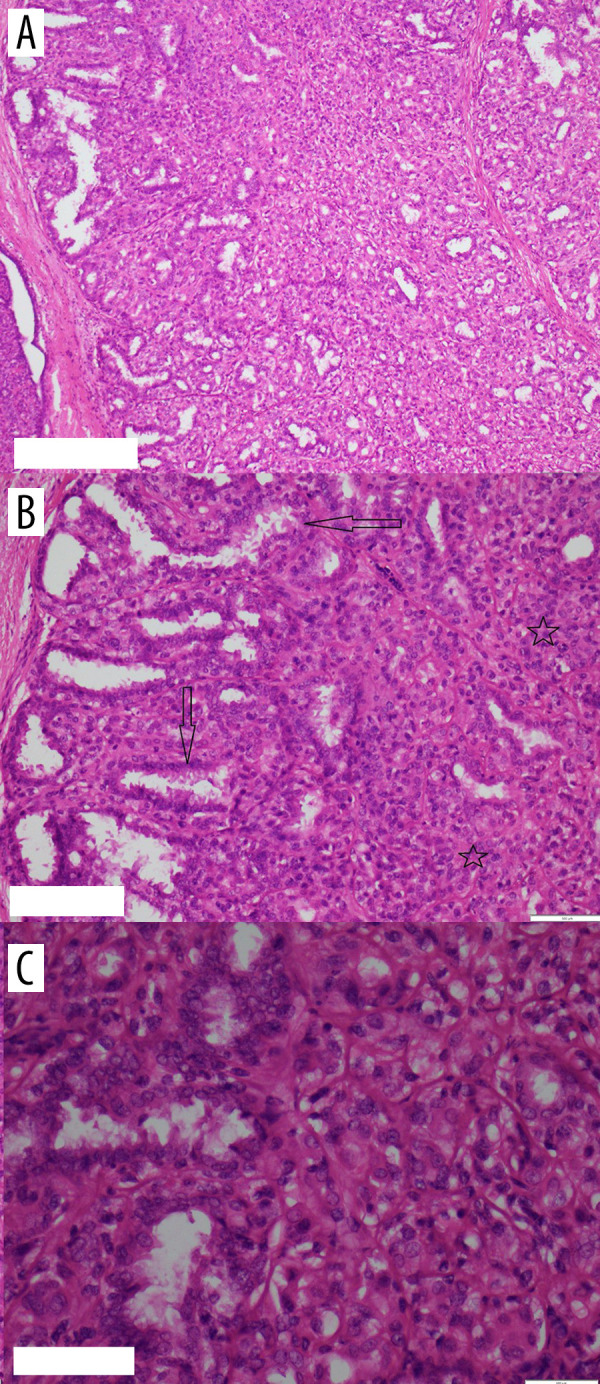
(A) H&E ×10; (B) H&E ×20; (C) H&E ×40, showing a biphasic tumor composed mainly of myoepithelial cells surrounding epithelial-lined spaces.
Figure 4.
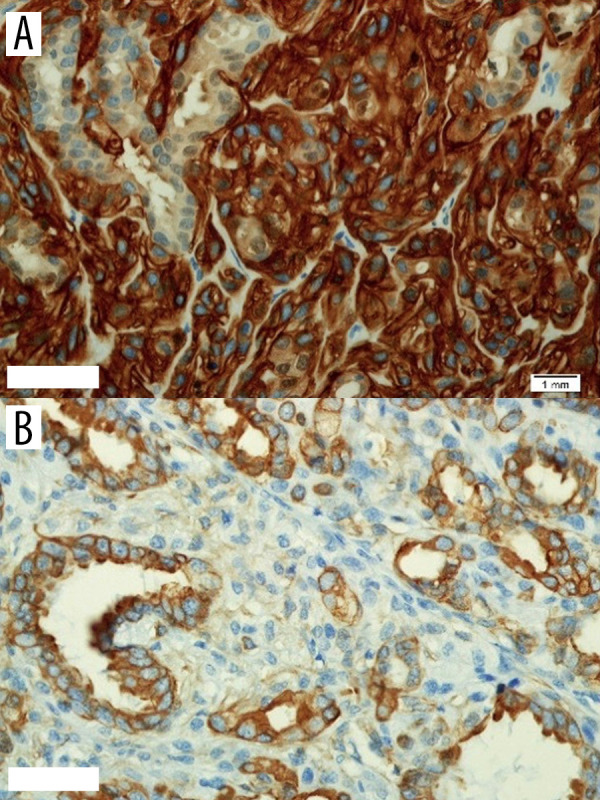
(A) Demonstrates CK7 in glandular components; (B) CD10 highlights the myoepithelial cells.
Case 3
A 49-year-old woman, who had cosmetic bilateral breast augmentation with saline implants at the age of 34, presented with a left breast mass and pain of 2-weeks duration. Local clinical examination of the breasts demonstrated moderate nodularity of both breasts with a non-tender, well-circumscribed, 2×3 cm left breast mass, with no palpable axillary lymph nodes. Mammography complemented by ultrasound revealed dense bilateral breasts with intact implants and left breast mass measuring 3×3 cm with internal vascularity. Furthermore, there was an incidental finding of suspicious calcifications occupying both upper quadrants of the left breast and were reported as BI-RADS 4. Breast MRI demonstrated an extensive area of microcalcification and architectural distortion, associated with a 4×4 cm mass in the upper outer quadrant, highly suggestive of malignancy (Figure 5). An ultrasound-guided core needle biopsy was obtained from both lesions and revealed AME in a background of fibrocystic changes, with usual ductal hyperplasia. However, the second lesion was reported as concomitant ductal carcinoma in situ. The multidisciplinary breast team meeting decided to remove implants, mastectomy, and sentinel lymph node biopsy.
Figure 5.
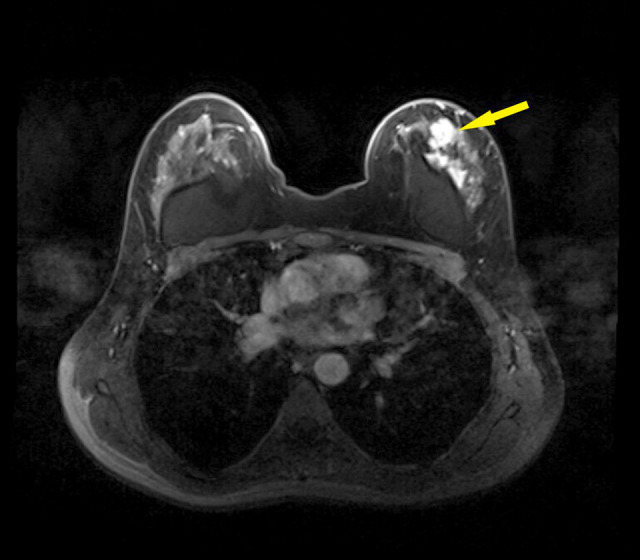
MRI shows a well-defined mass with a concomitant diffuse lesion on the surface of the intact implant on the left breast, denoted by the arrow.
Discussion
AME is a rare benign breast lesion that may pose both clinical and diagnostic challenges. It was first described by Hamperl in 1970 [5].
Histologically, it consists of biphasic proliferation of both myoepithelial and epithelial cells. AME lesions have been classified into tubular, lobulated, or spindle cell variants, and these growth patterns occasionally coexist [1]. Its papillary architecture in most masses propagated the theory of it being a variant of intraductal papilloma [2].
The benign nature of AME is comforting, but its low potential for malignancy incites extensive diagnostic and treatment plans [2].
The reported mean age at presentation is 59 years, yet it can occur at any age [6,7]. The 3 patients presented here demonstrated a wide range of disease patterns. Presentation varies from incidental findings on screening images to palpable, painless, well-defined masses [1]. Unilateral lesions are the most common. However, and to a lesser extent, bilateral lesions have also been reported [7]. The size variation as in this report ranged from 0.3 to 7 cm [1].
Based on heterogeneity and variability, diagnosis can be difficult and challenging [8]. The role of imaging cannot be overemphasized. Mammograms complemented by ultrasound can have limitations, as findings are occasionally nonspecific [9]. The addition of MRI can provide specific morphological and hemodynamic characteristics [10]. The usual appearance on mammograms is an oval or round isodense mass with clear or partially obscured or microlobulated margins; further, when associated with type C or type D breasts, it becomes more challenging as the heterogeneous density can obscure the visualization. Internal group macrocalcifications may be present in some lesions; however, associated microcalcification outside lesions can suggest a dual pathology, like case 3 in our series [7,9–12].
Ultrasound shows features such as solid, hypoechoic, oval-shaped nodules, and irregular, microlobulated, or indistinct margins. Color Doppler ultrasound can also be utilized to delineate lesion vascularity [7,9–12].
MRI supersedes other imaging techniques as it provides details of most lesions when increased homogeneously with the dynamic progressive enhancement curve, type I curve [10].
Another diagnostic method that has gained popularity in recent years is the sono-elastogram, which is used to assess the elasticity of normal breast tissue compared to tissue fibrosis, desmoplastic reaction, and malignant lesions [13].
The histological diagnosis of AME is also challenging. Diagnosis is difficult on cytology alone, as it can be confused with other neoplasms. On the other hand, core biopsy is more helpful and provides precision in diagnosis [14].
Various histological changes have been reported, from the less common presence of calcification and cystic appearance to areas of focal adenosis, sclerosing adenosis, and nodular sclerosis [15,16]. These changes have been documented in our series.
Special attention is required when various forms of metaplasia are present [1,17]. All 3 cases reported here showed some elements of metaplasia. Immunohistochemical staining is utilized to highlight both myoepithelial and epithelial cells of AME [8].
In our report, myoepithelial cells were stained by CK5/6, calponin, and p63, while epithelial cells were stained with CK and ER.
To date, the literature is devoid of established guidelines for the management of both benign and malignant AME [18]. The main treatment is surgical in the form of WLE, with special attention to achieve negative margins to avoid local recurrence [12]. Mastectomy with radiation is reserved for large malignant lesions.
Malignant transformation usually involves 1, or less commonly, both cellular components [2,19]. This is characterized by the rapid growth of a preexisting or newly developed mass [12]. Excisional biopsy usually discloses the diagnosis [20]. Typical histopathological features include a high mitotic rate, cellular atypia, and necrosis [21].
Malignant AME appears to spread through the hematogenous stream and may occur early with primary tumors ≥1.6 cm in size [3,22]. There are few reports of synchronous and meta-chronous breast malignancy [16,23,24].
As for any breast lesion, local recurrence of AME can occur with benign and malignant lesions due to insufficient or narrow margins of excision [1,21]. Surgical excision with wider margins is recommended for recurrent lesions [1].
The overall 5-year survival was 74.4% with studies reporting disease-free survival after 13 years of follow-up [23]. A poor prognosis was reported in patients ages 80 and older and those who chose nonsurgical treatment [25]. Postoperative chemotherapy, hormonal therapy, and radiation therapy were not associated with an improvement in overall survival [25].
Conclusions
Breast AME is a rare condition that can pose diagnostic challenges due to variable imaging presentations, necessitating percutaneous core biopsy for initial diagnosis. Clinical suspicion coupled with utilizing both radiological and histopathological facilities can aid in accurate diagnosis and management.
Abbreviations
- AME
adenomyoepithelioma;
- BI-RADS
breast imaging-reporting and data system category;
- MRI
magnetic resonance imaging;
- WLE
wide local excision;
- MLO
mediolateral oblique;
- CC
craniocaudal
Footnotes
Department and Institution Where Work Was Done
King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
Ethical approval
This report was approved by the Institutional Review Board, with the following number: IRB-2021-01-357.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Tavassoli FA. Myoepithelial lesions of the breast: Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15(6):554–6. doi: 10.1097/00000478-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rosen PP. Adenomyoepithelioma of the breast. Hum Pathol. 1987;18(12):1232–37. doi: 10.1016/s0046-8177(87)80406-9. [DOI] [PubMed] [Google Scholar]
- 3.Petrozza V, Pasciuti G, Pacchiarotti A, et al. Breast adenomyoepithelioma: A case report with malignant proliferation of epithelial and myoepithelial elements. World J Surg Oncol. 2013;11(1):2–7. doi: 10.1186/1477-7819-11-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan Z, Qu X, Zhang Z-T, Jiang WG. Lessons from managing the breast malignant adenomyoepithelioma and the discussion on treatment strategy. World J Oncol. 2017;8(4):126–31. doi: 10.14740/wjon1055e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- 6.McLaren BK, Smith J, Schuyler PA, et al. Adenomyoepithelioma: Clinical, histologic, and immunohistologic evaluation of a series of related lesions. Am J Surg Pathol. 2005;29(10):1294–99. doi: 10.1097/01.pas.0000164615.38200.86. [DOI] [PubMed] [Google Scholar]
- 7.Bajpai J, Punatar SB, Gupta A, et al. Bilateral adenomyoepithelioma of breast. J Cancer Res Ther. 2013;9(3):523–25. doi: 10.4103/0973-1482.119370. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JY, Chitale D. Adenomyoepithelioma of the breast: a brief diagnostic review. Arch Pathol Lab Med. 2013;137(5):725–29. doi: 10.5858/arpa.2011-0404-RS. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Delgado ML, López-Ruiz JA, Eizaguirre B, et al. Benign adenomyoepithelioma of the breast: Imaging findings mimicking malignancy and histo-pathological features. Acta radiol. 2007;48(1):27–29. doi: 10.1080/02841850601080432. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Qin G, He Z, et al. The mammography and MRI manifestations of adenomyoepithelioma of the breast. Clin Radiol. 2016;71(3):235–43. doi: 10.1016/j.crad.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Adejolu M, Wu Y, Santiago L, Yang WT. Adenomyoepithelial tumors of the breast: Imaging findings with histopathologic correlation. Am J Roentgenol. 2011;197(1):184–91. doi: 10.2214/AJR.10.6064. [DOI] [PubMed] [Google Scholar]
- 12.Howlett DC, Mason CH, Biswas S, et al. Adenomyoepithelioma of the breast: Spectrum of disease with associated imaging and pathology. Am. J. Roentgenol. 2003;180(3):799–803. doi: 10.2214/ajr.180.3.1800799. [DOI] [PubMed] [Google Scholar]
- 13.Hatzung G, Grunwald S, Zygmunt M, et al. Sonoelastography in the diagnosis of malignant and benign breast lesions: Initial clinical experiences. Ultraschall Med. 2010;31(6):596–603. doi: 10.1055/s-0029-1245526. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar P, Ali SZ, Brogi E. Fine-needle aspiration cytology of mammary adenomyoepithelioma: A study of 12 patients. Cancer. 2006;108(4):250–56. doi: 10.1002/cncr.21839. [DOI] [PubMed] [Google Scholar]
- 15.Trojani M, Guiu M, Trouette H, et al. Malignant adenomyoepithelioma of the breast: An immunohistochemical, cytophotometric, and ultrastructural study of a case with lung metastases. Am J Clin Pathol. 1992;98(6):598–602. doi: 10.1093/ajcp/98.6.598. [DOI] [PubMed] [Google Scholar]
- 16.Parikh P, Jameel Z, Falcon S, et al. Adenomyoepithelioma of the breast: Case series and literature review. Clin Imaging. 2021;75:157–64. doi: 10.1016/j.clinimag.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Cai RZ, Tan PH. Adenomyoepithelioma of the breast with squamous and sebaceous metaplasia. Pathology. 2005;37(6):557–59. doi: 10.1080/00313020500368394. [DOI] [PubMed] [Google Scholar]
- 18.Intagliata E, Gangi S, Trovato C, et al. Benign adenomyoepitelioma of the breast: Presentation of two rare cases and review of literature. Int J Surg Case Rep. 2020;67:1–4. doi: 10.1016/j.ijscr.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed AA, Heller DS. Malignant adenomyoepithelioma of the breast with malignant proliferation of epithelial and myoepithelial elements: A case report and review of the literature. Arch Pathol Lab Med. 2000;124(4):632–36. doi: 10.5858/2000-124-0632-MAOTBW. [DOI] [PubMed] [Google Scholar]
- 20.Hoda SA, Rosen PP. Observations on the pathologic diagnosis of selected unusual lesions in needle core biopsies of breast. Breast J. 2004;10(6):522–27. doi: 10.1111/j.1075-122X.2004.21412.x. [DOI] [PubMed] [Google Scholar]
- 21.Loose JH, Patchefsky AS, Hollander IJ, et al. Adenomyoepithelioma of the breast: A spectrum of biologic behavior. Am J Surg Pathol. 1992;16(9):868–76. doi: 10.1097/00000478-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Bult P, Verwiel JM, Wobbes T, et al. Malignant Adenomyoepithelioma of the breast with metastasis in the thyroid gland 12 years after excision of the primary tumor. Case report and review of the literature. Virchows Arch. 2000;436(2):158–66. doi: 10.1007/pl00008216. [DOI] [PubMed] [Google Scholar]
- 23.Moritz AW, Wiedenhoefer JF, et al. Breast adenomyoepithelioma and adenomyoepithelioma with carcinoma (malignant adenomyoepithelioma) with associated breast malignancies: A case series emphasizing histologic, radiologic, and clinical correlation. Breast. 2016;29:132–39. doi: 10.1016/j.breast.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Wiens N, Hoffman DI, Huang CY, et al. Clinical characteristics and outcomes of benign, atypical, and malignant breast adenomyoepithelioma: A single institution’s experience. Am J Surg. 2020;219(4):651–54. doi: 10.1016/j.amjsurg.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Haque W, Verma V, Suzanne Klimberg V, et al. Clinical presentation, national practice patterns, and outcomes of breast adenomyoepithelioma. Breast J. 2020;26(4):653–60. doi: 10.1111/tbj.13638. [DOI] [PubMed] [Google Scholar]