TO THE EDITOR:
Platins are effective chemotherapy drugs used to treat ovarian and colon cancers among others, but up to 25% of patients can develop life-threatening IgE-mediated hypersensitivity reactions (HSRs) after multiple exposures, which preclude first-line treatment prematurely and impact negatively in patients’ quality of life and life expectancy.1–4 Desensitization (DS) protocols induce a temporary tolerance state in which allergic patients with cancer can safely reintroduce their best treatment option. DS are safe procedures, but 10% to 30% of patients experience breakthrough reactions,1,5 and the severity of these reactions is unpredictable due to the absence of biomarkers that can identify patients at risk.1,6
Mast cells (MCs) are believed to be the main effector cells involved in allergic HSRs and DS. Inhibition of acute and late phase mediators, lack of FcεRI receptor internalization, and actin rearrangement have been shown in IgE desensitized MCs.7 Recent studies have shown that the soluble FcεRI (sFcεRI) is released during MC activation and prevents anaphylaxis in vivo.8 We hypothesized that DS might modulate sFcεRI levels protecting patients against anaphylaxis.
We recruited 14 platin allergic patients with cancer who underwent DS with a positive skin test (Materials and Methods and Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). sFcεRI was detectable in all patients and bound to IgE.9 No demographic, serological, or clinical characteristics correlated or modulated sFcεRI titers (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org). To rule out the effect of cancer or chemotherapy, an atopic cohort (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org) was compared with patients with cancer with no significant differences in sFcεRI or IgE levels (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org). DS successfully allowed all patients to receive their drug target dose. Serum samples obtained before and after completion of DS were analyzed (Figure 1). Tryptase levels were increased in 4 patients after DS, with 2 of them presenting with breakthrough reactions (patients 1 and 6). IgE levels were increased in 11 patients and sFcεRI increased in 9 patients after DS (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org).
FIGURE 1.
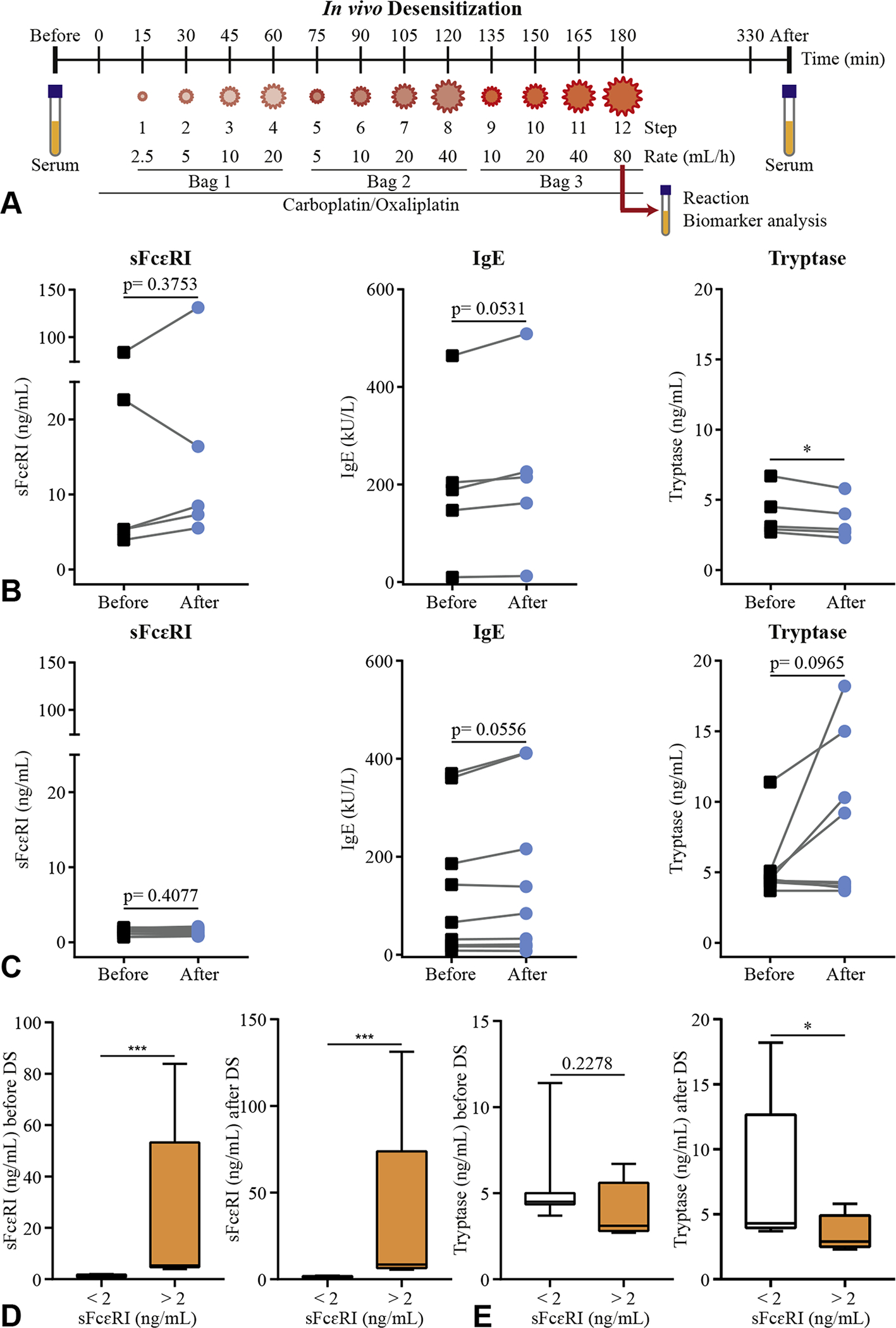
Baseline sFcεRI levels as a biomarker for risk of IgE-mediated reactions during DS. Outline of the in vivo DS protocol of 3 bags/ 12 steps (A). Levels of total sFcεRI, total IgE, and tryptase before (black squares) and after (blue circles) DS. Patients (n = 5) with sFcεRI levels >2 ng/mL (B) and patients (n = 9) with sFcεRI levels <2 ng/mL (C) are represented. Levels of total sFcεRI (D) and tryptase (E) before and after DS. Patients (n = 5) with sFcεRI levels >2 ng/mL (white bar) and patients (n = 9) with sFcεRI levels <2 ng/mL (orange bar) are represented (D) as box and whisker graphs (minimum to maximum). A paired t-test or a Mann-Whitney test was performed, where *P < .05, ***P < .001. DS, Desensitization; sFcεRI, soluble FcεRI.
Based on a recent study,9 2 ng/mL was used as a cutoff for clinically relevant sFcεRI levels, and thus we divided the cohort into 2 subgroups. In the first subgroup (sFcεRI >2 ng/mL), 80% of the patients (n = 4 of 5) presented a trend toward increased sFcεRI levels (mean = 32 ± 0.05%), increased IgE, and a significant decrease in tryptase levels after DS (Figure 1, B). In the second subgroup (sFcεRI <2 ng/mL), patients presented a trend toward increased IgE (n = 6 of 9) and tryptase (n = 4 of 9) levels, and decreased sFcεRI levels after DS (Figure 1, C). Increased tryptase levels were observed in the 2 patients with breakthrough reactions, who presented the highest tryptase titers and the lowest baseline sFcεRI titers (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). When sFcεRI, IgE, and tryptase titers after DS were compared between subgroups, a significant difference in sFcεRI and tryptase levels (Figure 1, D and E, and Figure E2, available in this article’s Online Repository at www.jaci-inpractice.org) was observed in the protected group.
To test our hypothesis of high sFcεRI as a predictor for protection during DS, a blinded clinical follow-up was performed during further DS (Table E3, available in this article’s Online Repository at www.jaci-inpractice.org). Overall, breakthrough reactions occurred in 29% of the patients (4 of 14), 3 of 4 patients with IgE-mediated type I reactions and 1 of 4 with a cytokine release reaction (Table E3, available in this article’s Online Repository at www.jaci-inpractice.org). The 3 patients belonged to the at-risk subgroup with the lowest sFcεRI titers (Figure E2, C, available in this article’s Online Repository at www.jaci-inpractice.org).
We investigated the modulation of sFcεRI levels in an in vitro DS model of murine humanized MCs. Activation and DS were evaluated using β-hexosaminidase release. β-Hexosaminidase and sFcεRI release correlated during activation (Figure E3, A–D, available in this article’s Online Repository at www.jaci-inpractice.org), and sFcεRI was significantly inhibited during DS by 86% (Figure E3, available in this article’s Online Repository at www.jaci-inpractice.org, and Figure 2, A–C). Control FcεRIα−/− (MuKO) cells had no β-hexosaminidase and sFcεRI release. Inhibition of sFcεRI release was achieved by cumulative doses of antigen during DS, whereas suboptimal single doses were able to trigger incremental sFcεRI release (Figure E3, available in this article’s Online Repository at www.jaci-inpractice.org, and Figure 2, D and E). In vitro DS successfully rendered humanized MCs unresponsive to activation leading to a lack of sFcεRI release, supporting previous results of the signaling requirements for sFcεRI release.8 Moreover, it might reflect a potential mechanistic approach to correlate the already known actions of in vitro DS and the signaling requirements for sFcεRI release.
FIGURE 2.
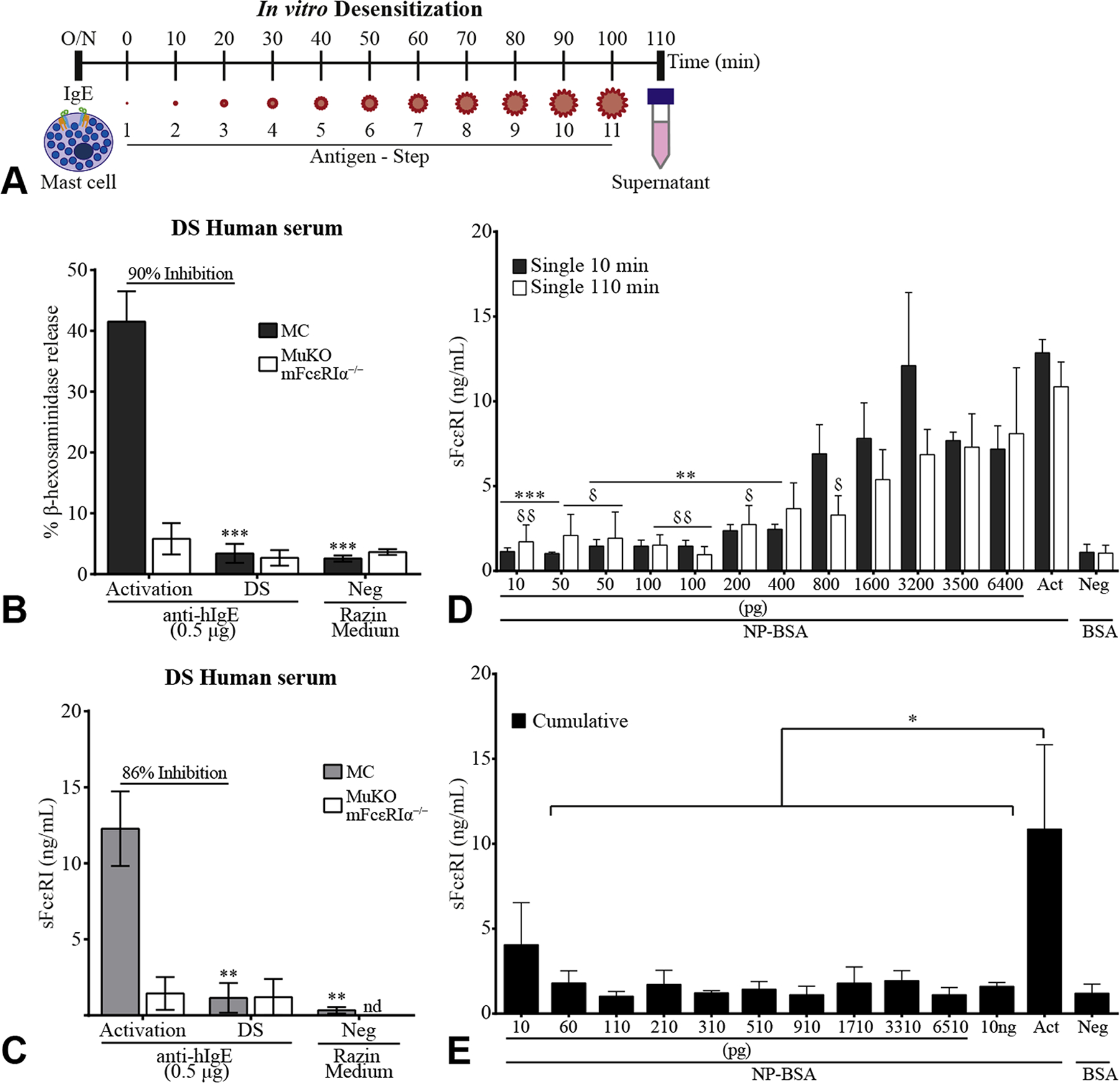
Rapid desensitization (DS) of mast cells (MCs) inhibits release of sFcεRI and it requires cumulative doses. Outline of the in vitro rapid DS protocol (A). A total of 1 × 106/mL MCs (humanized murine bone marrow–ederived MCs) or MuKO (mFcεRIα−/−) cells were loaded overnight with up to 10% allergic human serum in 200 μL (B, C). Percentage of β-hexosaminidase release (B) and total sFcεRI levels (C) were measured after DS with 0.5 μg/mL anti-hIgE. Razin Medium was used as control. A total of 1 × 106/mL MCs were loaded overnight with 0.5 μg/mL anti-NP cIgE (D, E). Total sFcεRI levels were measured after a single-dose challenge or cumulative doses (10 pg/mL to 10 ng/mL NP-BSA) for 10 or 110 minutes. A single challenge with 10 ng/mL NP-BSA (Act) or 10 ng/mL BSA (Neg) was used as a control. Data represent mean ± SEM of n = 3–8 independent experiments. A 1-way ANOVA test plus Tukey’s multiple correction (B-E) was performed, where */δP < .05, **/δδP < .01, and ***P < .001 compared with Neg (B, C) or Act (D, E). δRepresents statistics between conditions in white bars (D). ANOVA, Analysis of variance; BSA, bovine serum albumin; nd, not detected; NP-BSA, 4-hydroxy-3-nitrophenylacetyl bovine serum albumin; SEM, standard error of the mean; sFcεRI, soluble FcεRI.
To reconcile the in vitro results with the in vivo data, it is likely that complexes of sFcεRI/IgE are protective in vivo, decreasing the IgE binding to cell surface FcεRI, preventing MC activation and anaphylaxis. Because sFcεRI is not released without MC activation and during DS, a baseline level of subclinical sFcεRI release is necessary for protection.
We conclude that IgE, sFcεRI, and tryptase levels are useful biomarkers of DS (Figure E4, available in this article’s Online Repository at www.jaci-inpractice.org). Patients with sFcεRI levels above 2 ng/mL showed an increase in sFcεRI after DS and a significant decrease in tryptase levels. A protective function of sFcεRI was previously shown in murine models of anaphylaxis, in which administration of recombinant sFcεRI protected against anaphylaxis in naïve mice and diminished response severity in sensitized mice. Patients with higher sFcεRI levels were protected against HSRs during DS, and patients with low sFcεRI titers had increased tryptase levels and breakthrough reactions during DS.
Patients who suffered breakthrough IgE-mediated reactions (3 of 15) were classified as patients at risk and presented sFceRI levels <2 ng/mL. These patients might be at disadvantage as compared with patients with higher sFcεRI levels because free IgE is not captured by sFcεRI and can bind and activate MCs. These findings point to a delicate balance during DS between IgE and sFcεRI that likely determines the patient’s reactivity. Further studies would be needed in a larger population of platin IgE sensitized patients to confirm our findings.
In conclusion, patients with type I platin hypersensitivity phenotype presenting with an endotype with sFcεRI in serum of <2 ng/mL can be considered at risk for breakthrough reactions during DS. The use of tryptase, IgE, and sFcεRI serum titers has a promising value in providing personalized care in allergic patients with cancer, improving risk assessment. The availability of such biomarkers may be relevant in other immunotherapy protocols.
Supplementary Material
Clinical Implications.
Baseline serum soluble FcεRI titers are potential biomarkers to predict desensitization outcomes. Desensitization of humanized mast cells inhibits IgE-mediated activation correlating with the protection seen during desensitization protocols.
Acknowledgments
We thank all the members of the Szépfalusi, Fiebiger, and Castells laboratories for discussions and technical assistance. We also thank Klara Schmidthaler, Dr. Christina Bannert, Marlene García-Nueur, and Sahar Hamadi for their contribution in patient recruitment and Donna-Marie Lynch, MSN, FNP-BC, Desensitization nurse, for her help in the clinical assessment.
This work was supported by the Austrian Science Fund (FWF): DK W 1248-B13 (Z. Szépfalusi) and by the Harvard Digestive Diseases Center Grant P30DK034854, Cores B and C (E. Fiebiger). E. Fiebiger is supported by a Bridge Grant from the Research Council of Boston Children’s Hospital, an Emerging Investigator Award from FARE, a Senior research grant of the CCF, and an unrestricted gift from the Mead Johnson Nutrition Company. Additional funding for this study was provided by Ovations for the Cure. L. de las Vecillas was funded by a postresidency contract “Wenceslao López-Albo” provided by Instituto de Investigación Marqués de Valdecilla (Santander, Spain).
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Castells M Drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: the role of desensitizations. Front Immunol 2017;8:1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otani IM, Wong J, Banerji A. Platinum chemotherapy hypersensitivity: prevalence and management. Immunol Allergy Clin North Am 2017;37:663–77. [DOI] [PubMed] [Google Scholar]
- 3.Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, Lynch DM, Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol 2018;142:159–170. e2. [DOI] [PubMed] [Google Scholar]
- 4.Deak PE, Kim B, Adnan A, Labella M, De Las Vecillas L, Castells M, et al. Nanoallergen platform for detection of platin drug allergies. J Allergy Clin Immunol 2019;143:1957–1960. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altwerger G, Florsheim EB, Menderes G, Black J, Schwab C, Gressel GM, et al. Impact of carboplatin hypersensitivity and desensitization on patients with recurrent ovarian cancer. J Cancer Res Clin Oncol 2018;144:2449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy 2018;11:121–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Las Vecillas Sanchez L, Alenazy LA, Garcia-Neuer M, Castells MC. Drug hypersensitivity and desensitizations: mechanisms and new approaches. Int J Mol Sci 2017;18:E1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monino-Romero S, Erkert L, Schmidthaler K, Diesner SC, Sallis BF, Pennington L, et al. The soluble isoform of human FcepsilonRI is an endogenous inhibitor of IgE-mediated mast cell responses. Allergy 2019;74:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monino-Romero S, Lexmond WS, Singer J, Bannert C, Amoah AS, Yazdanbakhsh M, et al. Soluble FcepsilonRI: a biomarker for IgE-mediated diseases. Allergy 2019;74:1381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


