Abstract
Background
Increasing resistance to antibiotics poses medical challenges worldwide. Prospective data on carriage prevalence of multidrug resistant organisms (MDRO) in children at hospital admission are limited and associated risk factors are poorly defined.
Aim
To determine prevalence of MDRO carriage in children at admission to our paediatric hospital in Hamburg and to identify MDRO carriage risk factors.
Methods
We prospectively obtained and cultured nasal/throat and inguinal/anal swabs from children (≤ 18 years) at admission between September 2018 and May 2019 to determine prevalence of meticillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Gram-negative bacteria (MRGN) and vancomycin-resistant enterococcus (VRE) and associated species. We collected medical histories using a questionnaire and evaluated 31 risk factors using logistic regression models.
Results
MDRO carriage prevalence of 3,964 children was 4.31% (95% confidence interval (CI): 3.69–5.00). MRSA carriage prevalence was 0.68% (95% CI: 0.44–0.99), MRGN prevalence was 3.64% (95% CI: 3.07–4.28) and VRE prevalence 0.08% (95% CI: 0.02–0.22). MDRO carriage was associated with MRGN history (odds ratio (OR): 6.53; 95% CI: 2.58–16.13), chronic condition requiring permanent care (OR: 2.67; 95% CI: 1.07–6.13), antibiotic therapy (OR: 1.92, 95% CI: 1.24–2.94), living in a care facility (OR: 3.34; 95% CI: 0.72–12.44) and refugee status in previous 12 months (OR: 1.91; 95% CI: 0.27–8.02). Compared to established practice, screening using risk-factors had better diagnostic sensitivity (86.13%; 95% CI: 80.89–91.40) and specificity (73.54%; 95% CI: 72.12–74.97).
Conclusion
MRGN carriage was higher than MRSA and VRE. Extended risk-factor-based admission screening system seems warranted.
Keywords: multi-drug-resistant organisms, prevalence, colonization, childhood, risk factors
Introduction
Worldwide, multidrug-resistant organisms (MDRO) are an increasing medical and economic burden [1]. In particular, the increasing antibiotic resistance in Gram-negative bacteria poses major challenges to medical care [2,3]. Although meticillin-resistant Staphylococcus aureus (MRSA) colonisation shows a decreasing prevalence, the occurrence of multidrug resistant Gram-negative bacteria (MRGN), such as extended spectrum beta-lactamase (ESBL) Escherichia coli and Klebsiella pneumoniae, is increasing [2].
Different international prevalence estimates and risk factors for MDRO result in different strategies for MDRO management [4-8]. For example, our hospital isolates patients only after receiving a positive test result for MDRO, whereas other countries isolate newly admitted patients until a negative test result is received. While standardised MRSA screening is established in most European hospitals, the focus has changed to MRGN screening in adult patients in recent years [2,6,9-11]. Although recent studies published between 2013 and 2018, from Europe and the United States (US) have reported transmission routes for MRGN colonisation, such as direct person-to-person transmission, indirect transmission through contaminated areas or materials (e.g., kitchen towels), antibiotic use, contact with healthcare facilities in high MDRO prevalence countries and travel history to Africa and Asia, most risk factors seem unknown [2,6,12-14]. Because of belated or incomplete access to professional medical history at admission, MDRO detection is often delayed, which can increase morbidity and mortality [1].
European studies have shown an overall MRSA prevalence between 1.3% [7] and 2.0% [15], and a MRSA prevalence in children between 1.2% [15] and 2.7% (95% CI: 2.2–3.1) [16]. A meta-analysis conducted by Gesualdo et al. in 2013, ascertained a MRSA prevalence of 5.2% (95% CI: 3.1–7.3) in children with underlying medical conditions and chronic diseases, of 2.3% (95% CI: 1.8–2.7) in healthy children, and of 5.4% (95% CI: 3.1–7.7) in hospitalised children, which is higher than the 3.0% (95% CI: 2.4–3.6) in the general paediatric population [16]. According to a nationwide one-day point prevalence study in German hospitals focusing on adult care in 2014, MRSA prevalence was 1.64% (95% CI: 1.46–1.82). MRGN prevalence was 1.65% and of these were 1.4% 3MRGN and 0.25% 4MRGN. 3MRGN E. coli prevalence was 0.75% (95% CI: 0.60–0.89), Klebsiella spp. prevalence was 0.22% (95% CI: 0.15–0.29), Pseudomonas spp. prevalence was 0.21% (95% CI: 0.10–0.31), Enterobacter spp. prevalence was 0.10% (95% CI: 0.05–0.15), Acinetobacter spp. prevalence was 0.02% (95% CI: 0.00–0.03) and other non-specified 3MRGN prevalence was 0.10% (95% CI: 0.05–0.15). 4MRGN Pseudomonas spp. prevalence was 0.17% (95% CI:0.06–0.29), 4MRGN Klebsiella spp. prevalence was 0.04% (95% CI: 0.00–0.07) and other 4MRGN ranged between 0.01% and 0.04%. Vancomycin-resistant enterococcus (VRE) prevalence was 0.25% (95% CI: 0.13–0.37) [9]. Data published from 2013 from German hospitals reveal an increase in third-generation cephalosporin-resistant Enterobacteriaceae colonisation of up to 9.5%, and European studies show an increasing high ESBL carriage of up to 5.0% (95% CI: 3.4–6.6) [2] in adults [10,14,17-19].
With respect to MDRO prevalence, prospective data are scarce for children and adolescents [20]. In addition, changes in prevalence of MRSA and MRGN have not been adequately investigated. Furthermore, no uniform screening standards for MDRO detection in children have been established [6].
This study aimed to investigate the prevalence of MDRO carriage (MRSA, MRGN and VRE) at admission to the Altona Children’s Hospital (AKK) in Hamburg, Germany for scheduled or emergency hospital admissions as well as to determine clinical, epidemiological and microbiological risk factors associated with MDRO carriage. This information was used to critically evaluate the current MDRO screening at admission.
Methods
Study design
This prospective observational study was performed at one hospital. After written informed consent was obtained, participants were recruited over 9 months, between September 2018 and May 2019, at the AKK. With about 12,000 in-patient visits per year, the AKK is one of the largest paediatric care facilities in Germany, attracting patients from Hamburg and surrounding areas as well as from across Germany. The study included all in-patients (≤ 18 years, hereafter referred to as children) with scheduled or emergency admission from every department: general paediatrics, intensive care unit (ICU), neonatology, neurosurgery, otorhinolaryngology, orthopaedics, surgery, trauma surgery, urology, and Lufthafen, a special unit for children requiring permanent ventilation. Patients were excluded from the study if they were re-admitted to our clinic after initial recruitment.
Data and clinical sample collection
Pooled smear tests were taken from typical regions (nasal/throat and inguinal/anal) to determine MDRO occurrence (MRSA, MRGN and VRE) and associated species. In general, the samples were taken at admission. In special emergency situations, samples were taken after the patient was in a stable condition. Additionally, we collected clinical, epidemiological and microbiological data using a questionnaire for parents and attending physicians, which was completed during admission. The questionnaire was available in Arabic, English, French, German, Persian (Farsi), Russian and Turkish to ensure inclusion of different ethnicities. Based on recommendations from the German Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute (KRINKO) and on questionnaires from previous European studies [6,13-16,21], we evaluated six general demographic factors: (i) sex, (ii) age, (iii) department, (iv) emergency, (v) transfer (patient transferred from another hospital) and (vi) country of birth of the child and parents, and 25 specific factors (see Supplementary Tables S1–3). The patient questionnaire already in use and evaluating 17 risk factors was modified by adding eight additional risk factors: (i) history of VRE, (ii) contact with a family member treated at ICU for more than 7 days the previous 12 months, (iii) having a pet, (iv) being breastfed within the previous 12 months, (v) major surgery the previous 12 months, (vi) urogenital anomalies or recurrent urological infections, (vii) family member working in healthcare facilities with regular patient contact and (viii) stay at a neonatology unit for more than 7 days within the previous 12 months. For some questions (11 of 25), if the answer was affirmative, one to four sub-questions needed to be answered (a total of 18 sub-questions) – e.g., duration of treatment (see Supplementary Tables S1–3; 1.1, 2.1, 3.1, 4.1–2, 9.1–3, 17.1–2, 18.1, 20.1–4, 21.1, 22.1 and 24.1). Parents and attending physicians needed approximately 3 to 5 minutes to complete the whole questionnaire. Some information was derived from the medical history recorded during admission, including information from the physical examination.
Laboratory procedures
Pooled smear tests were taken using the eSwabÔ (Copan, Brescia, Italy) collection and transport device consisting of Nylon Flocked Swabs and 1ml of Liquid Amies. Most of the smears were taken by the nursing staff and rarely by doctors. One swab was used for sampling the throat and then the nasal cavity, and another swab was used for sampling the inguinal and then the anal region. The swabs were immersed in 1mL of Liquid Amies (eSwabÔ Copan, Brescia, Italy) until processed. Smears were analysed in the same diagnostic laboratory for the entire study period. In the laboratory, nasal/throat swabs were incubated on culture media for MRSA (MRSA Chromagar, Biomerieux, Nuertingen, Germany) and ESBL (ESBL Chromagar, Biomerieux). Inguinal/anal swabs were incubated on culture media for MRSA (MRSA Chromagar, Biomerieux), ESBL (ESBL Chromagar, Biomerieux) and VRE (VRE Chromagar, MAST Diagnostica, Reinfeld, Germany). All swabs were incubated for 48 h at 36 °C. Initial observations were made after 24 h. Growing cultures were isolated and selectively differentiated. The bacteria were identified by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight mass-spectrometer (MALDI-TOF Biotyper Systems, Bruker, US). Antimicrobial susceptibility testing was performed either by the disk diffusion method or by breakpoints in an automated system (Walkaway, Beckman Coulter, US), according to current European Committee on Antimicrobial Susceptibility Testing (EUCAST) susceptibility testing criteria. If an MDRO was isolated, the results were classified according to KRINKO recommendations: 2MRGN (resistant against piperacillin and cefotaxim and/or ceftazidim), 3MRGN (resistant against piperacillin, cefotaxim and/or ceftazidim and ciprofloxacin), or 4MRGN (3MRGN plus resistant against imipenem and/or meropenem) [22].
Statistical analysis
In 2013, Wegner et al. found an MDRO (MRSA, MRGN and VRE) prevalence of 3.04% in adults [23], and a slightly lower prevalence was expected in children [5,8,15]. Sample size estimation showed that based on 4,000 patients, a prevalence of at least 2.34% could be estimated – based on a 5% significance level and the conservative assumption that precision is at one-fifth of the expected prevalence [24,25]. Hence more than 4,000 patients were recruited (4,092).
The primary endpoint was MDRO carriage prevalence at admission to the AKK, and the secondary endpoint was the prevalence of individual pathogens (MRSA, MRGN and VRE). The 95% confidence intervals (CI) for these prevalence estimates were derived based on Clopper and Pearson [26]. To visualise the trend in prevalence over age groups, a locally weighted scatterplot smoothing (LOESS) estimate was used. Associations between individual risk factors and the primary endpoint, MDRO, as well as with the secondary endpoint, MRGN, were assessed with two multivariable logistic regression models. From these, odds ratio (OR) estimates along with 95% CIs were derived. Including all potential risk factors in the model resulted in the estimation of the direct effects of each of them, independent of the other factors. As analyses were based on all available cases, 3,851 (97.1%) observations contributed to the prevalence estimate of the primary and secondary endpoint. Sensitivity and specificity of the original screening survey were assessed as in the extended version and were reported along with 95% Wald CIs.
Data collection and processing based on questionnaires and microbiological results were performed using EpiData (EpiData Association, Odense, Denmark) [27]. Statistical analyses were performed using IBM SPSS Statistics 26 (IBM, Armonk, New York, US) and R (R-Foundation, Vienna, Austria) [28,29].
Results
Characteristics of the study population
During the study period, 4,092 children were recruited, but 124 patients had to be excluded as they were recruited twice during the study period and four additional children were excluded as data from their questionnaires could not be evaluated. As a result, 3,964 patients were included in the analyses (Table 1) and 3,851 (97.1%) observations contributed to the prevalence estimate of the primary and secondary endpoints
Table 1. General demographics, MDRO carriage status and MDRO prevalence in children at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 3,964).
| Characteristics | MDRO negative n = 3,685 |
MDRO positive n = 166 |
Missing n = 113 |
Total n = 3,964 |
MDRO Prevalence |
||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | n | n | % | % a | 95% CI | |
| Sex | |||||||||
| Male | 2,046 | 55.5 | 95 | 57.2 | 54 | 2,195 | 55.4 | 4.43 | 3.60–5.40 |
| Female | 1,639 | 44.5 | 71 | 42.8 | 59 | 1,769 | 44.6 | 4.15 | 3.26–5.21 |
| Age (years) | |||||||||
| Mean (SD) |
6.4 (5.7) |
NA | 4.4 (5.4) |
NA | NA | 6.4 (5.7) |
NA | NA | |
| Median (range: 0.0–18.0) | 5.0 | NA | 2.0 | NA | NA | 5.0 | NA | NA | |
| 0–4 years | 1,780 | 48.3 | 110 | 66.3 | 18 | 1,908 | 48.1 | 5.82 | 4.81–6.97 |
| 5–9 years | 651 | 17.7 | 19 | 11.4 | 29 | 699 | 17.6 | 2.84 | 1.72–4.39 |
| 10–14 years | 823 | 22.3 | 23 | 13.9 | 56 | 902 | 22.8 | 2.69 | 1.71–4.00 |
| 15–18 years | 421 | 11.4 | 14 | 8.4 | 20 | 455 | 11.5 | 3.22 | 1.77–5.34 |
| Department | |||||||||
| General paediatrics | 1,732 | 47.0 | 97 | 58.4 | 18 | 1,847 | 46.6 | 5.30 | 4.32–6.43 |
| Paediatric surgery | 714 | 19.4 | 23 | 13.9 | 1 | 738 | 18.6 | 3.12 | 1.99–4.65 |
| Orthopaedics | 566 | 15.4 | 12 | 7.2 | 89 | 667 | 16.8 | 2.08 | 1.08–3.60 |
| Trauma surgery | 343 | 9.3 | 14 | 8.4 | 2 | 359 | 9.1 | 3.92 | 2.16–6.49 |
| Neonatology | 125 | 3.4 | 4 | 2.4 | 0 | 129 | 3.3 | 3.10 | 0.85–7.75 |
| Otorhinolaryngology | 86 | 2.3 | 2 | 1.2 | 0 | 88 | 2.2 | 2.27 | 0.28–7.97 |
| Urology | 40 | 1.1 | 2 | 1.2 | 0 | 42 | 1.1 | 4.76 | 0.58–16.16 |
| Lufthafen | 32 | 0.9 | 4 | 2.4 | 0 | 36 | 0.9 | 11.11 | 3.11–26.06 |
| Neurosurgery | 29 | 0.8 | 3 | 1.8 | 3 | 35 | 0.9 | 9.38 | 1.98–25.02 |
| ICU | 18 | 0.5 | 5 | 3.0 | 0 | 23 | 0.6 | 21.74 | 7.46–43.70 |
| Emergency admission | |||||||||
| No | 1,420 | 38.5 | 43 | 25.9 | 95 | 1,558 | 39.3 | 2.94 | 2.14–3.94 |
| Yes | 2,118 | 57.5 | 115 | 69.3 | 18 | 2,251 | 56.8 | 5.15 | 4.27–6.15 |
| Missing information | 147 | 4.0 | 8 | 4.8 | 0 | 155 | 3.9 | NA | |
| Transferred from another hospital | |||||||||
| No | 3,315 | 90.0 | 141 | 84.9 | 112 | 3,568 | 90.0 | 4.08 | 3.44–4.79 |
| Yes | 222 | 6.0 | 17 | 10.2 | 1 | 240 | 6.1 | 7.11 | 4.20–11.14 |
| Missing information | 148 | 4.0 | 8 | 4.8 | 0 | 156 | 3.9 | NA | |
AKK: Altona Children's Hospital; ICU: intensive care unit; Lufthafen: special unit for children with permanent ventilation; MDRO: multidrug-resistant organisms; NA: not applicable; SD: standard deviation.
a Denominator for calculations = 3,851 patients with information on MDRO status.
Percentages may not add up to 100 because of rounding.
Of the closed-ended questions, 90.2% were answered. Open-ended questions about the country of birth of the child and the parents were answered by 28.4% (n = 1,125) of the parents; of these, 23.7% (n = 266) stated Germany as their birthplace. The participation rate of parents of male children was higher (55.4%) than parents of female children (44.6%). The mean age of participants was 6.4 (SD 5.7) years. Most participants were recruited from general paediatric wards (46.6%), followed by paediatric surgery (18.6%), orthopaedics (16.8%) and trauma surgery (9.1%) (Table 1). Over half of the participants were admitted via the emergency room (56.8%), and the rest were admitted electively (39.3%). Only a few were transferred from other hospitals (6.1%). The three risk factors with the highest positive response rates were (i) having a pet (30.4%), (ii) hospital stay within the previous 12 months (28.8%) and (iii) being breastfed within the previous 12 months (20.7%) (see Supplementary Tables S1–3). Of the 2.8% of participants with a previous positive history of MDRO, more than half had been tested positive for MRGN (1.8%) before the current study. In addition, a refugee status within the previous 12 months (n = 27) and living in a care facility (n = 31) resulted in a similar positive response rate. A higher proportion of participants indicated that they had a disability requiring permanent care (5.4%) or had received antibiotic therapy within the previous 6 months (16.6%). Other characteristics are shown in Table 2 (Supplementary Tables S1–3).
Table 2. Risk factors for MDRO colonisation in children, collected at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 3,964).
| Risk factor | MDRO negative n = 3,685 |
MDRO positive n = 166 |
Missing n = 113 |
Total n = 3,964 |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | n | % | |
| History of MRGN | |||||||
| No | 3,642 | 98.8 | 139 | 83.7 | 112 | 3,893 | 98.2 |
| Yes | 43 | 1.2 | 27 | 16.3 | 1 | 71 | 1.8 |
| Living in a care facility | |||||||
| No | 3,661 | 99.3 | 161 | 97.0 | 110 | 3,932 | 99.2 |
| Yes | 23 | 0.6 | 5 | 3.0 | 3 | 31 | 0.8 |
| Missing information | 1 | 0.0 | 0 | 0.0 | 0 | 1 | 0.0 |
| Refugee status within the previous 12 months | |||||||
| No | 3,656 | 99.2 | 161 | 97.0 | 113 | 3,930 | 99.1 |
| Yes | 25 | 0.7 | 2 | 1.2 | 0 | 27 | 0.7 |
| Missing information | 4 | 0.1 | 3 | 1.8 | 0 | 7 | 0.2 |
| Chronic condition requiring permanent care | |||||||
| No | 3,512 | 95.3 | 136 | 81.9 | 97 | 3,745 | 94.5 |
| Yes | 170 | 4.6 | 29 | 17.5 | 16 | 215 | 5.4 |
| Missing information | 3 | 0.1 | 1 | 0.6 | 0 | 4 | 0.1 |
| Antibiotic therapy within the previous 6 months | |||||||
| No | 2,961 | 80.4 | 104 | 62.7 | 89 | 3,154 | 79.6 |
| Yes | 590 | 16.0 | 51 | 30.7 | 19 | 660 | 16.6 |
| Missing information | 134 | 3.6 | 11 | 6.6 | 5 | 150 | 3.8 |
AKK: Altona Children’s Hospital; MDRO: multidrug-resistant organisms; MRGN: multidrug-resistant Gram-negative bacteria.
Percentages may not add up to 100 because of rounding.
MDRO prevalence
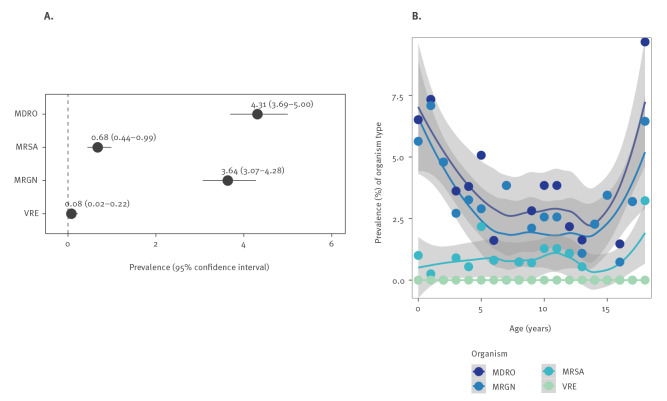
The prevalence of MDRO carriage at admission was 4.31% (95% CI: 3.69–5.00): MRGN prevalence was the highest (3.64%, 95% CI: 3.07–4.28), MRSA prevalence was lower (0.68%, 95% CI: 0.44–0.99) and VRE was the lowest (0.08%, 95% CI: 0.02–0.22) (Figure 1A).
Figure 1.
Overall MDRO prevalence (A) and age-specific MDRO prevalence (B) in children at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 3,851)a
AKK: Altona Children’s Hospital; LOESS: locally weighted scatterplot smoothing; MDRO: multidrug-resistant organisms; MRGN: multidrug-resistant Gram-negative bacteria; MRSA: meticillin-resistant Staphylococcus aureus; VRE: vancomycin-resistant enterococcus.
a Results for MDRO and MRGN are based on 3,851 observations, MRSA on 3,849 and VRE on 3,908.
b LOESS estimate for smoothed trend.
Comparison of screening algorithms at admission
Based on the AKK established admission screening using 17 risk factors, 109 (65.7%) of 166 MDRO cases would have been detected, as they reported at least one risk factor resulting in MDRO screening (Table 3). Based on the extended screening of 25 risk factors, as implemented in the current study (see methods), 143 (86.1%) MDRO cases would have been detected. Comparing both screening algorithms regarding their diagnostic performance, sensitivity (86.13%, 95% CI: 80.89–91.40) and specificity (73.54%, 95% CI: 72.12–74.97) were higher for the extended risk-factor-based screening compared with the established screening method.
Table 3. Diagnostic performance, detected MDRO cases and MDRO prevalence in children by MDRO screening algorithm at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 418 detected MDRO cases, results are based on 3,851 observations).
| Screening algorithm | Sensitivity |
Specificity |
Detected MDRO cases |
Estimated MDRO prevalence |
||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | n | % | |
| Established (17 factors) |
65.66 | 58.44–72.89 | 45.10 | 43.50–46.71 | 109 | 2.75 |
| Extended (25 factors) |
86.14 | 80.89–91.40 | 73.54 | 72.12–74.97 | 143 | 3.61 |
| Universal | NA | NA | 166 | 4.31 | ||
AKK: Altona Children’s Hospital; CI: confidence interval; MDRO: multidrug-resistant organisms; NA: not applicable.
Sensitivity and specificity were assessed using the original and extended screening survey along with 95% Wald CI.
MDRO species and frequency
Of the 30 participants with MRSA carriage, 21 were detected by nasal/throat screening; of the 153 participants with MRGN carriage, 130 (85.0%) were detected by inguinal/anal swabs (Table 4). of the 2MRGN (85 pathogen detection) was the highest MRGN colonisation, followed by 3MRGN (66 pathogen detection) and 4MRGN (1 participant two pathogen detection). The most frequent MRGN species were E. coli and Enterobacter spp. The 4MRGN were K. pneumoniae and Acinetobacter baumannii, both detected from nasal/throat swab. Carbapenemase of the OXA-48 group was detected in 4MRGN K. pneumoniae, and carbapenemase of the OXA-23 group was detected in 4MRGN A. baumannii. The child with detected 4MRGN had a chronic condition requiring permanent care and implanted medical devices and did not have refugee status. All three participants with VRE carriage swabbed positive for Enterococcus faecium. More than one different MDRO in the same patient was determined for three participants (0.08%) – one with MRSA and 2MRGN, one with MRSA and 3MRGN and one with two different 4MRGN.
Table 4. MDRO species frequencies in children by swab location at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 3,964 children).
| MDRO species | Swab location | Total swabs n = 7,928 |
||||
|---|---|---|---|---|---|---|
| Nasal/throat n = 3,964 |
Inguinal/anal n = 3,964 |
|||||
| n | % | n | % | n | % | |
| MRSA | 21 | 0.53 | 9 | 0.23 | 30 | 0.38 |
| MRGN | 23 | 0.58 | 130 | 3.28 | 153 | 1.92 |
| Escherichia coli | 9 | 0.23 | 99 | 2.50 | 108 | 1.36 |
| Enterobacter species | 1 | 0.03 | 8 | 0.20 | 9 | 0.11 |
| Klebsiella pneumoniae | 2 | 0.05 | 5 | 0.13 | 7 | 0.09 |
| Acinetobacter baumannii | 2 | 0.05 | 5 | 0.13 | 7 | 0.09 |
| Citrobacter species | 0 | 0.00 | 3 | 0.08 | 3 | 0.04 |
| Serratia species | 0 | 0.00 | 1 | 0.03 | 1 | 0.01 |
| Proteus species | 0 | 0.00 | 1 | 0.03 | 1 | 0.01 |
| Pseudomonas aeruginosa | 1 | 0.03 | 0 | 0.00 | 1 | 0.01 |
| Other | 8 | 0.20 | 8 | 0.20 | 16 | 0.20 |
| Not available | 4 | 0.10 | 111 | 2.80 | 115 | 1.45 |
| No MRSA or MRGN detected | 3,893 | 98.21 | 3,584 | 90.41 | 7,477 | 94.31 |
AKK: Altona Children's Hospital; MDRO: multidrug-resistant organisms; MRGN: multidrug-resistant Gram-negative bacteria; MRSA: meticillin-resistant Staphylococcus aureus.
Sex-, age- and department-specific MDRO prevalence
The prevalence of MDRO was similar in females (4.01%) and males (4.33%). Estimates of age-related prevalence revealed that MDRO and MRGN prevalence tended to be higher in the first years of life but also in adolescence (Figure 1B). MRSA carriage prevalence was constant over all ages (Figure 1B). The highest prevalence of MDRO carriage was observed in patients in the ICU (21.74%), the Lufthafen (the special unit for children on permanent ventilation) (11.11%) and the neurosurgical ward (9.38%) (Table 1).
Associations between risk factors and MDRO occurrence
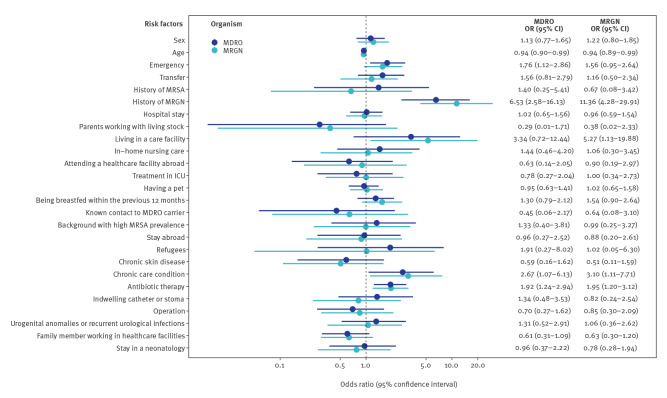
The most important risk factor for MDRO was MRGN history. An OR of 6.53 (95% CI: 2.58–16.13) indicated that the odds for MDRO occurrence in patients with previously reported MRGN was more than six times higher than for a patient without a previous MRGN history (Figure 2). Other clinically relevant associations with MDRO were living in a care facility (OR 3.34; 95% CI: 0.72–12.44), refugee status within the previous 12 months (OR: 1.91; 95% CI: 0.27–8.02), chronic condition requiring permanent care (OR: 2.67; 95% CI: 1.07–6.13) and antibiotic therapy within the previous 6 months (OR: 1.92; 95% CI: 1.24–2.94). Similar risk factors were also identified for MRGN colonisation, although more pronounced associations were identified for MRGN history (OR: 11.36; 95% CI: 4.28–29.91), living in a care facility (OR: 5.27; 95% CI: 1.13–19.88) and chronic care condition (OR: 3.10; 95% CI: 1.11–7.71) (Figure 2).
Figure 2.
Associations between risk factors and MDRO and MRGN in children at hospital admission, AKK Hamburg, Germany, September 2018–May 2019 (n = 3,017)
AKK: Altona Children's Hospital; ICU: intensive care unit; MDRO: multidrug-resistant organisms; MRGN: multidrug-resistant Gram-negative bacteria; MRSA: meticillin-resistant Staphylococcus aureus.
Odds ratio estimates were derived from logistic regression models as were 95% confidence intervals for MDRO and MRGN colonisation.
Results based on 3,017 complete observations.
In the figure, the risk factors are briefly summarized as keywords.
Discussion
In this study, we investigated the prevalence of MDRO carriage at admission to AKK, a major paediatric hospital in Hamburg, for scheduled or emergency admissions over 9 months.
We found an overall prevalence of MDRO carriage in children of 4.31% (95% CI: 3.69–5.00) at admission to hospital. This prevalence is higher than reported MDRO prevalence estimates in children in European studies up to 2014 [15,16], and similar to recent Danish and US studies examining paediatric and adult populations [10,13]. Previous European studies reported an MDRO prevalence in adults ranging from 3.04% [23], 3.46% [9], 5.2% (95% CI: 4.6–5.8 [10]), 7.2% [12] up to 10% [20].
The MRGN prevalence of 3.64% (95% CI: 3.07–4.28) in this study was higher than the observed prevalence in another German study examining adults (1.65%) [9] and similar to a recent Dutch study examining adults (5.0%, 95% CI: 3.4–6.6) [2]. Recent European studies have found an increasing ESBL carriage prevalence in preschool children, from 3.5% (95% CI: 2.5–4.8%) [13] to 16.8% [30], much greater than the MRSA carriage prevalence in children, from 1.2 [13] to 2.7% (95% CI: 2.2–3.1) [14]. This study confirmed a low prevalence of MRSA carriage of 0.68% (95% CI: 0.44–0.99) at hospital admission.
VRE had a reported low prevalence of 0.25% (95% CI: 0.13–0.37) [9] to 0.4% (95% CI: 0.3–0.6) [10] in previous German studies examining adults [9,23] and was only detected in three patients in this study (0.08%, 95% CI: 0.02–0.22).
The highest MDRO and MRGN carriage prevalence was observed in children aged 1–5 years and those 18 years of age. Possible reasons for the increased MRGN prevalence in the first years of life are physical contacts, crowding, difficulty in complying with basic hygiene routines, high likelihood for exposure to antimicrobial drugs, household-transmission and attendance at preschools and day care centres [22,30,31]. The increase in MDRO prevalence for 18-year-olds could be because most patients hospitalised at this age at a paediatric hospital have chronic paediatric diseases. That is, these patients may have had more contact with healthcare facilities in the past.
The difference in MDRO prevalence in Europe can be explained by the use of different MDRO definitions and by the evidence of a north-to-south and west-to-east gradient according to the latest surveillance report from the European Centre for Disease Prevention and Control (ECDC) and recent European studies [11,32,33]. As northern and western European countries seem to show a similar picture regarding MDRO occurrence, the results of this study are similar to the results of Danish and Dutch studies in adults [2,10]. Therefore, the results of this study might be primarily applicable to northwestern European regions; further Europe-wide children’s studies are required for confirmation of these findings.
The AKK is equipped with a hygiene commission consisting of four doctors and three trained hygiene staff who meet once a month to discuss hygiene complications and review existing screening forms against possible changes in KRINKO recommendations. To date, screening procedures at the AKK require nasal/throat or inguinal/anal swabs if patients reported one of 17 risk factors for MRSA or MRGN/VRE colonisation and nasal/throat swabs from all intensive care and neonatology patients. Isolation is performed after receiving a positive result, unless a colonisation was known beforehand. In this study, we extended our locally established questionnaire by adding additional eight risk factors and further subsequent questions, as there is no common agreement on relevant risk factors [12].
Similar to findings by Hamprecht et al. in 2016, the following risk factors for MDRO colonisation were identified: MRGN history, chronic condition requiring permanent care, antibiotic therapy within the previous 6 months, living in a care facility and refugee status within the previous 12 months [14]. Relevant risk factors for MRGN colonisation were the same. Because of the low number of cases, we could not provide relevant risk factors for MRSA and VRE colonisation.
The locally established screening at the AKK, which is similar to national and international standards, would have missed several MDRO cases (57 of 166) (Table 3). By adding eight risk factors, we were able to improve diagnostic performance (sensitivity: 86.13%, 95% CI: 80.89–91.40; specificity: 73.54%, 95% CI: 72.12–74.97). As screening all in-patients is not reasonable because of low prevalence and high screening costs, the best option might be an extended risk-factor-based admission screening system, as this increases the detection rate of MDRO carriers, secures adequate therapies and reduces health costs [12,34]. Moreover, it is also possible to use the extended risk-factor-based admission screening routinely at emergency units as the majority of the questions are relevant to the patient’s medical history, which would need to be reported in any case. As a consequence of this study’s findings, the extended screening system is now being established at the AKK.
When classifying MRGN cases according to antibiotic resistance, we primarily detected 2MRGN, followed by 3MRGN and 4MRGN. The detected carbapenemase of the OXA-48 group in 4MRGN K. pneumoniae and of the OXA-23 group in 4MRGN A. baumannii corresponds to the predominance in Europe [18] and Germany. According to KRINKO guidelines, 2MRGN colonisation is relevant in paediatric ICUs and other risk zones, such as neonatology units because of mandatory isolation and their same therapeutic status as 3MRGN [22]. Therefore, we also had the highest MDRO prevalence in our clinic risk zones, the ICU and the Lufthafen (Table 1).
Among the risk factors identified as having the highest positive response rate, being breastfed within the previous 12 months was identified in 20.7% of children with MDRO. Interestingly, Saporito et al. saw in 2020, a correlation between high MRGN colonisation and being breastfed (including breast milk feeding from bottles) [35] and recommend further evaluation of the handling and storage of the expressed breast milk before administration [35].
Lastly, 2MRGN colonisation must be viewed as a pre-stage to 3MRGN and 4MRGN stages. It is likely that the concerned participants in this study will develop bacterial infections that require antibiotic treatment, resulting in the development of further resistances within their positive MDRO colonisation status. It is known that positive MDRO colonisation can disappear for several years and return under selection pressure, such as exposure to antibiotics or travel to Africa and Asia [7,19,36,37]. Moreover, MRSA eradication treatments are available, but currently no therapeutic recommendations for MRGN colonisation exist. In line with our findings, other studies have also demonstrated only few E. coli or Enterobacter spp. in throat swabs. The clinical significance of E.coli (nasal/throat) as a pathogen is not clearly understood in paediatric patients [38], whereas sepsis caused by Enterobacter spp. and Escherichia spp. is associated with a high risk of mortality in the very low birth weight paediatric population [39].
Overall, this study had a high response rate: of the 4,092 patients recruited, 3,851 (97.1%) were included in our analyses. Although we generated high quality data, some questionnaires were incomplete, especially on days with a high patient influx at the clinic and thus some important risk factors, including patient/family member with treatment in an ICU, antibiotic treatments, parents working in healthcare facilities and country of birth, may have been missed. Because of the low number of cases or missing answers, five factors (country of birth of the child and parents, department, VRE history, dialysis and treatment of a family member in an ICU) and the 18 sub-questions could not be included in any of the logistic regression models. Data obtained from adolescent patients were generally more incomplete than from their younger counterparts as some adolescents refused anal swabs. This situation generated higher numbers of unknown anal and inguinal colonisation events, probably resulting in several missed MDRO cases. As previous studies have shown, taking a rectal swab has a higher certitude of detection than anal swabs. Because we already had a lower response for adolescence anal swabs, we would expect an even lower compliance if rectal swabs had been required. In addition, our results could have been influenced by the low number of participants who had refugee status (n = 27) [1] or who lived in a care facility (n = 31) [2], but it seems realistic to assume that the refugees are a minority among children being admitted at AKK. If the proportion of these had been higher, we would probably have had higher MDRO prevalence rates as other studies in Germany have shown among refugees and residents of care facilities [9,21,23,37,40].
Unfortunately, due to financial limitations, it was not possible to perform MDRO subtyping or to recruit participants over the entire year. Therefore, with regard to the described seasonal accumulations of, for example, ESBL occurrence in August and September, a year-round study would probably have shown an even higher MRGN prevalence [36].
Screening of parents was not performed and their medical history did not explicitly include questions about colonisation even though, as reported by van den Bunt et al. in 2016, parents of MDRO-positive children might also be carriers [31]. In any case, the source of parental colonisation would most likely be unknown. However, MDRO and especially ESBL transmission via contaminated items such as food and/or cloth (e.g., kitchen towels) has also been described as an MDRO risk factor [2,12].
Conclusion
Children and adolescents admitted to the AKK most likely represent the paediatric populations of Germany. This study has shown that the prevalence of colonisation of children with MRGN bacteria is higher than MRSA and VRE occurrence and that existing screening procedures can be optimised to decrease undetected MDRO colonisation events. Currently, the best option to detect MDRO in children from northern and western parts in Europe (or comparable low prevalence settings) might be an extended risk-factor-based admission screening system that considers healthcare-related risk factors. Therefore, there is a high need for further European studies on the modification of admission screening algorithms with a focus on MRGN in preschool children as this was the age group with the highest prevalence in our study.
Acknowledgements
The authors thank all participants, the management and clinical team at Altona Children's Hospital and the laboratory staff of laboratory Dr Fenner & colleagues for their support, furthermore Dr Laura Tegtmeyer for her help during the conception of the study at the very beginning.
Ethical statement
The study was approved by the Ethical Review Committee of the Medical Association Aerztekammer Hamburg in Germany (PV5792). Written informed consent was obtained from parents and patients over 7 years old before data collection.
Funding
This work was supported by Hamburg macht Kinder gesund e.V. (Martinistr. 52, 20246 Hamburg, Germany) and the Altona Children’s Hospital.
Supplementary Data
Conflict of interest: The authors declare no conflicts of interest. Study costs were limited to the microbiological laboratory tests. These amounted to 10.56 EUR per patient and were taken by a project-related donation by Hamburg macht Kinder gesund e.V. and by the Altona Children’s Hospital. The costs incurred for necessary isolation or treatment are covered by the Altona Children’s Hospital.
Authors’ contributions: Safiullah Najem, Dorothée Eick, Annette Aigner and Ingo Koenigs wrote and drafted the manuscript. Safiullah Najem, Johannes Boettcher and Annette Aigner performed statistical analyses. Safiullah Najem, Dorothée Eick, Johannes Boettcher, Annette Aigner and Ingo Koenigs analysed the data. Ingo Koenigs designed the study and raised the funding for the study. Safiullah Najem, Dorothée Eick, Johannes Boettcher, Annette Aigner, Mona Aboutara, Ines Fenner, Konrad Reinshagen and Ingo Koenigs participated in the conception of the study design. Safiullah Najem, Dorothée Eick, Mona Aboutara and Ines Fenner performed data collection. All authors participated in the review of the manuscript.
References
- 1. Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Burden of AMR Collaborative Group . Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56-66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Bunt G, van Pelt W, Hidalgo L, Scharringa J, de Greeff SC, Schürch AC, et al. Prevalence, risk factors and genetic characterisation of extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae (ESBL-E and CPE): a community-based cross-sectional study, the Netherlands, 2014 to 2016. Euro Surveill. 2019;24(41):1800594. 10.2807/1560-7917.ES.2019.24.41.1800594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walter J, Noll I, Feig M, Weiss B, Claus H, Werner G, et al. Decline in the proportion of methicillin resistance among Staphylococcus aureus isolates from non-invasive samples and in outpatient settings, and changes in the co-resistance profiles: an analysis of data collected within the Antimicrobial Resistance Surveillance Network, Germany 2010 to 2015. BMC Infect Dis. 2017;17(1):169. 10.1186/s12879-017-2271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gijón M, Bellusci M, Petraitiene B, Noguera-Julian A, Zilinskaite V, Sanchez Moreno P, et al. Factors associated with severity in invasive community-acquired Staphylococcus aureus infections in children: a prospective European multicentre study. Clin Microbiol Infect. 2016;22(7):643.e1-6. 10.1016/j.cmi.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 5. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing Susceptibility of Staphylococcus aureus in a US Pediatric Population. Pediatrics. 2016;137(4):e20153099. 10.1542/peds.2015-3099 [DOI] [PubMed] [Google Scholar]
- 6. Martischang R, Buetti N, Balmelli C, Saam M, Widmer A, Harbarth S. Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrob Resist Infect Control. 2019;8(1):37. 10.1186/s13756-019-0479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Bijnen EM, Paget J, de Lange-de Klerk ES, den Heijer CD, Versporten A, Stobberingh EE, et al. collaboration with the APRES Study Team . Antibiotic Exposure and Other Risk Factors for Antimicrobial Resistance in Nasal Commensal Staphylococcus aureus: An Ecological Study in 8 European Countries. PLoS One. 2015;10(8):e0135094. 10.1371/journal.pone.0135094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson K, Frei R, Heininger U. Prospective, cross-sectional study on MSSA and MRSA colonisation in hospitalised children. Eur J Pediatr. 2015;174(9):1255-62. 10.1007/s00431-015-2606-5 [DOI] [PubMed] [Google Scholar]
- 9. Huebner NO, Dittmann K, Henck V, Wegner C, Kramer A, Action Group Infection Prevention (AGIP) . Epidemiology of multidrug resistant bacterial organisms and Clostridium difficile in German hospitals in 2014: Results from a nationwide one-day point prevalence of 329 German hospitals. BMC Infect Dis. 2016;16(1):467. 10.1186/s12879-016-1756-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skjøt-Arkil H, Mogensen CB, Lassen AT, Johansen IS, Chen M, Petersen P, et al. Carrier prevalence and risk factors for colonisation of multiresistant bacteria in Danish emergency departments: a cross-sectional survey. BMJ Open. 2019;9(6):e029000. 10.1136/bmjopen-2019-029000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouchiat C, Curtis S, Spiliopoulou I, Bes M, Cocuzza C, Codita I, et al. ESCMID Study Group on Staphylococci and Staphylococcal Infections (ESGS) . MRSA infections among patients in the emergency department: a European multicentre study. J Antimicrob Chemother. 2017;72(2):372-5. 10.1093/jac/dkw431 [DOI] [PubMed] [Google Scholar]
- 12.Stoliaroff-Pépin A, Arvand M, Mielke M. Bericht zum Treffen der Moderatoren der regionalen MRENetzwerke am Robert Koch-Institut. [Report on the meeting of the moderators of the regional MDRO networks at the Robert Koch Institute]. Epidemiologisches Bulletin. 12 Oct 2017. German. Available from: https://edoc.rki.de/bitstream/handle/176904/2846/2377H38MG2d1U.pdf?sequence=1&isAllowed=y
- 13. Islam S, Selvarangan R, Kanwar N, McHenry R, Chappell JD, Halasa N, et al. Intestinal Carriage of Third-Generation Cephalosporin-Resistant and Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Healthy US Children. J Pediatric Infect Dis Soc. 2018;7(3):234-40. 10.1093/jpids/pix045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamprecht A, Rohde AM, Behnke M, Feihl S, Gastmeier P, Gebhardt F, et al. DZIF-ATHOS Study Group . Colonization with third-generation cephalosporin-resistant Enterobacteriaceae on hospital admission: prevalence and risk factors. J Antimicrob Chemother. 2016;71(10):2957-63. 10.1093/jac/dkw216 [DOI] [PubMed] [Google Scholar]
- 15. Otter JA, Herdman MT, Williams B, Tosas O, Edgeworth JD, French GL. Low prevalence of meticillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect. 2013;83(2):114-21. 10.1016/j.jhin.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 16. Gesualdo F, Bongiorno D, Rizzo C, Bella A, Menichella D, Stefani S, et al. MRSA nasal colonization in children: prevalence meta-analysis, review of risk factors and molecular genetics. Pediatr Infect Dis J. 2013;32(5):479-85. 10.1097/INF.0b013e3182864e4c [DOI] [PubMed] [Google Scholar]
- 17. Rohde AM, Zweigner J, Wiese-Posselt M, Schwab F, Behnke M, Kola A, et al. Prevalence of third-generation cephalosporin-resistant Enterobacterales colonization on hospital admission and ESBL genotype-specific risk factors: a cross-sectional study in six German university hospitals. J Antimicrob Chemother. 2020;75(6):1631-8. 10.1093/jac/dkaa052 [DOI] [PubMed] [Google Scholar]
- 18. Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, et al. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group . Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis. 2017;17(2):153-63. 10.1016/S1473-3099(16)30257-2 [DOI] [PubMed] [Google Scholar]
- 19. Otter JA, Natale A, Batra R, Tosas Auguet O, Dyakova E, Goldenberg SD, et al. Individual- and community-level risk factors for ESBL Enterobacteriaceae colonization identified by universal admission screening in London. Clin Microbiol Infect. 2019;25(10):1259-65. 10.1016/j.cmi.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 20. Fulchini R, Albrich WC, Kronenberg A, Egli A, Kahlert CR, Schlegel M, et al. Antibiotic-resistant pathogens in different patient settings and identification of surveillance gaps in Switzerland - a systematic review. Epidemiol Infect. 2019;147:e259. 10.1017/S0950268819001523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters C, Dulon M, Kleinmüller O, Nienhaus A, Schablon A. MRSA Prevalence and Risk Factors among Health Personnel and Residents in Nursing Homes in Hamburg, Germany - A Cross-Sectional Study. PLoS One. 2017;12(1):e0169425. 10.1371/journal.pone.0169425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon A, Tenenbaum T. Surveillance of multidrug-resistant Gram-negative pathogens in high-risk neonates--does it make a difference? Pediatr Infect Dis J. 2013;32(4):407-9. 10.1097/INF.0b013e3182875227 [DOI] [PubMed] [Google Scholar]
- 23. Wegner C, Hübner NO, Gleich S, Thalmaier U, Krüger CM, Kramer A. One-day point prevalence of emerging bacterial pathogens in a nationwide sample of 62 German hospitals in 2012 and comparison with the results of the one-day point prevalence of 2010. GMS Hyg Infect Control. 2013;8(1):Doc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79(11):1482-8. 10.1007/s12098-012-0763-3 [DOI] [PubMed] [Google Scholar]
- 25. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14-7. [PMC free article] [PubMed] [Google Scholar]
- 26. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-13. 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 27.Lauritsen J, Bruus M. EpiData: A Comprehensive Tool for Validated Entry and Documentation of Data Version 3. Odense, Denmark: The Epi Data Association. 2003. http://www.epidata.dk/ [Google Scholar]
- 28. Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- 29.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2021. https://www.R-project.org/
- 30. Kaarme J, Riedel H, Schaal W, Yin H, Nevéus T, Melhus Å. Rapid increase in carriage rates of Enterobacteriaceae producing extended-spectrum β-lactamases in healthy preschool children, Sweden. Emerg Infect Dis. 2018;24(10):1874-81. 10.3201/eid2410.171842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Bunt G, Liakopoulos A, Mevius DJ, Geurts Y, Fluit AC, Bonten MJ, et al. ESBL/AmpC-producing Enterobacteriaceae in households with children of preschool age: prevalence, risk factors and co-carriage. J Antimicrob Chemother. 2017;72(2):589-95. 10.1093/jac/dkw443 [DOI] [PubMed] [Google Scholar]
- 32. Wolfensberger A, Kuster SP, Marchesi M, Zbinden R, Hombach M. The effect of varying multidrug-resistence (MDR) definitions on rates of MDR gram-negative rods. Antimicrob Resist Infect Control. 2019;8(1):193. 10.1186/s13756-019-0614-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Centre for Disease Prevention and Control (ECDC). Surveillance of antimicrobial resistance in Europe: annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. Stockholm: ECDC; 15 Nov 2018. Available from: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017
- 34. Roope LSJ, Smith RD, Pouwels KB, Buchanan J, Abel L, Eibich P, et al. The challenge of antimicrobial resistance: What economics can contribute. Science. 2019;364(6435):eaau4679. 10.1126/science.aau4679 [DOI] [PubMed] [Google Scholar]
- 35. Saporito L, Graziano G, Mescolo F, Amodio E, Insinga V, Rinaudo G, et al. Efficacy of a coordinated strategy for containment of multidrug-resistant Gram-negative bacteria carriage in a Neonatal Intensive Care Unit in the context of an active surveillance program. Antimicrob Resist Infect Control. 2021;10(1):30. 10.1186/s13756-021-00902-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wielders CCH, Van Duijkeren E, Van Den Bunt G, Meijs AP, Dierikx CM, Bonten MJM, et al. Seasonality in carriage of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in the general population: a pooled analysis of nationwide cross-sectional studies. Epidemiol Infect. 2020;148:e68. 10.1017/S0950268820000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinheimer C, Abdollahi P, Zacharowski K, Meybohm P, Mutlak H, Klingebiel T, et al. Prevalence of multidrug-resistant organisms in refugee patients admitted to a German university hospital depending on duration of stay in Germany. GMS Hyg Infect Control. 2019;14:Doc07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Lastours V, Malosh RE, Aiello AE, Foxman B. Prevalence of Escherichia coli carriage in the oropharynx of ambulatory children and adults with and without upper respiratory symptoms. Ann Am Thorac Soc. 2015;12(3):461-3. 10.1513/AnnalsATS.201412-586LE [DOI] [PubMed] [Google Scholar]
- 39. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B, Israel Neonatal Network . Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis. 2005;40(2):218-24. 10.1086/426444 [DOI] [PubMed] [Google Scholar]
- 40. Tenenbaum T, Becker K-P, Lange B, Martin A, Schäfer P, Weichert S, et al. Prevalence of multidrug-resistant organisms in hospitalized pediatric refugees in an University Children’s Hospital in Germany 2015–2016. Infect Control Hosp Epidemiol. 2016;37(11):1310-4. 10.1017/ice.2016.179 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.