Abstract
Objective:
We quantified transdermal fentanyl prescribing in elderly nursing home residents without prior opioid use or persistent pain, and the association of individual and facility traits with opioid-naïve prescribing.
Design:
Cross-sectional study.
Setting:
Linked Minimum Data Set (MDS) assessments; Online Survey, Certification and Reporting (OSCAR) records; and Medicare Part D claims.
Participants:
From a cross-section of all long-stay US nursing home residents in 2008 with an MDS assessment and Medicare Part D enrollment, we identified individuals (≥65 years old) who initiated transdermal fentanyl, excluding those with Alzheimer disease, severe cognitive impairment, cancer, or receipt of hospice care.
Measurements:
We used Medicare Part D to select beneficiaries initiating transdermal fentanyl in 2008 and determined whether they were “opioid-naïve,” defined as no opioid dispensing during the previous 60 days. We obtained resident and facility characteristics from MDS and OSCAR records and defined persistent pain as moderate-to-severe, daily pain on consecutive MDS assessments at least 90 days apart. We estimated associations of patient and facility attributes and opioid-naïve fentanyl initiation using multilevel mixed effects logistic regression modeling.
Results:
Among 17,052 residents initiating transdermal fentanyl, 6190 (36.3%) were opioid-naïve and 15,659 (91.8%) did not have persistent pain. In the regression analysis with adjustments, residents who were older (ages ≥95 odds ratio [OR] 1.69, 95% confidence interval [CI] 1.46–1.95) or more cognitively impaired (moderate-to-severe cognitive impairment, OR 1.99, 95% CI 1.73–2.29) were more likely to initiate transdermal fentanyl without prior opioid use.
Conclusion:
Most nursing home residents initiating transdermal fentanyl did not have persistent pain and many were opioid-naïve. Changes in prescribing practices may be necessary to ensure Food and Drug Administration warnings are followed, particularly for vulnerable subgroups, such as the cognitively impaired.
Keywords: Nursing homes, fentanyl, prescription opioids, Food and Drug Administration
The US Food and Drug Administration (FDA) has focused important regulatory efforts on inappropriate prescribing.1 Prescription opioids have been of particular concern due to their associated morbidity and mortality.2 Although much of the focus regarding opioids has centered on misuse and abuse by young adults,3 the elderly, including nursing home residents, represent a vulnerable population, given the high prevalence of pain4-6 as well as their advanced age, comorbidities and frailty, and frequent polypharmacy.7-9 Nearly 3 million elderly Americans receive nursing home care each year,10 including many who receive opioids.11,12
Transdermal fentanyl, a long-acting opioid commonly prescribed for nursing home residents, is dangerous if inappropriately used.13 The FDA initially approved transdermal fentanyl in 1990 for patients with moderate-to-severe, continuous pain who have been receiving opioid therapy14; transdermal fentanyl is contraindicated in patients without moderate-to-severe, continuous pain or who are not opioid-tolerant.15,16 Because of reports of fatalities and life-threatening adverse events associated with the use of transdermal fentanyl among this contraindicated population, the FDA issued “Boxed Warnings” on the drug label and targeted risk communications in 2005 and 2007 to the general public and health care providers.17-20
One study assessed opioid-naïve prescribing for long-acting opioids, including transdermal fentanyl, in nursing home residents, but the study population was limited to Rhode Island during 2004 to 2005, just before these FDA actions.13 Another study assessed opioid-naïve prescribing for long-acting opioids in a more recent national sample of nursing home residents, but the study did not assess individual or facility factors that might be associated with opioid-naïve prescribing.21 In addition, the 2 studies did not assess whether prescribing in residents complied with FDA warnings that fentanyl be used only for moderate-to-severe, continuous pain. Our aim was to pursue these questions further using a comprehensive national nursing home population and examine the degree to which transdermal fentanyl use was consistent with FDA safety communications and the FDA-approved indications for use, particularly to assess disparities in inappropriate prescribing, including by socioeconomic factors and facility-level characteristics. To do so, we assessed data on nursing home residents, facilities, and medication prescribing in 2008 from the national Minimum Data Set (MDS), the Online Survey, Certification, and Reporting (OSCAR) database, and Medicare Part D. We evaluated the extent of transdermal fentanyl prescribing for elderly nursing home residents and determined whether residents receiving fentanyl prescriptions for the first time had moderate-to-severe, continuous pain and were opioid-naïve. We then assessed whether certain individual and facility-level factors were associated with opioid-naïve prescribing.
Methods
Participants
Our source population was the approximately 1.4 million individuals who resided in US nursing homes between January 1, 2008, and December 31, 2008, and had at least 1 prescription drug documented in a Part D record (Figure 1). We limited our sample to residents who received a transdermal fentanyl dispensing/claim in 2008 without having a transdermal fentanyl dispensing within the prior 2 months, and who had at least 1 MDS record and a 90-day continuous stay before fentanyl initiation. Only residents with at least one Part D claim during this 2-month prior window and were 65 years or older were eligible for our study. We excluded individuals with cancer or receiving hospice care, as well as Alzheimer disease or most severe cognitive impairment, defined as an MDS Cognitive Performance Scale (CPS) score of 5 or 6, because of the distinct pain management challenges.22 We also excluded residents in hospital-based facilities. Data were missing for at least 1 covariate for 561 of the remaining 18,800 individuals (3.2% of the sample), whom we excluded, leaving a study population of 17,052 residents (Figure 1).
Fig. 1.
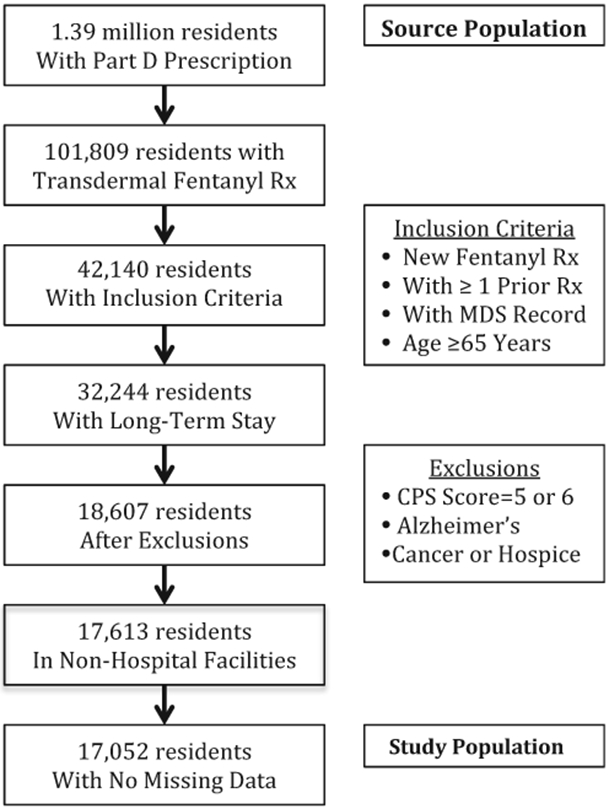
Source and study populations from Part D and the MDS (2008). Rx, prescription.
Measures
We analyzed data from the MDS, a standardized resident assessment instrument measuring each resident on 15 domains, including cognitive and physical functioning, psychosocial well-being, diseases, and pain.23 All US nursing homes certified for Medicare and/or Medicaid must use the MDS to periodically assess each resident.24 The MDS assessor, a trained nursing home staff person, relies on personal observation, resident and family interviews, medical records, and consultation with clinicians and other staff to complete MDS questions.23,25 This MDS information is used by nursing home staff to develop individual care plans for each resident.24,25 The nursing home evaluates each resident every 3 months for certain MDS measures (including cognitive and physical functioning and pain), annually for all MDS measures, and on any significant change in resident status.23 We relied on the MDS 2.0, which has been found to be generally valid and reliable for the domains when used by trained staff.26-28 We also relied on OSCAR data for facility factors, which the federal government compiles annually for each nursing home.29 Finally, we analyzed each resident’s drug dispensing by using Medicare Part D records.30
Opioid-naïve prescribing
We assessed whether each resident initiating transdermal fentanyl was opioid-naïve, defined as not having filed an opioid prescription with a duration end date within the 2 months prior to fentanyl initiation (ie, the 2-month window). We defined fentanyl initiation as the date of a resident’s first transdermal fentanyl prescription during 2008 and each prescription’s “duration end date” by adding the number of days’ supply to the prescription date. Our prescription opioid definition included the following: codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperedine, methadone, morphine, oxycodone, oxymorphone, and propoxyphene.
Persistent pain
We defined persistent pain as moderate-to-severe pain lasting 3 months or longer to follow the drug indication and other study approaches.12,15 Each nursing home resident is assessed in the MDS at least every 3 months for the frequency and intensity of any pain over the previous 7 days.23 This measurement has been found valid for measuring pain frequency and intensity in a scored scale.31 We based our assessment of persistent pain on the most recent MDS pain measurements before transdermal fentanyl initiation. Our study considered a nursing home resident in persistent pain if the individual had 2 consecutive MDS reports, at least 90 days apart but no more than 180 days apart, with moderate or severe pain daily during the prior 7-day period.
Statistical Analysis
We first assessed the proportion of residents in our study population receiving a transdermal fentanyl prescription who were (1) not in persistent pain or (2) opioid-naïve at fentanyl initiation. We then tested our hypothesis that individual factors (older age, poorer cognitive functioning, lower socioeconomic status (SES), and nonwhite race/ethnicity) and facility factors (smaller staff hours–to-resident ratio, fewer private pay residents, and for-profit status) would be associated with greater opioid-naïve prescribing based on prior literature about nursing home health disparities11,22,32-34 and quality of care.35-37 We used a multivariable logistic regression model with opioid-naïve prescribing (versus no opioid-naïve prescribing) as the outcome and included these individual and facility factors. We measured cognitive functioning in the MDS at persistent pain onset based on the CPS score from 0 (intact) to 4 (moderate severe impairment). We measured SES based on highest education level and whether the resident paid for nursing home services out-of-pocket (“self-pay”). We obtained the facility measurements from the most recent OSCAR survey before fentanyl initiation.
We also included in our model each of the following covariates that might be associated with any of our hypothesized factors and opioid-naïve transdermal fentanyl prescribing: resident gender, physical impairment, mood symptoms, family care involvement, facility compliance with federal law, and persistent pain. We measured physical impairment in the most recent MDS (at or before fentanyl initiation) by the degree of assistance needed for activities of daily living (ADLs) under the Morris additive scale from 0 (no help required) to 28 (most help needed); mood at the most recent MDS (at or before fentanyl initiation) by the MDS mood scale score from 0 (no mood symptoms) to 8 (most mood symptoms); family care involvement based on whether a family member or significant other participated in the most recent MDS care plan meeting (at or before fentanyl initiation); compliance with federal law based on whether any significant federal nursing home violations were outstanding, as recorded in the most recent OSCAR survey at or before fentanyl initiation; and whether the resident had persistent pain under our definition.
Because of resident clustering in each nursing home and nursing home clustering in each state, we included random intercepts in the model for these 2 levels to ensure more accurate standard errors. We also conducted sensitivity analyses using a more stringent definition of opioid-naïve status (ie, no opioid prescribing within a 6-month window before transdermal fentanyl initiation) and restricted to residents initiating fentanyl at the highest doses (≥50 mg/h) or whose initiation was within 10 days of their last pain assessment. Finally, we conducted subgroup analysis in those residents with arthritis and diabetes, as recorded in the MDS, because of their distinct pain experiences with nonopioid drug treatments (eg, corticosteroids for arthritis and gabapentin for diabetic pain). Data were analyzed using SAS, version 9.2 (SAS Institute, Inc, Cary, NC) and Stata version 13.0 (Stata Corp, College Station, TX) software. The study was approved by the Institutional Review Board of Johns Hopkins Bloomberg School of Public Health.
Results
Persistent Pain and Opioid-naïve Status
Only 8.2% of residents initiating transdermal fentanyl had persistent pain under our definition (Table 1). Furthermore, only 21.5% of residents had moderate or severe daily pain and 36.8% of residents had no pain at their last assessment before transdermal fentanyl initiation (Figure 2). More than one-third (36.3%) of residents initiating transdermal fentanyl were opioid-naïve (Table 1). Only 6.6% of residents had received an opioid before fentanyl initiation and had persistent pain (Table 1).
Table 1.
Numbers of Residents With Persistent Pain and Opioid-naïve Initiation of Transdermal Fentanyl (n = 17,052)
| Opioid-naïve Initiation | Total Population | ||
|---|---|---|---|
| No n (%) | Yes n (%) | ||
| Persistent pain | |||
| No n (%) | 9736 (57.1) | 5923 (34.7) | 15,659 (91.8) |
| Yes n(%) | 1126 (6.6)* | 267 (1.6) | 1393 (8.2) |
| Total population | 10,862 (63.7) | 6190 (36.3) | 17,052 (100) |
All percentages are based on total population of 17,052 residents in denominator.
Fentanyl prescribing complied with labeling conditions for persistent pain and no opioid-naïve initiation.
Fig. 2.
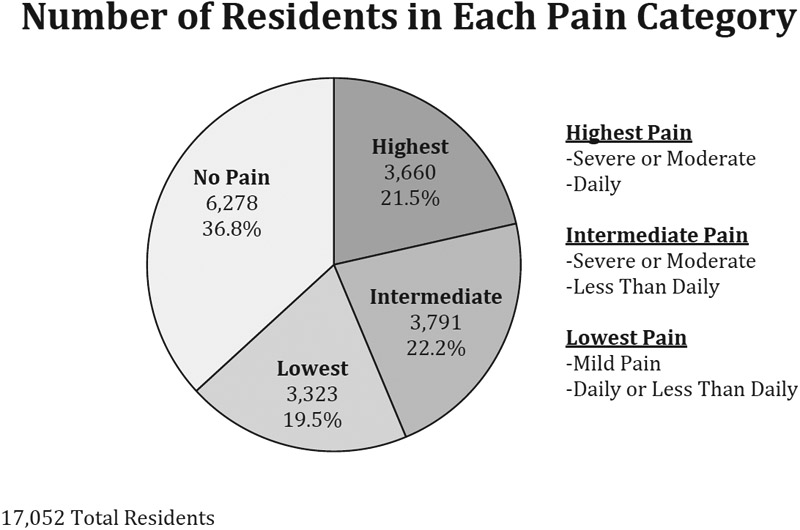
Number of residents in different pain categories at last assessment before fentanyl extended release initiation.
Patient and Facility Characteristics Associated With Fentanyl Use Among the Opioid-naïve
On bivariate analysis, several patient and facility characteristics were associated with the likelihood of transdermal fentanyl use among the opioid-naïve (Table 2). For example, nearly half (45.8%) of the oldest residents (≥95 years) were opioid-naïve, much greater than the proportion (29.7%) of youngest residents (65–75 years). Similarly, nearly half (46.9%) of the most cognitively impaired (CPS score = 4) were opioid-naïve, much greater than the proportion (28.2%) of cognitively intact residents who were opioid-naïve.
Table 2.
Fentanyl Extended Release (ER) Prescribing: Characteristics of Nursing Home Residents Receiving New Fentanyl ER Prescription in 2008 by Opioid-naïve Status (n = 17,052)
| Characteristics | Not Opioid-Naïve Initiation of Fentanyl ER, n (%)* |
Opioid-Naïve Initiation of Fentanyl ER, n (%)* |
χ2 Statistic, P† |
|---|---|---|---|
| Total population | 10,862 (63.7) | 6190 (36.3) | |
| Gender | .332 | ||
| Female | 8574 (63.9) | 4847 (36.1) | |
| Male | 2288 (63.0) | 1343 (37.0) | |
| Age, y | <.001 | ||
| 65–74 | 1981 (70.3) | 836 (29.7) | |
| 75–84 | 3771 (65.6) | 1979 (34.4) | |
| 85–94 | 4246 (61.6) | 2644 (38.4) | |
| ≥95 | 864 (54.2) | 731 (45.8) | |
| Race | <.001 | ||
| White | 9616 (64.0) | 5406 (36.0) | |
| Black | 850 (60.9) | 546 (39.1) | |
| Hispanic | 274 (64.0) | 154 (36.0) | |
| Asian | 70 (50.0) | 70 (50.0) | |
| Other | 52 (78.8) | 14 (21.2) | |
| Cognitive impairment by CPS score | <.001 | ||
| Intact = 0 | 2207 (71.8) | 867 (28.2) | |
| Borderline intact = 1 | 1714 (70.3) | 723 (29.7) | |
| Mild impairment = 2 | 2186 (65.2) | 1167 (34.8) | |
| Moderate impairment = 3 | 3768 (59.5) | 2561 (40.5) | |
| Moderate severe impairment = 4 | 987 (53.1) | 872 (46.9) | |
| Resident self-pay | <.001 | ||
| No | 9525 (64.3) | 5301 (35.8) | |
| Yes | 1337 (60.1) | 889 (39.9) | |
| Education level | .152 | ||
| Less than high school graduate | 4340 (64.3) | 2406 (35.7) | |
| High school graduate | 5923 (63.5) | 3406 (36.5) | |
| College graduate, graduate school | 599 (61.3) | 378 (38.7) | |
| Facility average staff hours per resident | .098 | ||
| <2.5 | 508 (62.5) | 305 (37.5) | |
| 2.5–3.0 | 1617 (65.7) | 843 (34.3) | |
| 3.0–3.5 | 3174 (62.5) | 1903 (37.5) | |
| 3.5–4.0 | 2964 (63.7) | 1688 (36.3) | |
| 4.0–4.5 | 1594 (63.6) | 912 (36.4) | |
| >4.5 | 1005 (65.1) | 539 (34.9) | |
| Facility’s proportion of residents’ self-pay, % | .001 | ||
| <10 | 1649 (61.6) | 1030 (38.5) | |
| 10–30 | 6408 (64.7) | 3502 (35.3) | |
| 30–50 | 2299 (63.6) | 1314 (36.4) | |
| >50 | 506 (59.5) | 344 (40.5) | |
| Facility for profit | .040 | ||
| No | 3449 (62.6) | 2060 (37.4) | |
| Yes | 7413 (64.2) | 4130 (35.8) | |
| ADL help: Morris additive scale | <.001 | ||
| 0 ADLs | 467 (73.7) | 167 (26.3) | |
| 1–7 ADLs | 1365 (71.0) | 558 (29.0) | |
| 8–14 ADLs | 1976 (66.6) | 990 (33.4) | |
| 15–21 ADLs | 4333 (63.1) | 2537 (36.9) | |
| 22–28 ADLs | 2721 (58.4) | 1938 (41.6) | |
| MDS mood scale | .362 | ||
| 0 | 5734 (63.2) | 3343 (36.8) | |
| 1–2 | 2712 (63.7) | 1543 (36.3) | |
| 3–4 | 1611 (65.3) | 858 (34.8) | |
| 5–6 | 690 (64.7) | 376 (35.3) | |
| 7–8 | 115 (62.2) | 70 (37.8) | |
| Family support | .012 | ||
| No | 5549 (64.6) | 3039 (35.4) | |
| Yes | 5313 (62.8) | 3151 (37.2) | |
| Facility compliant with federal law | .073 | ||
| Yes | 9788 (63.5) | 5630 (36.5) | |
| No | 1074 (65.7) | 560 (34.3) | |
| Persistent pain | <.001 | ||
| No | 9736 (62.2) | 5923 (37.8) | |
| Yes | 1126 (80.8) | 267 (19.2) |
ER, extended release.
Excludes 561 (3.2%) observations with missing values. Due to rounding, percentages do not all sum to 100.
All percentages correspond to row totals.
P value corresponds to the χ2 statistic.
Multivariable Predictors of Opioid-naïve Transdermal Fentanyl Use
After accounting for potentially confounding covariates, several patient and facility characteristics remained associated with opioid-naïve transdermal fentanyl use (Table 3). For example, such use was greater among patients who were older (compared with ages 65–74, ages 75–84 OR 1.16, 95% CI 1.04–1.29; ages 85–94 OR 1.30, 95% CI 1.16–1.44; ages >95 OR 1.69, 95% CI 1.46–1.95), more cognitively impaired (compared with intact cognitive functioning, borderline intact OR 1.06, 95% CI 0.93–1.20; mild impairment OR 1.31, 95% CI 1.16–1.47; moderate impairment OR 1.60, 95% CI 1.44–1.78; moderate severe impairment OR 1.99, 95% CI 1.73–2.29), or Asian (compared with white, OR 1.60, 95% CI 1.10–2.35).
Table 3.
ORs for Noncompliant Prescribing of Fentanyl Extended Release (ER) (No Opioid in 2 Months Before New Fentanyl ER Prescription)
| Characteristics | Multivariable Model* (n = 17,052) | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Gender | |||
| Female | (Ref) | ||
| Male | 1.08 | 0.99–1.18 | .069 |
| Age, y | |||
| 65–74 | (Ref) | ||
| 75–84 | 1.16 | 1.04–1.29 | .007 |
| 85–94 | 1.30 | 1.16–1.44 | <.001 |
| ≥95 | 1.69 | 1.46–1.95 | <.001 |
| Race | |||
| White | (Ref) | ||
| Black | 1.09 | 0.96–1.24 | .173 |
| Hispanic | 0.94 | 0.75–1.17 | .566 |
| Asian | 1.60 | 1.10–2.35 | .015 |
| Other | 0.50 | 0.27–0.94 | .033 |
| Cognitive impairment by CPS score | |||
| Intact = 0 | (Ref) | ||
| Borderline intact = 1 | 1.06 | 0.93–1.20 | .378 |
| Mild impairment = 2 | 1.31 | 1.16–1.47 | <.001 |
| Moderate impairment = 3 | 1.60 | 1.44–1.78 | <.001 |
| Moderate severe impairment = 4 | 1.99 | 1.73–2.29 | <.001 |
| Resident self-pay | |||
| No | (Ref) | ||
| Yes | 1.12 | 1.01–1.25 | .031 |
| Education level | |||
| Less than high school graduate | (Ref) | ||
| High school graduate | 1.09 | 1.01–1.17 | .026 |
| College graduate, graduate school | 1.17 | 1.01–1.37 | .041 |
| Facility average staff hours per resident | |||
| <2.5 | (Ref) | ||
| 2.5–3.0 | 0.83 | 0.69–1.00 | .048 |
| 3.0–3.5 | 0.95 | 0.79–1.14 | .577 |
| 3.5–4.0 | 0.87 | 0.73–1.05 | .146 |
| 4.0–4.5 | 0.90 | 0.74–1.10 | .305 |
| >4.5 | 0.86 | 0.69–1.06 | .157 |
| Facility’s proportion of residents self-pay, % | |||
| <10 | (Ref) | ||
| 10–30 | 0.85 | 0.77–0.95 | .003 |
| 30–50 | 0.86 | 0.75–0.98 | .022 |
| >50 | 0.95 | 0.78–1.16 | .644 |
| Facility for profit | |||
| No | |||
| Yes | 1.03 | 0.95–1.12 | .483 |
| ADL help: Morris additive scale | |||
| 0 ADLs | (Ref) | ||
| 1–7 ADLs | 1.06 | 0.85–1.31 | .606 |
| 8–14 ADLs | 1.24 | 1.01–1.53 | .039 |
| 15–21 ADLs | 1.35 | 1.11–1.65 | .003 |
| 22–28 ADLs | 1.47 | 1.20–1.81 | <.001 |
| MDS mood scale | |||
| 0 | (Ref) | ||
| 1–2 | 0.91 | 0.84–0.99 | .032 |
| 3–4 | 0.87 | 0.78–0.96 | .007 |
| 5–6 | 0.82 | 0.71–0.96 | .010 |
| 7–8 | 0.85 | 0.62–1.19 | .348 |
| Family support | |||
| No | (Ref) | ||
| Yes | 1.01 | 0.94–1.08 | .835 |
| Facility compliant with federal law | |||
| Yes | (Ref) | ||
| No | 1.00 | 0.88–1.14 | .981 |
| Persistent pain | |||
| No | (Ref) | ||
| Yes | 0.44 | 0.38–0.51 | <.001 |
Ref, reference.
Multivariable logistic regression model. Adjusted for gender; age; race; cognitive functioning; resident self-pay status; education; facility average staff hours per resident, facility percentage of residents who self-pay; facility for-profit status; resident number of ADLs on Morris additive scale requiring help; resident score on MDS mood scale, resident family support; facility compliance with federal law; and resident persistent pain. With multilevel modeling at the state and facility levels.
In addition, residents with higher SES (ie, resident self-pay and college/graduate school education) had greater odds for opioid-naïve prescribing. Few facility factors were statistically significantly associated with opioid-naïve prescribing, except opioid-naïve initiation was less likely for residents within facilities that had low (2.5–3.0) average staff hours per resident or between 10% and 30% and 30% and 50% self-pay residents. In sensitivity analysis with different persistent pain definitions and inclusion criteria, as well as subgroup analyses for residents with arthritis or diabetes, increasing age and greater cognitive impairment were also statistically significantly associated with increased odds for opioid-naïve fentanyl initiation.
Discussion
In our nationally representative analysis of nursing homes, more than 90% of residents prescribed transdermal fentanyl did not have persistent pain and more than one-third were opioid-naïve. More vulnerable nursing home residents, particularly the oldest-old and most cognitively impaired, were more likely to initiate transdermal fentanyl while opioid-naïve. These findings, which followed numerous FDA risk communications regarding transdermal fentanyl, highlight the importance of more effective risk communication and safer use of long-acting opioids in nursing homes.
We also determined that more than 1 in 3 residents initiating transdermal fentanyl were opioid-naïve, estimates similar to a 2004 to 2005 study of Medicaid residents in Rhode Island.13 These results are especially troubling in light of FDA-approved labeling that underscores that transdermal fentanyl should be initiated only in opioid-tolerant patients in persistent pain.16 These results are greater than another study’s estimate that 18.5% of residents who initiated a long-acting opioid any time after entering the nursing home were opioid-naïve (ie, not prescribed an opioid in a prior 60-day window).21 This finding was in a nursing home population from a national sample taken after our 2008 population, which could suggest improved adherence to the labeling condition in a subsequent time period. We note, though, that the 2 study populations differed in important ways. For example, we excluded individuals with cancer and hospice care, as well as Alzheimer disease and severe cognitive impairment, from the analysis. These individuals were 42.3% of residents who met the initial inclusion criteria (Figure 1). If these excluded residents had a lower rate of opioid-naïve prescribing than the remaining population, then including all of these residents in the analysis would reduce the prevalence finding for inappropriate prescribing. Furthermore, we found that 20% of opioid-naïve initiators in our study were prescribed transdermal fentanyl at higher doses (≥50 μg/h), inconsistent with additional precautions in drug labeling and practice guidelines to initiate prescription opioids at the lowest doses and titrate upward if necessary.5,16,38
Our study results that inappropriate prescribing was greater in older residents and those with cognitive impairment is noteworthy. These patient characteristics are well-described barriers to high-quality care in nursing homes.11,12,32 In some settings, they may be associated with lower likelihood of adequate analgesia.11,12,32 So these same characteristics may contribute not only to analgesic underuse in nursing homes, but also to misuse, as we describe in this report.
Our finding that only 8.2% of residents in our study initiating transdermal fentanyl had persistent pain is extremely low for transdermal fentanyl initiators. In fact, this figure is not much greater than another study’s finding that 4.8% of residents in the general nursing home population had daily pain on 2 consecutive MDS assessments within 120 days (similar to our persistent pain definition).39 Our sensitivity analysis results that at least 30% of residents did not have any pain within 10 days before transdermal fentanyl initiation reinforces this concern. These findings are limited, though, by any inaccuracies or biases in assessing and reporting pain in the MDS.40,41
Our study results that residents with higher SES (self-pay status and more education) had greater odds for opioid-naïve initiation is counter to our hypothesis and should be explored further. In addition, in contrast to our hypotheses, we did not find that most examined facility characteristics were statistically significantly associated with lower odds of opioid-naïve prescribing. A recent systematic review, though, cautioned against overreliance on staff numbers for determining nursing home care quality because of other important staffing factors that may account for such associations, such as turnover rates.36
Our study has important strengths, particularly the findings’ generalizability, because the source population included all US nursing home residents who received a transdermal fentanyl prescription in 2008. In addition, we based our measures on comprehensive data for prescribing, resident traits, and facility characteristics. We were able to examine individual and facility-level attributes, as well as control for other unique factors in our model, such as residents’ family involvement in care and facilities’ compliance with federal law.
Our study also has several limitations. First, nursing home practices, including transdermal fentanyl prescribing, may have changed since the time of our study population in 2008. For example, the American Geriatrics Society revised its clinical practice guidelines for pain management in 20095 and the American Medical Directors Association published its guideline for nursing home pain management in 2011.38 One study assessing long-acting opioid initiation in a national nursing home population in 2011 found lower rates of opioid-naïve prescribing.21 However, our study provides important findings about transdermal fentanyl prescribing after the FDA issued its public warnings in 2005 and 2007 about inappropriate use and can be used as a baseline for additional analyses in more recent populations. In addition, even if inappropriate fentanyl prescribing has decreased in more recent years, the disparities we have identified for nursing home residents may still apply, particularly that inappropriate use is associated with older age and greater cognitive impairment.
Second, we did not assess whether those who had evidence of an opioid before fentanyl initiation were opioid-tolerant, and thus, our findings provide a conservative estimate of the magnitude of inappropriate transdermal fentanyl use.16 Third, there was an average 33-day time lag between the last MDS measurement and fentanyl initiation date for each resident, and thus some residents may have developed persistent pain after their last MDS assessment. However, significant changes in resident pain status, such as the development of persistent pain requiring a long-acting opioid, should have triggered an MDS assessment if considered a significant change in resident status.23 Also, in sensitivity analyses, we restricted the sample to residents with an MDS assessment no more than 10 days before fentanyl initiation and found similar results. Fourth, we may have mis-classified some patients as not having persistent pain, because physicians might consider some individual residents to be in persistent pain before 90 days have elapsed. However, even if 50% of patients were erroneously misclassified, it would still suggest that nearly half of residents (46%) were inappropriately prescribed these products.
The high rates of inappropriate prescribing that we describe raise several questions. First, to address potential pain misclassification, future studies could assess residents directly in nursing homes rather than rely on MDS measurements. Second, research should explore the underlying mechanisms for inappropriate fentanyl prescribing in nursing homes, particularly the circumstances for transdermal fentanyl initiation in residents without prior opioid use, especially older and more cognitively impaired residents. The mechanisms for disparities are complex and can be grounded in implicit bias on the part of health care providers.42,43 Third, the Institute of Medicine expressed alarm with medicines used to “chemically restrain” nursing home residents, such as psychotropic drugs.24 Future research should explore if transdermal fentanyl is being used for similar purposes.
Based on our study findings, policy makers and practitioners should consider further steps to ensure appropriate transdermal fentanyl use in nursing home residents.44 Our findings are more troubling given that the FDA took additional actions to warn the public and practitioners about the serious dangers from initiating transdermal fentanyl in patients who are opioid-naïve or without persistent pain.17-20 The FDA has more recently implemented a Risk Evaluation and Mitigation Strategy (REMS) for long-acting opioids, including transdermal fentanyl, requiring more precautionary steps for prescribing, including health care provider training.45 However, little is known regarding any effect that the REMS program may have had on reducing opioid-related injuries and deaths,46 and the REMS program for opioids is targeted primarily at opioid abuse in the general population and does not include additional steps for the elderly or nursing home patients. The FDA could tailor transdermal fentanyl safety communications and the REMS plan more specifically for vulnerable populations, such as nursing home residents.
Nursing home practices and policies also could require specific additional steps to ensure transdermal fentanyl is not prescribed to residents who do not have persistent pain and are opioid-naïve. Many recent efforts have focused on improving nursing home pain care and prescribing by multimodal approaches incorporating more accurate pain assessment methods, improved clinician-staff communications, pharmacological and nonpharmacological treatments, and education and training.47-49 These efforts also should ensure that more vulnerable subpopulations within nursing homes (eg, more cognitively impaired and older) are not inappropriately prescribed transdermal fentanyl.
Conclusion
We examined transdermal fentanyl prescribing in a large, vulnerable population and assessed whether the prescribing followed FDA requirements. Our findings, from a study population drawn from all nursing home residents in the United States in 2008, indicate that a large proportion of nursing home residents received transdermal fentanyl inappropriately because they were not in persistent pain and were opioid-naïve. In addition, older residents and those who were cognitively impaired were more likely to be inappropriately prescribed these products. These results support the need for the FDA, health care organizations, and nursing homes to continue their efforts to ensure appropriate transdermal fentanyl prescribing, with particular care for these more neglected subpopulations.
Acknowledgments
KMF was supported as a Sommer Scholar at the Johns Hopkins Bloomberg School of Public Health during the time of this study. GCA is supported by the Agency for Healthcare Research and Quality (RO1 HS0189960) and the National Heart, Lung, and Blood Institute (R01 HL107345). The funding sources had no role in the design and conduct of the study, analysis, or interpretation of the data and preparation or final approval of the manuscript before publication.
Footnotes
The authors declare no conflicts of interest. GCA is Chair of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; serves as a paid consultant to PainNavigator, a mobile startup to improve patient self-management of pain; serves as a paid consultant to IMS Health; and serves on an IMS Health scientific advisory board. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Fain K, Alexander GC. Are Food and Drug Administration prescription drug safety plans working? A case study of isotretinoin. Pharmacoepidemiol Drug Saf 2013;22:1258–1262. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Califf, FDA top officials call for sweeping review of agency opioid policies. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm484765.htm; 2016. Accessed September 22, 2016.
- 3.Centers for Disease Control and Prevention. Increases in drug and opioid overdose deaths: United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–1382. [DOI] [PubMed] [Google Scholar]
- 4.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ 2014;348:g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older adults. J Am Geriatr Soc 2009;57:1331–1346. [DOI] [PubMed] [Google Scholar]
- 6.Reisner L. Pharmacological management of persistent pain in older persons. J Pain 2011;12:S21–S29. [DOI] [PubMed] [Google Scholar]
- 7.Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse events among nursing home residents. Arch Intern Med 2001;161:1629–1631. [DOI] [PubMed] [Google Scholar]
- 8.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000;109:87–94. [DOI] [PubMed] [Google Scholar]
- 9.Cool C, Cestac P, Laborde C, et al. Potentially inappropriate drug prescribing and associated factors in nursing homes. J Am Med Dir Assoc 2014;15:850. e1–850.e9. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services. Nursing Home Compendium. Washington, DC: US Centers for Medicare and Medicaid; 2010. [Google Scholar]
- 11.Lapane KL, Quilliam BJ, Chow W, Kim MS. Pharmacologic management of non-cancer pain among nursing home residents. J Pain Symptom Manage 2013;45: 33–42. [DOI] [PubMed] [Google Scholar]
- 12.Won AB, Vallow S, Schein J, et al. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc 2004; 52:867–874. [DOI] [PubMed] [Google Scholar]
- 13.Dosa DM, Dore DD, Mor V, Teno JM. Frequency of long-acting opioid analgesic initiation in opioid-naive nursing home residents. J Pain Symptom Manage 2009;38:515–521. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. FDA News Release: FDA Issues Second Safety Warning on Fentanyl Skin Patch. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109046.htm; 2007. Accessed February 27, 2016.
- 15.US Food and Drug Administration. FDA approved Duragesic labeling. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/19813s039lbl.pdf; 2005. Accessed February 27, 2016.
- 16.US Food and Drug Administration. FDA approved Duragesic labeling. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019813s033lbl.pdf; 2008. Accessed February 27, 2016.
- 17.US Food and Drug Administration. Public health advisory: Safety warnings regarding use of fentanyl transdermal (skin) patches. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm051739.htm; 2005. Accessed February 27, 2016.
- 18.US Food and Drug Administration. FDA public health advisory: Important information for the safe use of fentanyl transdermal system (patch). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm051257.htm; 2007. Accessed February 27, 2016.
- 19.US Food and Drug Administration. Information for healthcare professionals: Fentanyl transdermal system (marketed as Duragesic and generics) 07/15/05. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125844.htm; 2005. Accessed February 26, 2016.
- 20.US Food and Drug Administration. Information for healthcare professionals: Fentanyl transdermal system (marketed as Duragesic and generics) - 12/21/2007 Update. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm084307.htm; 2007. Accessed February 26, 2016.
- 21.Pimentel CB, Gurwitz JH, Tjia J, et al. New initiation of long-acting opioids in long-stay nursing home residents. J Am Geriatr Soc 2016;64:1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds KS, Hanson LC, DeVellis RF, et al. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Symptom Manage 2008;35:388–396. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare and Medicaid Services. Resident Assessment Instrument 2.0 Manual. Washington, DC: US Centers for Medicare and Medicaid; 2002. [Google Scholar]
- 24.Institute of Medicine. Improving the quality of long-term care. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 25.Straker JK, Bailer AJ. A review and characterization of the MDS process in nursing homes. J Gerontol Nurs 2008;34:36–44. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter GI, Hastie CL, Morris JN, et al. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC Geriatr 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JN, Fries BE, Mehr DR, et al. MDS cognitive performance scale. J Gerontol 1994;49:M174–M182. [DOI] [PubMed] [Google Scholar]
- 28.Mor V. A Comprehensive clinical assessment tool to inform policy and practice. Med Care 2004;42. III-50–III-59. [DOI] [PubMed] [Google Scholar]
- 29.Kash BA, Hawes C, Phillips CD. Comparing staffing levels in the Online Survey Certification and Reporting (OSCAR) system with the Medicaid cost report data: Are differences systematic? Gerontologist 2007;47:480–489. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare and Medicaid Services. CMS Guide to Requests for Medicare Part D Prescription Drug Event (PDE) Data. Washington, DC: US Centers for Medicare and Medicaid; 2008. [Google Scholar]
- 31.Fries BE, Simon SE, Morris JN, et al. Pain in U.S. nursing homes: Validating a pain scale for the Minimum Data Set. Gerontologist 2001;41:173–179. [DOI] [PubMed] [Google Scholar]
- 32.Won A, Lapane K, Gambassi G. Correlates and management of nonmalignant pain in the nursing home. J Am Geriatr Soc 1999;47:1–7. [DOI] [PubMed] [Google Scholar]
- 33.Luo H, Zhang X, Cook B, et al. Racial/ethnic disparities in preventive care practice among U.S. nursing home residents. J Aging Health 2014;26:519–539. [DOI] [PubMed] [Google Scholar]
- 34.Cai S, Feng Z, Fennell ML, Mor V. Despite small improvement, black nursing home residents remain less likely than whites to receive flu vaccine. Health Aff (Millwood) 2011;30:1939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comondore VR, Devereaux PJ, Zhou Q, et al. Quality of care in for-profit and not-for-profit nursing homes: Systematic review and meta-analysis. BMJ 2009; 339:b2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spilsbury K, Hewitt C, Stirk L, Bowman C. The relationship between nurse staffing and quality of care in nursing homes: A systematic review. Int J Nurs Stud 2011;48:732–750. [DOI] [PubMed] [Google Scholar]
- 37.Mor V, Zinn J, Angelelli J, et al. Driven to tiers: Socioeconomic and racial disparities in the quality of nursing home care. Milbank Q 2004;82:227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Medical Directors Association. Pain management in the long-term care setting. Washington, DC: National Guideline Clearinghouse; 2011. [Google Scholar]
- 39.Lapane KL, Quilliam BJ, Chow W, Kim M. The association between pain and measures of well-being among nursing home residents. J Am Med Dir Assoc 2012;13:344–349. [DOI] [PubMed] [Google Scholar]
- 40.Lin WC, Lum TY, Mehr DR, Kane RL. Measuring pain prevalence and intensity in nursing home residents. J Am Med Dir Assoc 2006;7:147–153. [DOI] [PubMed] [Google Scholar]
- 41.Engle VF, Graney MJ, Chan A. Accuracy and bias of licensed practical nurse and nursing assistant ratings of nursing home residents’ pain. J Gerontol A Biol Sci Med Sci 2000;56:M405–M411. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine. Unequal Treatment. Washington, DC: National Academy of Sciences; 2003. [Google Scholar]
- 43.van Ryn M, Fu SS. Paved with good intentions: Do public health and human service providers contribute to racial/ethnic disparities in health? Am J Public Health 2003;93:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander GC, Frattaroli S, Gielen AC, editors. The Prescription Opioid Epidemic: An Evidence-Based Approach. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; 2015. [Google Scholar]
- 45.US Food and Drug Administration. Risk evaluation and mitigation strategy for extended-release and long-acting opioids. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf; 2014. Accessed February 27, 2016.
- 46.Qato DM, Alexander GC. Post-marketing drug safety and the Food and Drug Administration’s risk evaluation and mitigation strategies. JAMA 2011;306: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 47.Ersek M, Jablonski A. A mixed-methods approach to investigating the adoption of evidence. J Gerontol Nurs 2014;40:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swafford KL, Miller LL, Tsai PF, et al. Improving the process of pain care in nursing homes: A literature synthesis. J Am Geriatr Soc 2009;57:1080–1087. [DOI] [PubMed] [Google Scholar]
- 49.Stevenson KM, Dahl JL, Berry PH, et al. Institutionalizing effective pain management practices: Practice change programs to improve the quality of pain management in small health care organizations. J Pain Symptom Manage 2006;31:248–261. [DOI] [PubMed] [Google Scholar]


