Abstract
Background
Sperm quality evaluation is the logical first step in increasing field fertility. Spermatozoa contain cytoplasmic organelles and biomolecules known as sperm-intrinsic factors, which play key roles in sperm maturation, sperm-oocyte fusion, and embryo development. In particular, sperm membrane proteins [e.g., arginine vasopressin receptor 2, beta-actin, prohibitin, and heat shock protein family D member 1 (HSPD1)] and RNA could be used as functional indicators of male fertility. We sought to clarify the effects of differential mRNA expression of selected genes on several fertilisation parameters, including sperm motility, motion kinematics, capacitation, and litter size, in a porcine model.
Results
Our results demonstrated that HSPD1 expression was significantly correlated with male fertility, as measured by the litter size of inseminated sows. The expression of HSPD1 mRNA was linked to sperm motility and other motion kinematic characteristics. Furthermore, HSPD1 had a 66.7% overall accuracy in detecting male fertility, and the high-litter size group which was selected with the HSPD1 marker had a 1.34 greater litter size than the low-litter size group.
Conclusions
Our findings indicate that HSPD1 might be a helpful biomarker for superior boar selection for artificial insemination, which could boost field fertility.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-022-00689-0.
Keywords: Fertilisation, HSPD1, Male fertility, Sperm motility, Sperm RNA
Background
Artificial insemination (AI) has been applied globally to breed various types of livestock. More than 80% of cattle and swine production worldwide depends on AI [1]. Therefore, AI failure can cause considerable economic damage to the animal industry. Moreover, approximately 50% of all infertility cases in humans and animals are caused by male factors [1], and only 50% of inseminations result in successful full-term pregnancies [2]. Thus, male fertility is a critical factor to consider when assessing AI failure in the livestock industry. To increase the success rate of AI, new technologies must be developed to optimise sperm quality.
Spermatozoa carry not only the paternal genome, but also several intrinsic factors that modulate early development after fertilisation, including cytoplasmic organelles and biomolecules, such as proteins and RNAs [3–5]. These sperm-intrinsic factors (SIFs) are involved in critical steps of development, such as sperm maturation, sperm-oocyte fusion, and embryo development [4, 6]. Proteins and RNAs in spermatozoa directly affect pre- and post-fertilisation processes. For instance, proteins secreted from the intraluminal compartment of the epididymis interact with sperm surface proteins and induce sperm maturation [7]. Moreover, several sperm cell functions and developmental milestones, such as capacitation, acrosome reaction, sperm penetration, and sperm-oocyte fusion, are strictly controlled by sperm proteins [8]. Several studies have suggested that sperm proteins and RNAs play important roles in fertilisation and pre-implantation embryonic development [8–11]. Several transcriptomic studies of spermatozoa have shown that differential expression of RNA is related to diverse semen traits [12–16]. Although numerous RNAs have been associated with male fertility, further research is needed before changes can be implemented in the field.
Several compelling studies have successfully demonstrated the applicability of sperm proteins and RNA as biomarkers of male fertility [2, 17–20]. The vast majority of sperm membrane proteins have been identified as biomarkers of male fertility outcomes in these studies. Sperm membrane proteins play crucial roles in developmental biology, including protein receptor signalling, flagella movement control, ion homeostasis regulation, and sperm-zona pellucida interactions [20]. In particular, differential expression of arginine vasopressin receptor 2 (AVPR2), beta-actin (ACTB), prohibitin (PHB), and heat shock protein family D member 1 (HSPD1) proteins, all of which are classified by the Gene Ontology database as integral membrane components, has been linked to male fertility outcomes [17]. AVPR2, ACTB, and PHB are closely related to sperm motility and affect male fertility. Arginine vasopressin affects the male reproductive tract, sperm count, and motility in mice [21]. AVPR2 is the G-protein-coupled receptor of arginine vasopressin, and AVPR2 mRNA is found in the vas deferens epithelium of humans and pigs [22] and the tail mid-piece and the acrosome region of mouse spermatozoa [21]. ACTB is a major component of the cytoskeleton and is involved in many crucial cellular processes [23]. Differential expression of the ACTB protein has been linked to male fertility outcomes in both human and porcine spermatozoa [17, 24, 25]. PHB is a sperm mitochondrial protein that modulates mitochondrial structure and functions as a molecular chaperone [26]. A recent study demonstrated that PHB interacts with protein kinase B in the mitochondrial sheath of murine spermatozoa and controls motility by activating the phosphoinositide 3-kinase/serine-threonine kinase (PI3K/AKT) signalling pathway [27]. HSPD1 is a major target for capacitation-associated tyrosine phosphorylation, which exposes the zona pellucida receptor to the cell surface of spermatozoa [28, 29].
Although AVPR2, ACTB, PHB, and HSPD1 are known to be linked to male fertility, the effects of their corresponding mRNA levels on fertility are not well understood. Considering the crucial role of proteins in male fertility, we hypothesised that the mRNAs encoding these proteins may play a key role in pre- and post-fertilisation processes. To test this hypothesis, we used boar sperm samples. Pigs offer unique advantages over other well-established species for developmental biology studies because pigs share major characteristics with humans, despite being a polytocous (i.e., multiparous) species. Thus, this polytocous trait provides more comprehensive and convincing information on male fertility after AI in a large number of sows. To investigate the physiological role of sperm mRNA, we focused on elucidating the direct relationship between the mRNA expression of selected genes in pre-fertilisation parameters (sperm motility, motion kinematics, and capacitation status) and in vivo fertility.
Methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Chung-Ang University (Approval No. 2017–00018) and performed in accordance with the corresponding guidelines. All methods were performed according to relevant guidelines and regulations.
Experimental design
All semen samples were obtained and processed as described below. Semen samples (n = 27) were acquired from a farm (Sunjin Co., Danyang, Korea).
First, to compare the parameters in representative groups, samples from high- (average litter size 13.15 ± 0.39, n = 3) and low- (average litter size 11.50 ± 0.10, n = 3) litter sizes were selected based on the average litter size (total piglets/total breeding). Pre-fertilisation parameters (motility, motion kinematics, and capacitation status) and mRNA expressions of AVPR2, ACTB, PHB, and HSPD1 were assessed in the high- and low-litter size groups.
Subsequently, randomly selected boar sperm samples (n = 21) were examined to elucidate the correlation between mRNA expression levels and pre-fertilisation parameters (sperm motility, motion kinematics, and capacitation status) and in vivo fertility.
Boar sperm preparation
Upon collection, the sperm samples were immediately transferred to a constant temperature container (17 °C) and stored until the downstream processing steps [30]. All semen samples were centrifuged at 500×g for 20 min with a discontinuous 70% (v/v) and 35% (v/v) Percoll gradient (Sigma-Aldrich, St Louis, MO, USA) to remove seminal plasma as well as immotile and dead spermatozoa [31]. The isolated live spermatozoa were then incubated at 37 °C in 5% CO2 modified tissue culture medium 199 (mTCM 199; 0.91 mmol/L sodium pyruvate, 3.05 mmol/L d-glucose, 2.92 mmol/L calcium lactate, and 2.2 g/L sodium bicarbonate; Sigma-Aldrich) for 30 min [30, 32].
Computer-assisted sperm analysis (CASA)
Boar sperm motility (%) and motion kinematics were analysed using a CASA system (SAIS-PLUS VERSION 10.1; Medical Supply, Seoul, Korea) [33]. After incubation, 10 μL of the sperm sample was placed in a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel), which was then placed on a preheated heat block at 37 °C [32, 34, 35]. Sperm motility, hyperactivated motility (HYP), curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), mean amplitude of lateral head displacement (ALH), beat cross frequency (BCF), linearity (LIN), and wobble (WOB) were determined [32]. Each sample was observed using phase-contrast microscopy with a 10× objective lens [36].
Combined Hoechst 33258/chlortetracycline fluorescence assessment of capacitation status
The capacitation status of boar spermatozoa was examined with a dual staining method using a combined Hoechst 33258/chlortetracycline fluorescence staining process [30, 37]. The samples were first incubated in mTCM 199 and centrifuged at 400×g for 10 min at room temperature. The supernatant was discarded, and then 135 μL of phosphate-buffered saline (PBS) and 15 μL of H33258 solution were added. The samples were gently mixed and incubated for 10 min at room temperature. Excess dye was inactivated with 250 μL of 2% (w/v) polyvinylpyrrolidone (Sigma-Aldrich) in PBS. After centrifugation at 400×g for 10 min, the supernatant was discarded, and then the pellet was resuspended in 600 μL of PBS and 600 μL of chlortetracycline (CTC) fluorescence solution (750 mmol/L CTC in 5 μL buffer; 20 mmol/L Tris, 130 mmol/L sodium chloride (NaCl), and 5 mmol/L cysteine, pH 7.4; Sigma-Aldrich) [38]. The stained samples were counted using a Microphot-FXA microscope (Nikon, Tokyo, Japan) under epifluorescence illumination using ultraviolet BP 340–380/LP 425 and BP 450–490/LP 515 excitation/emission filters for H33258 and CTC, respectively. Capacitation status was quantified on approximately 400 spermatozoa per slide for each sample. Capacitation status was further classified into four categories: live non-capacitated (F; green fluorescence distributed evenly throughout the sperm head), live capacitated (B; green fluorescence over the acrosome region and a dark post-acrosome region), acrosome-reacted (AR; showing no fluorescence over the head), and dead (D; nuclei with blue fluorescence within the sperm head) [37].
RNA extraction, cDNA synthesis, and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA extraction, cDNA synthesis, and RT-qPCR were conducted as described previously [39]. Briefly, all samples were washed with PBS, centrifuged at 10,000×g for 10 min, and stored at − 80 °C prior to RNA extraction. Each sperm sample was counted, and then sperm concentrations were adjusted to 50 × 106 cells/mL with fresh PBS. The samples were then centrifuged at 13,000×g for 10 min at 4 °C, and the supernatant was then removed. Sperm pellets were lysed using a lysis buffer (PureLink™ RNA Mini Kit; Invitrogen, Carlsbad, CA, USA) containing 40 μL/mL β-mercaptoethanol (Sigma-Aldrich) and then homogenised with 20 G needles. After mixing the homogenised mixture for 2 min, 500 μL of TRIzol reagent (Invitrogen) was added to the sperm sample. The sample was then kept at room temperature for 5 min, and then 200 μL of chloroform (Sigma-Aldrich) was added, and the samples were mixed vigorously by hand for 20 s. The samples were incubated at room temperature for another 5 min and then centrifuged at 12,000×g for 25 min at 4 °C. Next, 500 μL of the upper phase (which contained RNA) was carefully transferred to a fresh 1- mL tube, and an equal amount of 100% pure ethanol was then added. The mixture was then mixed by pipetting and processed according to the manufacturer’s instructions. RNA was eluted in 20 μL nuclease-free water. RNA concentrations and 260/280 ratios were measured using an Epoch microplate spectrophotometer (BioTek, Winooski, VT, USA). cDNA synthesis was performed using the PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio, Inc., Shiga, Japan) following the manufacturer’s instructions. RT-qPCR was performed using the AVPR2-, ACTB-, PHB-, HSPD1-, glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-, and peptidylprolyl isomerase A (PPIA)-specific primers, which were designed for Sus scrofa (Additional file 1: Table S1). GAPDH and PPIA were used as reference genes [40], and RT-qPCR data were analysed using the delta-delta Cq method [41].
The standardised annealing temperature of the designed primers was 60 °C. A standard curve analysis was performed for each gene to determine the PCR efficiency. Melt curve analysis was conducted to examine the single amplification, and the size of the PCR products was determined using gel electrophoresis [39].
Western blotting
Protein quantification of HSPD1 was performed by Western blotting [21]. Briefly, 100 × 106 sperm cells were lysed with a buffer containing 5% 2-mercaptoethanol (Sigma-Aldrich). The lysate was centrifuged at 10,000×g for 10 min at room temperature. The supernatant was boiled and stored for electrophoresis. Proteins were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were transferred onto polyvinylidene fluoride membranes (Amersham, Piscataway, NJ, USA). For protein detection, anti-HSPD1 antibody (Abcam, Cambridge, UK) was used at a 1:20,000 ratio. Horseradish peroxidase (HRP)-conjugated rabbit IgG (1:3000; Cell Signaling Technology, Danvers, MA, USA) was used to detect HSPD1 level. Protein α-tubulin was quantified as an internal control with anti-α-tubulin mouse antibody (1:10,000; Abcam) and HRP-conjugated mouse IgG (1:3000; Cell Signaling Technology). The HRP signal was visualised on an X-ray film using chemiluminescence. The X-ray film was scanned using a GS-800-calibrated imaging densitometer (Bio-Rad, Hercules, CA, USA) and analysed using Quantity One software (Bio-Rad). The ratio of HSPD1/α-tubulin levels was calculated as the relative expression level of each sample.
Measurement of male fertility
AI was conducted to measure the male fertility outcomes. Environmental conditions were maintained at 20 ± 5 °C with ventilation and a 16 h light and 8 h dark photoperiod. The average number of AI per boar was 22.95 ± 1.24 (18–38). Semen samples from boars were diluted with Beltsville thawing solution (100 mL per 30 × 106 sperm cells) and stored at 17 °C until required for insemination.
Quality assessment of genes as the indicators of male fertility
Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were evaluated using screening tests [37, 42, 43]. Sensitivity was defined as the ratio of boars showing true-positive results (i.e., the percentage of boars for which we could accurately identify the litter sizes). In contrast, specificity was defined as the percentage of boars that exhibited true-negative results. PPV and NPV were defined as the rate of boars exhibiting positive or negative results, respectively, when the litter size was ≥ 12.68 or < 12.68 (average litter size of samples).
Statistical analysis
All data were analysed using SPSS v1.8 (SPSS Inc., Chicago, IL, USA), and all parameters were confirmed for normality using the Shapiro–Wilk test. All comparisons between two groups were analysed using Student’s two-tailed t-tests and the homogeneity of variance test (Levene’s test). Correlations were identified using Pearson’s correlation coefficients for the groups that exhibited a normal distribution (P ≥ 0.05). In groups that failed the normality tests (P < 0.05), the Spearman correlation coefficient was used [44]. The prognostic power of fertility parameters as a function of HSPD1 mRNA expression was evaluated using the receiver operating characteristic (ROC) curve, and optimal cut-off values were generated based on the highest sensitivity and specificity values determined from ROC analysis [45, 46]. The accuracy of HSPD1 in assessing male fertility outcomes was determined based on sensitivity and specificity. All numerical data are reported as the mean ± standard error of the mean (SEM), and P < 0.05 were considered statistically significant.
Results
Pre-fertilisation parameters (motility, motion kinematics, and capacitation status) and gene expression in the high- and low-litter size boar groups
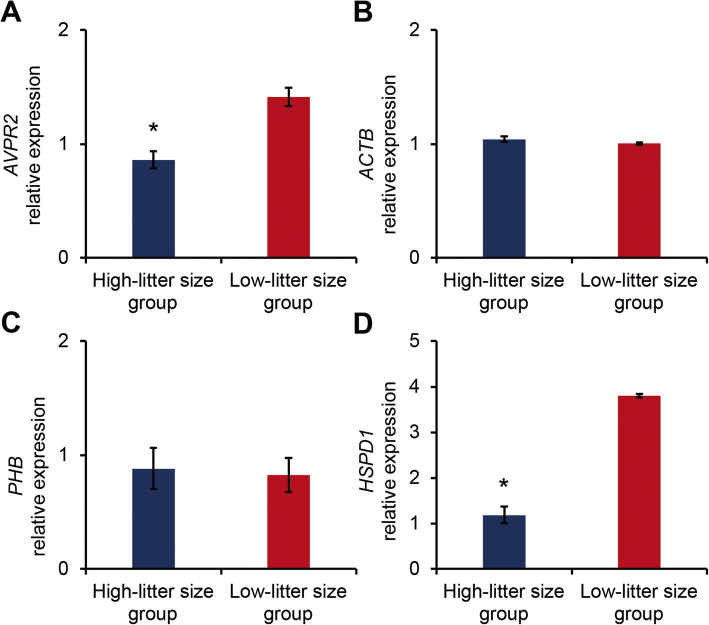
CASA and H33285/CTC dual staining were conducted to assess sperm motility, motion kinematics, and capacitation status as the pre-fertilisation potential of boar spermatozoa. As summarised in Table 1, no significant differences in sperm motility, motion kinematics, and capacitation status were observed between the high- and low-litter size boar groups (P < 0.05). The PCR efficiency of the designed primers ranged from 89% to 107% (Additional file 1: Fig. S1). PCR amplicon size after reaction with the designed primers matched the predicted size, and all melt curve analyses showed a single peak (Additional file 1: Fig. S2). Among the selected genes, AVPR2 and HSPD1 mRNA expression levels were significantly different between sperm samples (Fig. 1 and Additional file 1: Fig. S3). Specifically, AVPR2 and HSPD1 mRNA expression levels were higher in the low-litter size boar group (P < 0.05; Fig. 1A and D and Additional file 1: Fig. S3A and D).
Table 1.
Male fertility parameters of high- and low-litter size spermatozoa
| Parameter | High-litter size group | Low-litter size group | |
|---|---|---|---|
| Litter size | 13.15 ± 0.39* | 11.50 ± 0.10 | |
| Motility and motion kinematics | MOT, % | 84.13 ± 2.21 | 90.7 ± 0.68 |
| HYP, % | 13.28 ± 0.55 | 15.66 ± 2.15 | |
| VCL, μm/s | 141.05 ± 10.69 | 149.68 ± 4.73 | |
| VSL, μm/s | 66.29 ± 17.43 | 71.02 ± 1.01 | |
| VAP, μm/s | 76.33 ± 13.51 | 83.23 ± 1.39 | |
| LIN, % | 45.73 ± 8.27 | 47.49 ± 0.91 | |
| BCF, Hz | 11.93 ± 0.44 | 11.49 ± 0.27 | |
| WOB, % | 53.34 ± 5.18 | 55.67 ± 0.27 | |
| ALH, μm/s | 6.37 ± 0.62 | 6.64 ± 0.13 | |
| Capacitation status | AR, % | 0.22 ± 0.22 | 1.28 ± 0.51 |
| F, % | 91.48 ± 1.02 | 91.51 ± 3.4 | |
| B, % | 8.28 ± 1.07 | 7.2 ± 3.39 | |
MOT motility, HYP hyperactivated motility, VCL curvilinear velocity, VSL straight-line velocity, VAP average path velocity, BCF beat cross frequency, LIN linearity, WOB wobble, ALH amplitude of lateral head displacement. AR, acrosome-reacted spermatozoa; F, non-capacitated spermatozoa; B, capacitated spermatozoa; *P < 0.05
Fig. 1.
Beta-actin (ACTB), prohibitin (PHB), heat shock protein family D member 1 (HSPD1), and arginine vasopressin receptor 2 (AVPR2) mRNA expression in high- and low-litter size boar spermatozoa. Differences in marker candidate gene expression in the high-litter size (n = 3) and low-litter size (n = 3) spermatozoa groups based on the average litter sizes. A AVPR2 mRNA expression in boar spermatozoa with high- and low-litter sizes. B ACTB mRNA expression in boar spermatozoa with high- and low-litter sizes. C PHB mRNA expression in boar spermatozoa with high- and low-litter sizes. D HSPD1 mRNA expression in boar spermatozoa with high- and low-litter sizes. Relative expression was normalised to GAPDH expression. The data are expressed as the mean ± standard error of the mean (SEM); *P < 0.05
Correlation analysis between gene expression and pre-fertilisation parameters and litter size
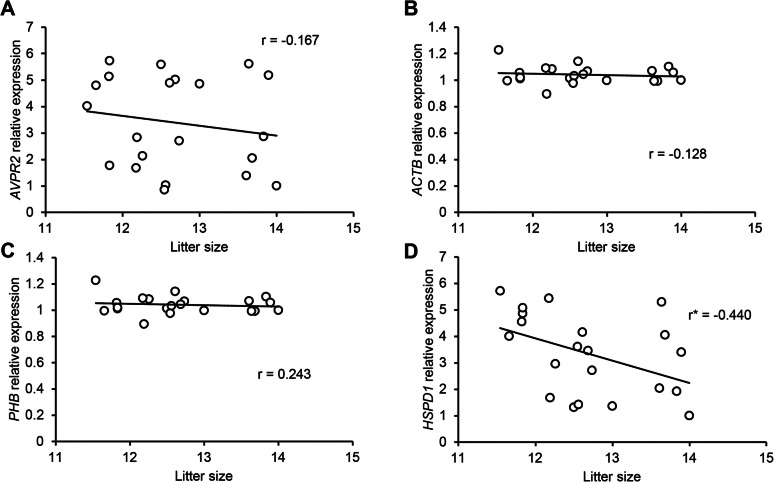
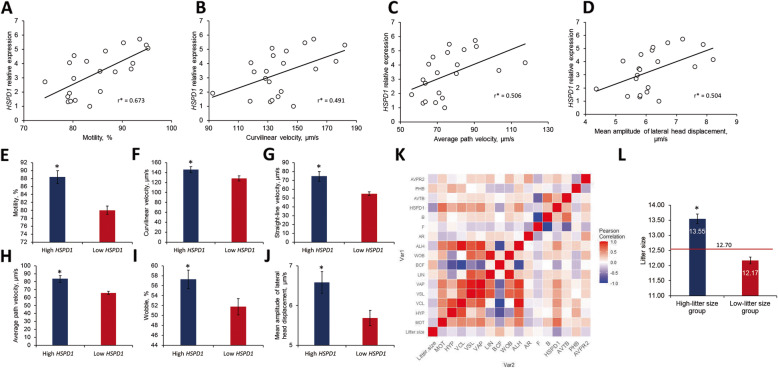
The results for the normality test of every parameter (pre-fertilisation parameters, litter size, and gene expression) from 21 boars are provided in Additional file 1: Table S2. A correlation analysis was conducted to identify the interactions between all parameters in 21 randomly selected boars (Figs. 2 and 4A–D and K). The mRNA expression of HSPD1 was significantly correlated with litter size (R = − 0.440; Fig. 2D and Additional file 1: Table S3). Moreover, HSPD1 expression was positively correlated with sperm motility (%) and several motion kinematics, including VCL (μm/s), VAP (μm/s), and ALH (μm/s) (Fig. 4A–D and Additional file 1: Table S3).
Fig. 2.
Correlation analysis between gene expression and pre-fertilisation parameters. A Linear regression of AVPR2 mRNA expression and litter size. B Linear regression of ACTB mRNA expression and litter size. C Linear regression of PHB mRNA expression and litter size. D Linear regression of HSPD1 mRNA expression and litter size. r, Pearson correlation coefficient; *P < 0.05, calculated via the linear regression test
Fig. 4.
Fertility parameters linked to gene expression dysregulation. A Linear regression of HSPD1 expression and motility (%). B Linear regression of HSPD1 expression and curvilinear velocity (μm/s). C Linear regression of HSPD1 expression and average path velocity (μm/s). D Linear regression of HSPD1 expression and mean amplitude of lateral head displacement (μm/s). E Difference of motility in high- and low-HSPD1 expression groups. F Difference of curvilinear velocity (μm/s) in high- and low-HSPD1 expression groups. G Difference of straight-line velocity (μm/s) in high- and low-HSPD1 expression groups. H Difference of average path velocity (μm/s) in high- and low-HSPD1 expression groups. I Difference of wobble (%) in high- and low-HSPD1 expression groups. J Difference of mean amplitude of lateral head displacement (μm/s) in high- and low-HSPD1 expression groups. The average values of each fertility parameter were compared based on the cut-off values of HSPD1 expression (3.1798) from the ROC curves. K Correlation heatmap of all parameters. L Average litter size of high- and low-litter size groups separated by HSPD1 mRNA expression. The data are expressed as the mean ± SEM; *P < 0.05
Quality of genes as the indicators of male fertility
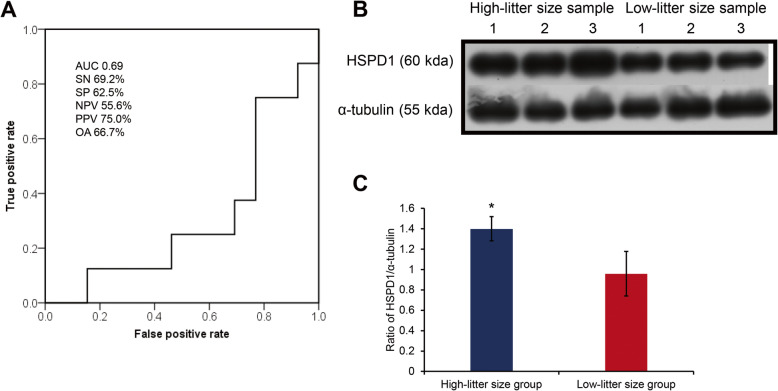
HSPD1 was characterised for quality assurance. The sensitivity, specificity, NPV, PPV, and overall litter size prediction accuracy of HSPD1 were 69.2%, 62.5%, 55.6%, 75.0%, and 66.7%, respectively (Fig. 3A). The area under the curve (AUC) for the HSPD1 ROC curve was 0.69 (Fig. 3A). The samples were divided into high- and low-HSPD1 expression groups based on the cut-off value of the ROC curve. In the HSPD1 high-expression group, the motility (%), VCL (μm/s), VSL (μm/s), VAP (μm/s), WOB (%), and ALH (μm/s) were significantly different (P < 0.05) from the those of the HSPD1 low-expression group (Fig. 4E–J and Additional file 1: Table S4). According to the cut-off value of HSPD1 expression, the litter size difference between the high- and low-litter size groups was 1.34 (P < 0.05; Fig. 4L). HSPD1 protein was highly expressed in the low-litter size group (P < 0.05; Fig. 3B and C).
Fig. 3.
Quality assessment of HSPD1 mRNA marker and protein expression. A Receiver operating characteristic (ROC) curve of HSPD1 mRNA expression versus litter size. All the predictive parameters were calculated based on the average litter size (12.68) of samples. AUC, Area under the curve. Sensitivity (SN) is the percentage of boars showing true-positive results when tested with mRNA expression. Specificity (SP) is the percentage of boars showing true-negative results. The positive predictive value (PPV) is the percentage of boars that tested positive and also exhibited a true-positive litter size. The negative predictive value (NPV) is the percentage of boars that tested negative or simultaneously had a true-negative litter size. OA, Overall accuracy. B Western blotting image of HSPD1 and α-tubulin proteins. C Relative expression of HSPD1 in high- and low-litter size groups. The data are expressed as the mean ± SEM; *P < 0.05
Discussion
A substantial proportion of the livestock industry relies on AI-based breeding, and the fertilisation success rate can be affected by sperm quality. Therefore, sperm quality assessment is critical for predicting male fertility. To accurately investigate male fertility markers to increase field fertility, a porcine model was selected for the current study. The importance of the porcine model for biomedical research has been widely acknowledged [47]. Pigs are also uniquely well-suited for developmental studies because of the similarity between porcine and human genomes and the availability of numerically well-organised AI fertility data [48, 49]. The AI of pigs has rendered a plethora of valuable information on male fertility. Therefore, the porcine model provides unique advantages over other species in sperm RNA functional research on male fertility. The litter size of pigs is the endpoint of a successful developmental process. The present study analysed sperm function and in vivo fertility data to reveal whether the selected genes have practical importance and attempted to develop precise markers to increase field fertility.
High throughput “omics” technologies have provided growing evidence indicating that SIFs, particularly protein and RNA, are involved in the fertilisation process. Numerous proteomic technologies have aided in elucidating the vital role of sperm proteins in developmental biology, and several functional biomarkers have been validated for use in the biomedical field. However, owing to the seminal nature of sperm RNA research, the function of sperm RNA remains a subject of debate. Furthermore, despite the existence of compelling sperm transcriptome studies [50–54], identifying functional biomarkers that can be used in the biomedical field remains a challenge. Functional studies of sperm RNA have mainly focused on small RNAs, including tRNA-derived small RNAs and small interfering RNAs [55–57]. Nonetheless, the function of sperm mRNA in the fertilisation process remains controversial. Pang et al. [39] recently optimised an RT-qPCR-based method to characterise mRNA expression in boar spermatozoa and identified several functional mRNA biomarkers. Interestingly, mRNA markers exhibited a 60–95% overall accuracy in predicting male fertility and significantly increased the litter size by a maximum of 1.61 piglets per insemination in field trials [18, 39, 58]. However, examining only a few functional biomarkers cannot explain the entire fertilisation process. Therefore, it would be desirable to use additional markers to predict male fertility.
Our selected genes were classified using the Gene Ontology database as integral membrane components. Among the genes in this category, AVPR2, ACTB, PHB, and HSPD1 are considered mRNA markers of male fertility in pigs based on relevant literature. To comprehensively understand the effect of the differential mRNA expression of the selected genes on male fertility, several parameters (sperm motility, motion kinematics, capacitation, and litter size) were analysed against the mRNA expression of the aforementioned genes.
Sperm motility and motion kinematics are important characteristics that can enable sperm to travel through the female reproductive tract to fertilise the oocyte. Additionally, sperm cells must undergo essential maturation changes, such as capacitation and acrosome reaction, to fertilise the oocytes [59]. Therefore, we used sperm motility, motion kinematics, and capacitation status as the pre-fertilisation features of spermatozoa. Although numerous studies have addressed the relationship between conventional semen analyses and male fertility, the exact relationship between these parameters remains not fully understood [32, 60, 61]. In the present study, neither of these pre-fertilisation parameters exhibited a clear correlation with litter size. This was consistent with a previous study [44], which reported that sperm motility, motion kinematics, and capacitation status are better suited for the acquisition of preliminary and quantitative sperm fertility data than of qualitative information.
Transcriptomic biomarkers can explain many biological processes through high-throughput data and are more cost-effective than other biomarkers [62]. Although spermatozoa contain a small quantity of RNA, functional RNAs synthesised during spermatogenesis are transferred to oocytes during fertilisation [63]. The present study suggests that HSPD1 mRNA plays a functional role in male fertility and could be a biomarker for successful fertilisation.
HSPD1 plays important roles in various biological functions associated with fertilisation. In mouse spermatozoa, tyrosine phosphorylation of the HSPD1 protein was observed, and this protein is involved in receptor-mediated interactions with female gametes [29]. In the present study, the protein level of HSPD1 was checked after the evaluation of HSPD1 as a sperm mRNA marker. HSPD1 protein was highly expressed in the high-litter size group. High expression of HSPD1 might facilitate receptor-mediated interactions between spermatozoa and oocytes and consequently increase litter size. In the present study, the expression patterns of HSPD1 protein and mRNA were inversely related. On the genomic scale, protein abundance and mRNA expression levels show poor correlation [64]. Moreover, in spermatozoa, neither mRNA functions nor mRNA-protein interactions are known. Evaluation from an evolutionary perspective can help us understand how HSPD1 mRNA expression is associated with fertilisation [65]. During male gametogenesis in Arabidopsis, short suspensor transcripts are generated and transferred to female gametes for zygotic translation. As such, HSPD1 mRNA might have a separate function in addition to its protein translation, but further research is required.
HSPD1 plays an important role in embryonic development [66]. In the present study, despite the significant correlation between HSPD1 mRNA expression and sperm motility, motility parameters were not correlated to litter size. This result suggests that HSPD1 mRNA affects male fertility, irrespective of sperm function. Moreover, the current study suggests that there are sperm variables that impact fertilisation ability or embryo development that are not detectable using standard sperm evaluation methods. Our study demonstrates the relationship between gene expression and the endpoint of the fertilisation process, suggesting that HSPD1 mRNA expression might be transferred to the oocytes and modulate the entire developmental process, including implantation, foetal development, and successful birth.
Conclusions
Among the several known sperm membrane protein genes, HSPD1 mRNA levels were found to be crucial indicators of male fertility. Most importantly, these transcriptomic markers were more closely related to male fertility parameters, especially the litter size of inseminated sows, with HSPD1 being particularly correlated with sperm motility and motion kinematics. Our findings suggest that HSPD1 plays a crucial role in fertilisation and developmental processes beyond fertilisation. Therefore, HSPD1 mRNA could be used in sperm evaluation to predict male fertility before AI which would lead to further improvements in field fertility.
Supplementary Information
Additional file 1: Table S1. Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) primers. Table S2. The results for the normality test of every parameters (pre-fertilization parameters, litter size, and gene expression) from 21 boars. Table S3. Correlation between pre-fertilisation parameters (sperm motility, motion kinematics, and capacitation status) and gene expression. Table S4. Comparison between all parameters in the heat shock protein family D member 1 (HSPD1) high and low expression groups. Fig. S1. The standard curve and qPCR efficiency of studied genes. Fig. S2. The melt curve and gel electrophoresis image of qPCR products. Fig. S3. ACTB, PHB, HSPD1, and AVPR2 mRNA expression in high- and low-litter size boar spermatozoa.
Acknowledgements
Not applicable.
Abbreviations
- HSPD1
Heat shock protein family D member 1
- SIFs
Sperm intrinsic factors
- AVPR2
Arginine vasopressin receptor 2
- ACTB
Beta-actin
- PHB
Prohibitin
- PI3K/AKT
Phosphoinositide 3-kinase/serine-threonine kinase
- ROC
Receiver operating characteristic
- CASA
Computer-assisted sperm analysis
- HYP
Hyperactivated motility
- VCL
Curvilinear velocity
- VSL
Straight-line velocity
- VAP
Average path velocity
- ALH
Mean amplitude of lateral head displacement
- BCF
Beat cross frequency
- LIN
Linearity
- WOB
Wobble
- PBS
Phosphate-buffered saline
- CTC
Chlortetracycline
- NaCl
Sodium chloride
- RT-qPCR
Quantitative reverse transcription-polymerase chain reaction
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- HRP
Horseradish peroxidase
- SEM
Standard error of the mean
- NPV
Negative predictive value
- PPV
Positive predictive value
- AUC
Area under the curve
Authors’ contributions
This study was conceived and designed by WKP and MGP. WKP, JHS, DYR, and YJP performed the experiments. The data were analysed by JHS and WKP. The manuscript was mainly written by WKP and MGP with the assistance of JHS, DYR, MSR, and YJP. All authors read and approved the final manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2018R1A6A1A03025159).
Availability of data and materials
The datasets from the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Experimental procedures involving animals were performed in accordance with the Institutional Animal Care and Use Committee of Chung-Ang University guidelines and were approved by the corresponding committee (Approval No. 2017–00018).
Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vishwanath R. Artificial insemination: the state of the art. Theriogenology. 2003;59(2):571–584. doi: 10.1016/S0093-691X(02)01241-4. [DOI] [PubMed] [Google Scholar]
- 2.Kwon WS, Rahman MS, Lee JS, Yoon SJ, Park YJ, Pang MG. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol Cell Proteomics. 2015;14(5):1230–1240. doi: 10.1074/mcp.M114.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Blerkom J, Davis P, Merriam J, Sinclair J. Nuclear and cytoplasmic dynamics of sperm penetration, pronuclear formation and microtubule organization during fertilization and early preimplantation development in the human. Hum Reprod Update. 1995;1(5):429–461. doi: 10.1093/humupd/1.5.429. [DOI] [PubMed] [Google Scholar]
- 4.Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int Rev Cytol. 2000;195:1–65. doi: 10.1016/s0074-7696(08)62703-5. [DOI] [PubMed] [Google Scholar]
- 5.Sutovsky P. Review: sperm-oocyte interactions and their implications for bull fertility, with emphasis on the ubiquitin-proteasome system. Animal. 2018;12(s1):s121–s132. doi: 10.1017/S1751731118000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56(5):334–348. doi: 10.3109/19396368.2010.512377. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146(1):R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 8.Castillo J, Jodar M, Oliva R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum Reprod Update. 2018;24(5):535–555. doi: 10.1093/humupd/dmy017. [DOI] [PubMed] [Google Scholar]
- 9.Yuan S, Schuster A, Tang C, Yu T, Ortogero N, Bao J, Zheng H, Yan W. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development. 2016;143(4):635–647. doi: 10.1242/dev.131755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boerke A, Dieleman SJ, Gadella BM. A possible role for sperm RNA in early embryo development. Theriogenology. 2007;68(Suppl 1):S147–S155. doi: 10.1016/j.theriogenology.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Godia M, Swanson G, Krawetz SA. A history of why fathers' RNA matters. Biol Reprod. 2018;99(1):147–159. doi: 10.1093/biolre/ioy007. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Rodriguez M, Martinez C, Wright D, Barranco I, Roca J, Rodriguez-Martinez H. The transcriptome of pig spermatozoa, and its role in fertility. Int J Mol Sci. 2020;21(5):1572. 10.3390/ijms21051572 . [DOI] [PMC free article] [PubMed]
- 13.Li Y, Li RH, Ran MX, Zhang Y, Liang K, Ren YN, He WC, Zhang M, Zhou GB, Qazi IH, Zeng CJ. High throughput small RNA and transcriptome sequencing reveal capacitation-related microRNAs and mRNA in boar sperm. BMC Genomics. 2018;19(1):736. doi: 10.1186/s12864-018-5132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai DH, Qazi IH, Ran MX, Liang K, Zhang Y, Zhang M, et al. Exploration of miRNA and mRNA profiles in fresh and frozen-thawed boar sperm by transcriptome and small RNA sequencing. Int J Mol Sci. 2019;20(4):802. 10.3390/ijms20040802. [DOI] [PMC free article] [PubMed]
- 15.Fraser L, Brym P, Pareek CS, Mogielnicka-Brzozowska M, Paukszto L, Jastrzebski JP, et al. Transcriptome analysis of boar spermatozoa with different freezability using RNA-Seq. Theriogenology. 2020;142:400–413. doi: 10.1016/j.theriogenology.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Godia M, Estill M, Castello A, Balasch S, Rodriguez-Gil JE, Krawetz SA, et al. A RNA-Seq analysis to describe the boar sperm transcriptome and its seasonal changes. Front Genet. 2019;10:299. doi: 10.3389/fgene.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon WS, Oh SA, Kim YJ, Rahman MS, Park YJ, Pang MG. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci Rep. 2015;5(1):13821. doi: 10.1038/srep13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KU, Pang WK, Kang S, Ryu DY, Song WH, Rahman MS, Kwon WS, Pang MG. Sperm solute carrier family 9 regulator 1 is correlated with boar fertility. Theriogenology. 2019;126:254–260. doi: 10.1016/j.theriogenology.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA, Reproductive Medicine N The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013;19(6):604–624. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashrafzadeh A, Karsani SA, Nathan S. Mammalian sperm fertility related proteins. Int J Med Sci. 2013;10(12):1649–1657. doi: 10.7150/ijms.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon WS, Park YJ, Kim YH, You YA, Kim IC, Pang MG. Vasopressin effectively suppresses male fertility. PLoS One. 2013;8(1):e54192. doi: 10.1371/journal.pone.0054192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagedorn TM, Carlin RW, Schultz BD. Oxytocin and vasopressin stimulate anion secretion by human and porcine vas deferens epithelia. Biol Reprod. 2007;77(3):416–424. doi: 10.1095/biolreprod.106.056762. [DOI] [PubMed] [Google Scholar]
- 23.Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146(2):267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23(4):783–791. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 25.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31(6):1395–1401. doi: 10.1007/s00345-013-1023-5. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Z, Zhang H, Huang B, Liu Q, Wang Y, Shi D, Li X. Expression pattern of prohibitin, capping actin protein of muscle Z-line beta subunit and tektin-2 gene in Murrah buffalo sperm and its relationship with sperm motility. Asian-Australas J Anim Sci. 2018;31(11):1729–1737. doi: 10.5713/ajas.18.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XH, Chai RR, Chen GW, Zhang LF, Tan-Tai WJ, Shi HJ, et al. Prohibitin (PHB) interacts with AKT in mitochondria to coordinately modulate sperm motility. Asian J Androl. 2020;22(6):583-9. 10.4103/aja.aja_46_20. [DOI] [PMC free article] [PubMed]
- 28.Ecroyd H, Jones RC, Aitken RJ. Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation. Biol Reprod. 2003;69(6):1801–1807. doi: 10.1095/biolreprod.103.017350. [DOI] [PubMed] [Google Scholar]
- 29.Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117(Pt 16):3645–3657. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- 30.Kwon WS, Rahman MS, Lee JS, You YA, Pang MG. Improving litter size by boar spermatozoa: application of combined H33258/CTC staining in field trial with artificial insemination. Andrology. 2015;3(3):552–557. doi: 10.1111/andr.12020. [DOI] [PubMed] [Google Scholar]
- 31.Flesch FM, Colenbrander B, van Golde LM, Gadella BM. Capacitation induces tyrosine phosphorylation of proteins in the boar sperm plasma membrane. Biochem Biophys Res Commun. 1999;262(3):787–792. doi: 10.1006/bbrc.1999.1300. [DOI] [PubMed] [Google Scholar]
- 32.Oh SA, Park YJ, You YA, Mohamed EA, Pang MG. Capacitation status of stored boar spermatozoa is related to litter size of sows. Anim Reprod Sci. 2010;121(1–2):131–138. doi: 10.1016/j.anireprosci.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Paston MJ, Sarkar S, Oates RP, Badawy SZ. Computer-aided semen analysis variables as predictors of male fertility potential. Arch Androl. 1994;33(2):93–99. doi: 10.3109/01485019408987809. [DOI] [PubMed] [Google Scholar]
- 34.Sukcharoen N, Ngeamjirawat J, Chanprasit Y, Aribarg A. A comparison of Makler counting chamber and improved Neubauer hemocytometer in sperm concentration measurement. J Med Assoc Thail. 1994;77(9):471–476. [PubMed] [Google Scholar]
- 35.Yoon SJ, Kwon WS, Rahman MS, Lee JS, Pang MG. A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS One. 2015;10(5):e0126232. doi: 10.1371/journal.pone.0126232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrell PB, Presicce GA, Brockett CC, Foote RH. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology. 1998;49(4):871–879. doi: 10.1016/S0093-691X(98)00036-3. [DOI] [PubMed] [Google Scholar]
- 37.Kwon WS, Shin DH, Ryu DY, Khatun A, Rahman MS, Pang MG. Applications of capacitation status for litter size enhancement in various pig breeds. Asian-Australas J Anim Sci. 2018;31(6):842–850. doi: 10.5713/ajas.17.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon WS, Park YJ, Mohamed el SA, Pang MG. Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril. 2013;99(2):354–361. doi: 10.1016/j.fertnstert.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Pang WK, Kang S, Ryu DY, Rahman MS, Park YJ, Pang MG. Optimization of sperm RNA processing for developmental research. Sci Rep. 2020;10(1):11606. doi: 10.1038/s41598-020-68486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng C, He L, Peng W, Ding L, Tang K, Fang D, Zhang Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology. 2014;68(1):113–121. doi: 10.1016/j.cryobiol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79(1):16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 43.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev. 2008;29(Suppl 1):S83–S87. [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon WS, Rahman MS, Ryu DY, Khatun A, Pang MG. Comparison of markers predicting litter size in different pig breeds. Andrology. 2017;5(3):568–577. doi: 10.1111/andr.12332. [DOI] [PubMed] [Google Scholar]
- 45.Kwon WS, Rahman MS, Ryu DY, Park YJ, Pang MG. Increased male fertility using fertility-related biomarkers. Sci Rep. 2015;5(1):15654. doi: 10.1038/srep15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 47.Tumbleson ME, Schook LB. Advances in swine in biomedical research. Vols 1 and 2. New York: Plenum Press; 1996:1–4.
- 48.Broekhuijse ML, Feitsma H, Gadella BM. Artificial insemination in pigs: predicting male fertility. Vet Q. 2012;32(3–4):151–157. doi: 10.1080/01652176.2012.735126. [DOI] [PubMed] [Google Scholar]
- 49.Humphray SJ, Scott CE, Clark R, Marron B, Bender C, Camm N, Davis J, Jenks A, Noon A, Patel M, Sehra H, Yang F, Rogatcheva MB, Milan D, Chardon P, Rohrer G, Nonneman D, de Jong P, Meyers SN, Archibald A, Beever JE, Schook LB, Rogers J. A high utility integrated map of the pig genome. Genome Biol. 2007;8(7):R139. doi: 10.1186/gb-2007-8-7-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hua M, Liu W, Chen Y, Zhang F, Xu B, Liu S, Chen G, Shi H, Wu L. Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization. Cell Discov. 2019;5(1):20. doi: 10.1038/s41421-019-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrido N, Martinez-Conejero JA, Jauregui J, Horcajadas JA, Simon C, Remohi J, et al. Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril. 2009;91(4 Suppl):1307–1310. doi: 10.1016/j.fertnstert.2008.01.078. [DOI] [PubMed] [Google Scholar]
- 52.Bissonnette N, Levesque-Sergerie JP, Thibault C, Boissonneault G. Spermatozoal transcriptome profiling for bull sperm motility: a potential tool to evaluate semen quality. Reproduction. 2009;138(1):65–80. doi: 10.1530/REP-08-0503. [DOI] [PubMed] [Google Scholar]
- 53.Godia M, Castello A, Rocco M, Cabrera B, Rodriguez-Gil JE, Balasch S, et al. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility. Sci Rep. 2020;10(1):7985. doi: 10.1038/s41598-020-64711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lalancette C, Platts AE, Johnson GD, Emery BR, Carrell DT, Krawetz SA. Identification of human sperm transcripts as candidate markers of male fertility. J Mol Med (Berl) 2009;87(7):735–748. doi: 10.1007/s00109-009-0485-9. [DOI] [PubMed] [Google Scholar]
- 55.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, Qian J, Duan E, Zhai Q, Zhou Q. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351(6271):397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016;17(12):733–743. doi: 10.1038/nrg.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429(6988):154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- 58.Kang S, Pang WK, Ryu DY, Song WH, Rahman MS, Park YJ, Pang MG. Porcine seminal protein-I and II mRNA expression in boar spermatozoa is significantly correlated with fertility. Theriogenology. 2019;138:31–38. doi: 10.1016/j.theriogenology.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 59.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108(12):4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis SE. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134(1):31–40. doi: 10.1530/REP-07-0152. [DOI] [PubMed] [Google Scholar]
- 61.Khatun A, Rahman MS, Pang MG. Clinical assessment of the male fertility. Obstet Gynecol Sci. 2018;61(2):179–191. doi: 10.5468/ogs.2018.61.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lowe R, Shirley N, Bleackley M, Dolan S, Shafee T. Transcriptomics technologies. PLoS Comput Biol. 2017;13(5):e1005457. doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 2013;41(7):4104–4117. doi: 10.1093/nar/gkt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W. Paternal control of embryonic patterning in Arabidopsis thaliana. Science. 2009;323(5920):1485–1488. doi: 10.1126/science.1167784. [DOI] [PubMed] [Google Scholar]
- 66.Christensen JH, Nielsen MN, Hansen J, Fuchtbauer A, Fuchtbauer EM, West M, et al. Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones. 2010;15(6):851–863. doi: 10.1007/s12192-010-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) primers. Table S2. The results for the normality test of every parameters (pre-fertilization parameters, litter size, and gene expression) from 21 boars. Table S3. Correlation between pre-fertilisation parameters (sperm motility, motion kinematics, and capacitation status) and gene expression. Table S4. Comparison between all parameters in the heat shock protein family D member 1 (HSPD1) high and low expression groups. Fig. S1. The standard curve and qPCR efficiency of studied genes. Fig. S2. The melt curve and gel electrophoresis image of qPCR products. Fig. S3. ACTB, PHB, HSPD1, and AVPR2 mRNA expression in high- and low-litter size boar spermatozoa.
Data Availability Statement
The datasets from the current study are available from the corresponding author upon reasonable request.