Abstract
BACKGROUND
The relative efficacy and safety of allopurinol and febuxostat when used according to current guidelines for the treatment of hyperuricemia are unknown. This double-blind noninferiority trial examined these issues.
METHODS
Participants with gout and hyperuricemia (with at least 33% having stage 3 chronic kidney disease) were randomly assigned to allopurinol or febuxostat in this 72-week trial, with doses titrated to target serum urate. The trial had three phases: titration (weeks 0 to 24), maintenance (weeks 25 to 48), and observation (weeks 49 to 72). Allopurinol and febuxostat were initiated at daily doses of 100 and 40 mg, with maximum titration to 800 and 120 mg, respectively. Antiinflammatory prophylaxis was given during phases 1 and 2. The primary end point was the proportion of patients experiencing one or more flares during phase 3, with a prespecified noninferiority margin of less than 8 percentage points between allopurinol and febuxostat. Secondary end points included efficacy in patients with chronic kidney disease, proportion achieving target serum urate levels, and serious adverse events.
RESULTS
This study included 940 participants; 20.1% withdrew, with similar proportions in treatment arms. During phase 3, 36.5% of allopurinol-treated participants had one flare or more compared with 43.5% of febuxostat-treated participants (P<0.001 for noninferiority). Overall, 80% of participants achieved mean target urates during phase 2 with no differences by treatment. There were no treatment differences (including cardiovascular events) in serious adverse events.
CONCLUSIONS
Allopurinol and febuxostat achieved serum urate goals in patients with gout; allopurinol was noninferior to febuxostat in controlling flares. Similar outcomes were noted in participants with stage 3 chronic kidney disease. (Funded by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development; ClinicalTrials.gov identifier, NCT02579096.)
Introduction
Gout, the most common inflammatory arthritis, affects 4% of adults or more than 9 million people in the United States alone,1 with an incidence that has doubled in the past 30 years.2 The clinical impact of gout is underscored by its strong associations with hypertension, obesity, diabetes, and renal and cardiovascular disease, in addition to accelerated mortality.3–6 Population data suggest that gout is significantly under-treated, with urate-lowering therapy (the cornerstone of appropriate gout management) either frequently not used or underdosed if used.7
A paucity of data exists regarding the relative efficacy and safety of the two major oral urate-lowering therapies, allopurinol and febuxostat, when administered as part of a titrate-to-target approach to achieve a serum urate below 6 mg/dl as recommended by guidelines from both the American College of Rheumatology8,9 and the European League Against Rheumatism (EULAR).10 In contrast to these recommendations, regulatory trials that led to the approval of febuxostat compared fixed doses of febuxostat against a maximum fixed dose of 300 mg of allopurinol.11–13 Evidence regarding comparative efficacy and safety is particularly important in the context of chronic kidney disease, one of the most common comorbid conditions among patients with gout.4,14 While guidelines recommend both therapies in patients with chronic kidney disease, only febuxostat’s labeling provides guidance around use. Furthermore, the Food and Drug Administration (FDA) recently issued a boxed warning concerning the cardiovascular safety of febuxostat15 on the basis of the results of the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout (CARES) trial.16 Therefore, there exists substantial uncertainty and controversy regarding which urate-lowering therapy to use. This multicenter, randomized, double-blind, noninferiority trial was designed to examine the comparative efficacy and safety of allopurinol and febuxostat when appropriately titrated in participants with gout, including participants with chronic kidney disease (stage 3).
Methods
STUDY DESIGN
Detailed methods of the study design were published previously.17 Briefly, 950 participants fulfilling American College and Rheumatology gout classification criteria18 with a serum urate concentration of 6.8 mg/dl or greater were enrolled from 21 sites between 2017 and 2019. This noninferiority, double-blind, double-dummy, 72-week trial (CSP594 Comparative Effectiveness in Gout: Allopurinol versus Febuxostat [ClinicalTrials.gov identifier, NCT02579096], funded by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development) randomly assigned participants 1:1 to receive allopurinol or febuxostat at doses uptitrated according to a standard protocol to achieve a serum urate target of 6 mg/dl or lower (<5 mg/dl or lower if tophi present). Participants with gout — based on satisfaction of the American College of Rheumatology9 and EULAR10 gout classification criteria and hyperuricemia, defined as 6.8 mg/dl or greater — were eligible for this study as long they had not previously been treated with more than 300 mg of allopurinol daily. Full inclusion and exclusion criteria are listed in Figure S1 in the Supplementary Appendix. The protocol additionally specified that one third or more of enrolled participants would have stage 3 chronic kidney disease (estimated glomerular filtration rate of <60 and ≥30 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula).19 Treatment assignment was stratified by site, and trial recruitment was monitored to ensure accrual of the appropriate proportion of participants with chronic kidney disease as well as balanced accrual of participants with chronic kidney disease, markedly elevated urate levels (>9 mg/dl), and previous allopurinol use (Figure S6). HLA-B*5801 testing of participants (an indicator of possible risk of allopurinol hypersensitivity) was performed at the discretion of each site investigator. The study protocol was approved by the Veterans Affairs Central Institutional Review Board. All participants gave written informed consent.
TREATMENT PROTOCOL
The treatment protocol consisted of three phases: urate-lowering therapy titration (weeks 0 to 24), maintenance (weeks 25 to 48), and observation (weeks 49 to 72). During phase 1, those randomly assigned to allopurinol or febuxostat were initiated at daily doses of 100 and 40 mg, respectively, with therapies titrated until a serum urate below 6.0 mg/dl (<5.0 mg/dl if tophi were present) was achieved or the maximal dose was reached. Participants taking allopurinol before the trial continued taking their pretrial allopurinol dose if randomly assigned to that arm, with dose titration delayed for those receiving 200 mg (to week 6) or 300 mg (to week 9) or, if randomly assigned to febuxostat, started at 40 mg per day. Titration followed the 2012 American College of Rheumatology gout guidelines,8 with maximum daily doses of 800 mg of allopurinol and 120 mg of febuxostat. The timing of febuxostat dose escalation was adjusted to parallel that of equivalent doses of allopurinol (Table S1). The original maximal daily febuxostat dose of 120 mg per day was chosen to conform to EULAR guidelines but was reduced to 80 mg per day in 2019 per FDA request around safety concerns and updated labeling (Fig. S2).15 All participants received guideline-directed antiinflammatory prophylaxis with colchicine, nonsteroidal antiinflammatory drugs, or glucocorticoids per investigator choice8 during phases 1 and 2. Treatment dose titration occurred primarily in phase 1; however, because a single target serum urate is not indicative of permanent control, allopurinol and febuxostat dose adjustments were permitted until week 33 in order to achieve the serum urate goal. During phase 3, no study drug dose adjustments were allowed, and all prophylactic antiinflammatory treatments were discontinued, although prophylaxis could be restarted in the event of a gout flare. Information regarding gout flares and medication use was collected in a patient diary through questionnaires that were gathered by site staff at scheduled in-person and telephone visits.
STUDY OUTCOMES
The primary outcome was the proportion of participants experiencing one or more gout flares during phase 3. Participants were defined as having experienced a gout flare if they met three of four participant-reported criteria20 — warm joint(s), swollen joint(s), pain (>3) at rest on a scale of 0 to 10 (with higher numbers indicating more severe pain), or self-identified gout flare — or reported use of standard antiinflammatory medications to treat a flare on a participant-reported questionnaire collected every 6 weeks.
Secondary outcomes included efficacy and side-effect profiles in participants with chronic kidney disease, the proportion of participants achieving the predefined serum urate goal at the end of phase 2, and serious adverse events.
Acute kidney injury in a post hoc analysis was classified using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria of at least a 50% increase in creatinine relative to the most recent creatinine values or any increase of at least 0.3 mg/dl within a 48-hour period.21
TRIAL CONDUCT
The trial was temporarily halted twice (Fig. S2): first with the publication of the CARES trial,16 which suggested an increased association with cardiovascular mortality with febuxostat compared with allopurinol, and then when the resulting boxed warning was issued by the FDA.15 After consultation with the FDA and the Veterans Affairs Central Institutional Review Board, the trial resumed after convening an independent board of experts to adjudicate all cardiovascular events in a blinded fashion, reducing the maximal allowable febuxostat dose (from 120 to 80 mg), and reconsenting all participants. Participants receiving 120 mg of febuxostat or placebo had their dose blindly decreased to 80 mg at the time of this change. Furthermore, the Covid-19 pandemic affected the later part of in-person data accumulation. This did not greatly influence ascertainment of our primary outcome, which was conducted through phone visits.
STATISTICAL ANALYSIS
Our one-sided null hypothesis posited that allopurinol was inferior to febuxostat. We compared the proportion of participants with one or more gout flares during phase 3 between the two treatments. A noninferiority bound of 8 percentage points was chosen. The criterion for noninferiority would be met if the upper bound of the 95% confidence interval (CI) of the difference (the percentage of participants assigned to allopurinol who had a flare minus the percentage of participants assigned to febuxostat who had a flare) was less than 8 percentage points. This 8-percentage-point margin was established during the trial design by a panel of gout experts on the basis of clinical significance. (See the NEJM Evidence “Stats, STAT!” on noninferiority trials at https://evidence.nejm.org/doi/full/10.1056/EVIDstat2100040.) A one-sided alpha level was set at 0.05. We planned to enroll 950 participants to have 90% power to detect noninferiority with the stated margin of 8 percentage points, assuming a 10% loss to follow-up. Our analysis excluded participants who either never received the trial interventions or were identified as ineligible after random assignment. According to our prespecified analysis plan, the primary analysis excluded dropouts; thus, our primary analysis was a complete case analysis. As a sensitivity analysis, we performed a worst-case scenario analysis by assigning a flare outcome to phase 3 participant dropouts in the allopurinol group and assigning no flare to participant dropouts in the febuxostat group. A more rigorous analysis imputing missing outcomes would produce a result between these two analyses. A two-sided Cox model analysis was performed to account for dropouts as having censored outcomes.
Other than noninferiority tests, all other test comparisons were two sided. The primary outcome was similarly assessed in the prespecified subgroups defined by chronic kidney disease status. All secondary outcomes were compared between treatment groups using two-sided chi-square tests or Student t-tests. Statistical analyses were performed using SAS (version 9.4; SAS Institute) and R (version 4.0.0; R Project for Statistical Computing) software.
Results
PARTICIPANT CHARACTERISTICS
Characteristics of the 940 participants with gout receiving at least one dose of study medication are shown in Table 1. There were no striking imbalances in the treatment groups, and no active adjustments were required to achieve evenly randomly assigned treatment groups. Comorbidities were common, and participants in the allopurinol group were slightly older and slightly more likely to have chronic kidney disease, hypertension, diabetes, and a history of cardiovascular disease. Early termination before completion occurred in 20.1% of participants, with similar proportions by treatment arm (Fig. 1 and Table S6). Prophylactic medications prescribed were similar in both treatment groups, with 91.2% of participants receiving colchicine (Table S2). Participants randomly assigned to febuxostat were potentially affected by the FDA-mandated change in maximum febuxostat dose. Twelve participants in the febuxostat arm finished the trial with the 120-mg dose before the change, 10 participants had their dose decreased from 120 to 80 mg, and 10 participants had urate levels greater than 6 mg/dl but continued to take 80 mg of febuxostat per day without further up-titration.
Table 1.
Baseline Characteristics of the Total Participants Enrolled.*
| Characteristic | Allopurinol (n=468) | Febuxostat (n=472) | Total (n=940) |
|---|---|---|---|
| Demographics | |||
| Age — yr | 62.9 (11.8) | 61.3 (12.9) | 62.1 (12.4) |
| Men | 98.5 | 98.3 | 98.4 |
| Race and ethnicity | |||
| White/Caucasian | 67.3 | 68.2 | 67.8 |
| Black/African American | 22.2 | 21.6 | 21.9 |
| Asian | 3.0 | 3.2 | 3.1 |
| Native Hawaiian/Pacific Islander/Maori | 2.1 | 1.9 | 2.0 |
| American Indian | 0.6 | 0.4 | 0.5 |
| Other | 4.7 | 4.7 | 4.7 |
| Comorbidity† | |||
| Chronic kidney disease, eGFR — ml/min | |||
| ≥90 | 12.2 | 12.7 | 12.4 |
| 60–89 | 49.1 | 51.3 | 50.2 |
| 30–59 | 38.7 | 36.0 | 37.3 |
| Serum creatinine — mg/dl | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| Hypertension‡ | 78.0 | 74.8 | 76.4 |
| Diabetes | 35.0 | 31.6 | 33.3 |
| Cardiovascular disease§ | 30.1 | 23.5 | 26.8 |
| Body-mass index¶ | 33.6 (6.6) | 33.7 (6.6) | 33.7 (6.6) |
| C-reactive protein — mg/l | 9.6 (18.7) | 8.2 (15.4) | 8.9 (17.1) |
| Gout-related factors | |||
| Serum urate | |||
| Mean — mg/dl | 8.6 (1.4) | 8.5 (1.3) | 8.5 (1.4) |
| ≥9 | 34.2 | 31.4 | 32.8 |
| Allopurinol use ≤ 300 mg/d | 38.0 | 35.4 | 36.7 |
| Gout duration — yr | 9.7 (10.6) | 10.2 (11.4) | 10.0 (11.0) |
| Presence of tophi | 17.3 | 15.0 | 16.2 |
Values are presented as percentages or means (SD). eGFR denotes estimated glomerular filtration rate.
Chronic kidney disease was defined by an eGFR of 30 to 60 ml/min at enrollment. Participants were excluded if they had prior febuxostat use or an eGFR of less than 30 ml per minute.
Hypertension and diabetes were documented for this study using participants’ problem lists in the medical record review.
Cardiovascular disease indicates a history of coronary artery disease, myocardial infarction, or heart failure.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Figure 1.
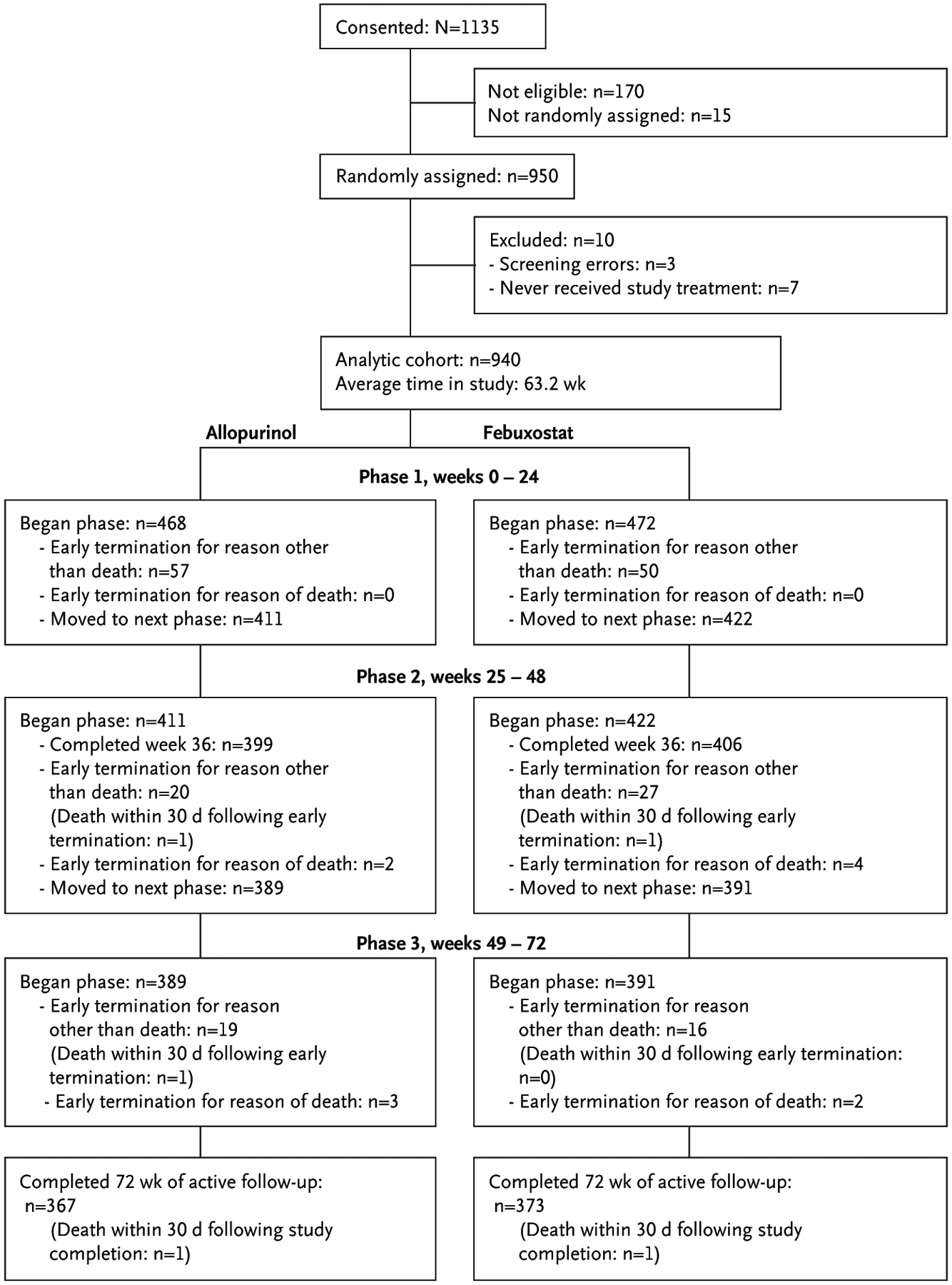
CONSORT Flow Diagram.
CONSORT denotes Consolidated Standards of Reporting Trials.
PRIMARY OUTCOME
Of the 749 evaluable participants in phase 3 (Table S3), 36.5% of participants treated with allopurinol experienced one or more flares compared with 43.5% of participants treated with febuxostat; the difference in the percentage of patients with one or more flares was −7 (95% CI, −∞ to −1.2) percentage points, with the minus sign indicating fewer events with allopurinol (P<0.001 for noninferiority of allopurinol; Table 2). The lower percentage of flare events with allopurinol was observed despite similar serum urate values achieved between the treatment groups (mean 5.2 mg/dl for both groups entering phase 3). Using a worst-case scenario sensitivity analysis, the results were similar to those in the primary analysis (risk difference, −2.6%; upper boundary of the one-sided 95% CI, 3.2%, which also supports our noninferiority claim). To account for missing primary end points, a confirmatory analysis was performed with the use of the Cox model. The time to the first flare event from all of the patients who had entered phase 3 was used as the outcome. The analysis yielded similar results (hazard ratio, 0.76; 95% CI, 0.60 to 0.95; P=0.02). Finally, because of the FDA-mandated febuxostat dose reduction, an analysis was performed assuming that none of the affected 20 participants taking febuxostat flared (result: 39.8% flare rate); therefore, allopurinol (36.5% flare rate) remained noninferior to febuxostat.
Table 2.
Study Results.
| End Point* | Allopurinol | Febuxostat | Risk Difference or Risk Ratio (95% CI)† |
|---|---|---|---|
| Primary | |||
| ≥1 gout flare in phase 3 | 36.5 (135/370) | 43.5 (165/379) | −7 (−∞ to −1.2) |
| Secondary | |||
| All study participants | |||
| Serum urate in phase 2 < 6.0 mg/dl‡ | 81.1 (318/392) | 78.4 (308/393) | 1.04 (0.96 to 1.11) |
| Serum urate in phase 2 < 6.8 mg/dl‡ | 92.4 (362/392) | 91.1 (358/393) | 1.01 (0.97 to 1.06) |
| Serious adverse event | 26.7 (125/468) | 26.1 (123/472) | 1.02 (0.83 to 1.27) |
| Early study termination | 20.5 (96/468) | 19.7 (93/472) | 1.04 (0.81 to 1.34) |
| Rate of gout flares — events/person-years | |||
| During whole study | 1.73 | 1.97 | 0.88 (0.81 to 0.96) |
| During phase 1 | 2.09 | 2.25 | 0.93 (0.81 to 1.06) |
| During phase 2 | 1.60 | 1.59 | 1.00 (0.85 to 1.18) |
| During phase 3 | 1.48 | 2.02 | 0.73 (0.63 to 0.86) |
| Cardiovascular event§ | 8.1 (38/468) | 6.8 (32/472) | 1.20 (0.76 to 1.88) |
| C-reactive protein — mg/l¶ | 7.0 (12.3) | 6.5 (11.3) | N/A |
| Serum creatinine — mg/dl¶ | 1.2 (0.4) | 1.2 (0.4) | N/A |
| Serum urate in phase 2 — mg/dl‡ | 5.2 (1.2) | 5.2 (1.3) | N/A |
| Serum urate at study end — mg/dl | 5.1 (1.4) | 5.3 (1.8) | N/A |
| Week 48 medication dosage — mg∥ | 400 (300–500) | 40 (40–80) | N/A |
| Participants with stage 3 chronic kidney disease | |||
| ≥1 gout flares in phase 3 | 31.9 (44/138) | 45.3 (63/139) | 13.4 (−∞ to −3.9)** |
| Serious adverse events | 38.1 (69/181) | 35.9 (61/170) | 1.06 (0.81 to 1.40) |
| Serum urate < 6.0 mg/dl in phase 2‡ | 78.8 (119/151) | 81.3 (117/144) | 0.97 (0.87 to 1.09) |
| Serum urate < 6.8 mg/dl in phase 2‡ | 92.1 (139/151) | 93.1 (134/144) | 0.99 (0.93 to 1.06) |
Primary end point was assessed for 749 patients entering phase 3 (P<0.001). Values are presented as percentages (proportions) unless indicated otherwise.
Risk differences (95% confidence intervals [CIs]) are presented for the primary end point, whereas risk ratios are presented for the secondary end points unless indicated otherwise. N/A indicates not applicable.
Serum urate in phase 2 was defined as the mean concentration at weeks 36, 42, and 48; 46 (5.9%) at week 36, 144 (18.5%) at week 42, and 33 (4.2%) serum urate measurements are missing.
Table shows adjudicated cardiovascular events.
Mean (SD) C-reactive protein and serum creatinine laboratory values were calculated from participants within the week 48 visit window.
Values are presented as the median (interquartile range).
This result is the risk difference. All other results below in this column are risk ratio results.
PRESPECIFIED SECONDARY OUTCOMES
Among participants with chronic kidney disease, allopurinol was noninferior to febuxostat (risk difference, 13.4; 95% CI, −∞ to −3.9), with 31.9% and 45.3% of allopurinol- and febuxostat-treated participants experiencing a flare, respectively (Fig. S3). Considering all enrolled participants, 79.8% achieved the serum urate goal during phase 2, with 81.1% of patients for allopurinol and 78.4% for febuxostat (Table 2). Participants with chronic kidney disease were similarly successful in achieving target serum urate (80.0% overall), with no differences between treatment groups.
Table 2 details the flare rates during the three phases by treatment. Flare rates did not differ between allopurinol and febuxostat during phases 1 and 2, but there were 1.48 and 2.02 events per person-year with allopurinol and febuxostat, respectively, with a risk ratio of 0.73 (0.63 to 0.86) favoring allopurinol during phase 3. There was a trend for flare rates to decrease as the trial progressed for allopurinol but not for febuxostat (Table 2).
POST HOC SECONDARY OUTCOMES
Other outcomes of interest (Table 2 and Table S4) included declines in mean serum urate levels from baseline to week 48, no changes in serum creatinine, and declines in C-reactive protein levels, although none of these parameters differed by treatment group. Further, more than 99% of participants remaining in the trial at the end of phase 1 (titration phase) had at least one serum urate below 6.0 mg/dl during the trial, and this did not differ across treatment groups. The median allopurinol dose at the end of phase 2 was 400 mg per day and the median febuxostat dose was 40 mg per day (Table 2). Titrated doses greater than 300 mg of allopurinol per day were required in 54.5% of participants and doses of at least 80 mg of febuxostat per day were required in 47.7% of participants (Fig. S4).
SAFETY
There were no differences in serious adverse events (including the percentage of participants with cardiovascular events or death) across treatment groups as a whole or in the subset with chronic kidney disease (Table 3). Adverse events leading to study withdrawal were also similar across treatment groups (Table 3). Adjudicated major adverse cardiovascular events occurred with similar frequency in both treatment groups, while hospitalization for heart failure was more common in participants treated with allopurinol (23 vs. 10).
Table 3.
Safety Results.*
| Outcome | Allopurinol (n=468) | Febuxostat (n=472) | Total (n=940) |
|---|---|---|---|
| Adverse events leading to discontinuation of the study | 21 | 15 | 36 |
| Deaths | |||
| While active in the study | 5 | 6 | 11 |
| Within 30 days of participant’s last visit | 3 | 2 | 5 |
| Total | 8 | 8 | 16 |
| Serious adverse events (total events) | 330 | 273 | 603 |
| Unique participants (>2% total) without CKD and with the following† | |||
| Cellulitis | 0 | 8 | 8 |
| Pneumonia | 4 | 1 | 5 |
| Acute kidney injury‡ | 3 | 3 | 6 |
| Unique participants (>2% total) with CKD and with the following† | |||
| Cellulitis | 6 | 4 | 10 |
| Pneumonia | 6 | 8 | 14 |
| Acute kidney injury‡ | 15 | 4 | 19 |
| Rashes | |||
| Severe | 1 | 2 | 3 |
| Total (mild, moderate, and severe) | 63 | 52 | 115 |
| Unique participant adjudicated cardiovascular events | |||
| Arrhythmias not associated with ischemia | 14 | 11 | 25 |
| Hospitalization for heart failure | 23 | 10 | 33 |
| Transient ischemic attack | 0 | 3 | 3 |
| Venous and peripheral arterial thromboembolic events | 1 | 1 | 2 |
| MACEs | 10 | 10 | 20 |
| Total (unique participants) | 38 | 32 | 70 |
| Unique participant adjudicated MACEs | |||
| Cardiovascular death | 3 | 1 | 4 |
| Nonfatal myocardial infarction | 2 | 4 | 6 |
| Stroke | 1 | 2 | 3 |
| Unstable angina requiring urgent revascularization | 4 | 3 | 7 |
| Total | 10 | 10 | 20 |
Values are presented as the number of participants. CKD denotes chronic kidney disease and MACE major adverse cardiovascular events.
Only serious adverse events that occurred in more than 2% of study participants are included.
Acute kidney injury was determined per post hoc definition.
Among participants with chronic kidney disease, serious adverse events for superimposed acute kidney injury were more common in the allopurinol group. Applying a post hoc definition of acute kidney injury,21 15 participants in the allopurinol arm and 4 in the febuxostat arm experienced acute-on-chronic kidney injury. Among these 19 participants, most events were related to volume depletion or congestive heart failure and none were considered by the site investigators to be related to the study drug. All but three events were transient (subsequent nadir serum creatine results no higher than 0.3 mg/dl above baseline); of these three participants, two were assigned to allopurinol (respective outcomes of need for chronic hemodialysis and death attributable to cirrhosis shortly after renal injury) and one to febuxostat (outcome of death from pancreatic cancer 2 months after renal injury).
Rashes were equally common in both treatment groups. A single case of possible allopurinol hypersensitivity syndrome was observed, and the participant was removed from the study. This severe rash occurred shortly after the 6-week up-titration to 300 mg of allopurinol per day and resolved promptly with allopurinol discontinuation. Eleven participants died during the study, with an additional five deaths occurring within 30 days of study end or withdrawal. Of the 16 deaths during the study or within 30 days of study termination or completion, 8 were in the allopurinol group and 8 were in the febuxostat group (Table S5); none were reported as related to study treatment on the basis of the known adverse event profile of both agents.
Discussion
Our randomized double-blind trial demonstrates that allopurinol, when dosed appropriately as part of a titrate-to-target strategy, is noninferior to febuxostat with respect to flares of gout. Our primary outcome was based on the proportion of participants experiencing a flare, an important, clinically relevant outcome. This finding has both important economic implications, because febuxostat is roughly 19 times more costly than allopurinol,22 and important safety implications, with the recent FDA boxed warning around the cardiovascular safety of febuxostat15 and the uncertain cardiovascular safety given the conflicting results of the CARES trial16 and the Febuxostat versus Allopurinol Streamlined Trial (FAST).23 Our findings of the noninferiority of allopurinol support the recent guidelines of the American College of Rheumatology recommending allopurinol as the initial urate-lowering therapy for gout.9 Twenty febuxostat-treated participants were affected by the FDA-mandated change of the maximal allowed febuxostat dose. However, an analysis assuming none of these febuxostat-treated participants flared showed that allopurinol was still noninferior to febuxostat for flare prevention.
Importantly, both allopurinol and febuxostat were highly and equally efficacious in controlling hyperuricemia when used in this titrate-to-target protocol, with 80% of patients achieving and maintaining serum urate levels at goal at 1 year and more than 91% of participants achieving a mean serum urate of less than 6.8 mg/dl, which is the serum level at which urate becomes supersaturated. Our findings are comparable to those published by Doherty et al.,24 in which allopurinol was titrated to target according to a nurse-led protocol and 95% of participants achieved target serum urate at 2 years. Our success with both allopurinol or febuxostat when used in a titrate-to-target protocol has substantial implications in this painful and chronic disease, where available data suggest that with usual care, 30% of patients with gout, or fewer, achieve target serum urate levels.24,25 In practice, the most common allopurinol dose is 300 mg daily. In this study, the median dose of allopurinol to achieve target was 400 mg, while 29% of participants needed 500 mg or higher. Given the tremendous burden of gout in terms of painful morbidity, health care costs, and work loss,1–3 the demonstration of the ability to successfully achieve target serum urate levels in the majority of participants with either agent when appropriately titrated has significant implications. If strategies can be employed to operationalize our findings to clinical practice, the reduction in morbidity and economic burden secondary to gout would be dramatic.
Chronic kidney disease is present in more than one third of all patients with gout,14 representing both a major risk factor for the development of hyperuricemia and gout as well as a potential complicating factor for optimal dosing of urate-lowering therapies. Early recommendations for dosing of allopurinol in participants with chronic kidney disease26 were based on scant data and have contributed to the undertreatment and poor control of gout in these participants.27 Current recommendations from the American College of Rheumatology9 and EULAR10 advocate for more aggressive dosing in chronic kidney disease but are also based on few data. This trial, with 351 participants with gout and stage 3 chronic kidney disease, is among the largest of such populations ever studied in a blinded titrate-to-target trial. By following titrate-to-target guidelines that initiated urate-lowering therapy at 100 mg of allopurinol or 40 mg of febuxostat, we were able to titrate urate-lowering therapy and achieve target urate levels without undue toxicity or impairment of kidney function in the majority of participants with chronic kidney disease stage 3. In post hoc analysis applying KDIGO criteria, acute kidney injury occurred in 15 allopurinol-treated participants compared with 4 febuxostat-treated participants, a numerical difference that is being investigated. Patients with more severe chronic kidney disease were excluded from the trial, so no conclusions can be drawn regarding the efficacy or safety in this population.
During our study, the findings of the CARES trial were published16; these findings suggested that febuxostat increased cardiovascular and all-cause mortality compared with allopurinol. On the basis of these results, the FDA issued a boxed warning in 2019.15 These events led to the temporary interruptions of our trial, which resumed for the second time in April 2019 with modifications that included decreasing the maximum allowable daily dose of febuxostat to 80 mg and blindly adjudicating all cardiovascular events. Subsequently, the FAST trial was published,22 which indicated in a study of a size similar to that of CARES that there was no differential safety signal for febuxostat compared with allopurinol. Our results, in a gout population with a high comorbidity burden, also show no indication that febuxostat increases the risk of major adverse cardiovascular events or is associated with greater cardiovascular mortality or overall mortality. Conversely, our results suggest that allopurinol may be associated with an increased risk of heart failure hospitalization. However, definitive conclusions cannot be drawn from these numerical differences because our study was powered for efficacy and not safety. Previously, allopurinol was studied in two trials of hyperuricemia in participants with heart failure, with conflicting results.28,29 These observations warrant further study.
An important difference between our trial and the larger CARES trial16 was that the retention rate was less than 50% in CARES. Subsequent publications about CARES30 that have included analysis of participants who dropped out strongly suggested that the high dropout rate may have biased the findings. Colchicine has been shown to protect against cardiovascular events in participants without gout in multiple recent publications.31–34 This is relevant because colchicine was used for prophylaxis in more than 90% of participants in our trial, and its use did not differ across treatment groups. Prophylaxis was not allowed with any medication in phase 3 of our trial. We speculate that if colchicine use is prophylactic not only against gout flares but also against any adverse cardiovascular outcomes with febuxostat, we may have mitigated this risk with the near ubiquitous use of colchicine in phases 1 and 2. Even though our trial is relatively small, our data, taken together with those from FAST, strongly suggest that febuxostat is not associated with significant excess cardiovascular risk relative to allopurinol even in the high-risk population that we studied.
Participants who were taking doses of 300 mg of allopurinol or less and still not at urate goals were considered good candidates for this trial. This included 37% of randomly assigned participants, which could have affected the trial results in both directions. These participants had shown the ability to tolerate allopurinol but had a suboptimal response at low doses. Importantly, participants were equally distributed across treatments. Our withdrawal rate of 20% was higher than we had predicted but significantly lower than that found in other gout trials.16,23 Early terminations were similar in both arms (Table S6). With the high precision of our primary outcome results and the sensitivity analysis, we do not believe that this modest early termination rate biased our results. In addition, a diverse population was enrolled, making our results generalizable to patients with gout within the United States (Fig. S5).
In conclusion, this trial demonstrates that allopurinol is noninferior to febuxostat in flare reduction in participants with gout and that either urate-lowering therapy, when used in a titrate-to-target approach, is highly and nearly uniformly effective in getting participants below serum urate goals. Further, the comparative efficacy of these therapies has been extended to participants with chronic kidney disease stage 3, a common comorbidity in gout. Finally, we found no evidence that febuxostat increases cardiovascular morbidity or overall mortality compared with allopurinol.
Supplementary Material
Acknowledgments
Supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (grant 9025CSP825). The opinions expressed in this article are those of the authors and do not necessarily represent those of the Department of Veterans Affairs or the United States Government.
Footnotes
Disclosures
Author disclosures and other supplementary materials are available at evidence.nejm.org.
References
- 1.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the united states and decadal trends: the National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol 2019;71:991–9 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol 2018;45:574–9 10.3899/jrheum.170806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2007;57:109–15 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan E Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One 2012;7:e50046 10.1371/journal.pone.0050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarson LE, Chandratre P, Hider SL, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol 2015;22:335–43 10.1177/2047487313514895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher MC, Rai SK, Lu N, Zhang Y, Choi HK. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis 2017;76:1289–94 10.1136/annrheumdis-2016-210588. [DOI] [PubMed] [Google Scholar]
- 7.Stamp LK, Merriman TR, Barclay ML, et al. Impaired response or insufficient dosage? Examining the potential causes of “inadequate response” to allopurinol in the treatment of gout. Semin Arthritis Rheum 2014;44:170–4 10.1016/j.semarthrit.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–60 10.1002/acr.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 11.Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008; 59:1540–8 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 14.Jing J, Kielstein JT, Schultheiss UT, et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant 2015;30:613–21 10.1093/ndt/gfu352. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. FDA adds boxed warning for increased risk of death with gout medicine Uloric (febuxostat). February 21, 2019. (https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat).
- 16.White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378:1200–10 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 17.Timilsina S, Brittan K, O’Dell JR, et al. Design and rationale for the Veterans Affairs “Cooperative Study Program 594 Comparative Effectiveness in Gout: Allopurinol vs. Febuxostat” trial. Contemp Clin Trials 2018;68:102–8. [DOI] [PubMed] [Google Scholar]
- 18.Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative [published correction appears in Arthritis Rheumatol. 2016;68:515]. Arthritis Rheumatol 2015;67:2557–68 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch KE, Chang JW, Matheny ME, et al. Comparison of automated and retrospectively calculated estimated glomerular filtration rate in electronic health record data. BMC Nephrol 2018;19:380 10.1186/s12882-018-1179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaffo AL, Dalbeth N, Saag KG, et al. Brief report: validation of a definition of flare in patients with established gout. Arthritis Rheumatol 2018;70:462–7 10.1002/art.40381. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 22.Lexicomp for Dentistry [online]. Hudson, OH: UpToDate, 2021. [Google Scholar]
- 23.Mackenzie IS, Ford I, Nuki G, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, noninferiority trial. Lancet 2020;396:1745–57 10.1016/s0140-6736(20)32234-0. [DOI] [PubMed] [Google Scholar]
- 24.Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018; 392:1403–12 10.1016/s0140-6736(18)32158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikuls TR, Cheetham TC, Levy GD, et al. Adherence and outcomes with urate-lowering therapy: a site-randomized trial. Am J Med 2019;132:354–61 10.1016/j.amjmed.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984;76:47–56 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 27.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol 2006; 33:1646–50. [PubMed] [Google Scholar]
- 28.Hare JM, Mangal B, Brown J, et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 2008;51:2301–9 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 29.Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study. Circulation 2015;131:1763–71 10.1161/circulationaha.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi H, Neogi T, Stamp L, Dalbeth N, Terkeltaub R. New perspectives in rheumatology: implications of the cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular morbidities trial and the associated Food and Drug Administration public safety alert. Arthritis Rheumatol 2018;70:1702–9 10.1002/art.40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381: 2497–505 10.1093/eurheartj/ehaa659. [DOI] [PubMed] [Google Scholar]
- 32.Tong DC, Quinn S, Nasis A, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS Randomized Clinical Trial. Circulation 2020;142:1890–900. [DOI] [PubMed] [Google Scholar]
- 33.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–47 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 34.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–10 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


