Abstract
In an open, randomized, six-period crossover study, the pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin were compared after a single oral dose in 12 healthy volunteers (6 men and 6 women). The volunteers received 250 mg of ciprofloxacin, 400 mg of gatifloxacin, 600 mg of grepafloxacin, 500 mg of levofloxacin, 400 mg of moxifloxacin, and 200 mg of trovafloxacin. The concentrations of the six fluoroquinolones in serum and urine were measured by a validated high-performance liquid chromatography method. Blood and urine samples were collected before and at different time points up to 48 h after medication. Levofloxacin had the highest peak concentration (Cmax, in micrograms per milliliter) (6.21 ± 1.34), followed by moxifloxacin (4.34 ± 1.61) and gatifloxacin (3.42 ± 0.74). Elimination half-lives ranged from 12.12 ± 3.93 h (grepafloxacin) to 5.37 ± 0.82 h (ciprofloxacin). The total areas under the curve (AUCtot, in microgram-hours per milliliter) for levofloxacin (44.8 ± 4.4), moxifloxacin (39.3 ± 5.35), and gatifloxacin (30 ± 3.8) were significantly higher than that for ciprofloxacin (5.75 ± 1.25). Calculated from a normalized dose of 200 mg, the highest Cmaxs (in micrograms per milliliter) were observed for levofloxacin (2.48 ± 0.53), followed by moxifloxacin (2.17 ± 0.81) and trovafloxacin (2.09 ± 0.58). The highest AUCtot (in microgram-hours per milliliter) for a 200-mg dose were observed for moxifloxacin (19.7 ± 2.67) and trovafloxacin (19.5 ± 3.1); the lowest was observed for ciprofloxacin (4.6 ± 1.0). No serious adverse event was observed during the study period. The five recently developed fluoroquinolones (gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin) showed greater bioavailability, longer half-lives, and higher Cmaxs than ciprofloxacin.
Fluoroquinolones are widely used alternatives to β-lactam agents in the treatment of many bacterial infections. Their antimicrobial activity results from a selective antagonism between host DNA and bacterial DNA without interfering with eucaryotic topoisomerases. The early fluoroquinolones, from the early 1960s, had a limited spectrum of antibacterial activity, mainly against gram-negative pathogens. Further observations of structure-related increases in activity, changes in pharmacokinetic characteristics, and reduced toxicity were followed by numerous chemical modifications of the quinolone molecule. The resulting new fluoroquinolone antimicrobial agents have enhanced activity against gram-positive organisms and anaerobes (1, 2) and improved pharmacokinetic parameters in comparison to previous derivatives. However, only reports on single-drug kinetics have been published so far, and there have been no direct comparisons between the new drugs and the standard drug, ciprofloxacin (1–3).
We therefore evaluated and compared the pharmacokinetics of six fluoroquinolones after a single oral dose in the same volunteers.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999 [I. Keller et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1190, 1999].)
MATERIALS AND METHODS
Volunteers.
Six male and six female young healthy Caucasians between 21 and 35 years old (mean, 28.4 ± 4.3 years) and with an average weight of 67.4 ± 10.6 kg and an average body surface of 1.81 ± 0.16 m2 participated in the study. All had normal renal and hepatic functions; the mean creatinine clearance was 113 ± 19.5 ml/min/1.73 m2. The volunteers were included in the study protocol after normal findings from physical examination, electrocardiogram (ECG), and laboratory tests (including hematological and biochemical parameters, hepatitis and human immunodeficiency virus serological tests, tests for drug abuse, urinalysis, and pregnancy test). Exclusion criteria were regular use of medications; abuse of alcoholic beverages; symptoms of significant illness within 3 months before the study period; history of gastrointestinal, liver, or kidney disease potentially interfering with absorption, metabolism, or excretion of drugs; history of central nervous system (CNS) disorders; allergy or hypersensitivity to the study drugs; blood donation of more than 500 ml during the previous 3 months; participation in a clinical trial within 3 months before the study period; and pregnancy. Written informed consent was obtained from all volunteers prior to inclusion in the study protocol. The study was approved by the Ethics Committee of Benjamin Franklin Hospital, Free University of Berlin, Berlin, Germany.
Study design.
The study was performed in an open, randomized, six-period crossover design. Each volunteer received each drug once, followed by a 2-week washout period. The tablets were administered with 240 ml of water after an overnight fasting period of 12 h and contained the following dosages: 250 mg of ciprofloxacin (Bayer AG, Leverkusen, Germany), 400 mg of gatifloxacin (Grünenthal GmbH, Aachen, Germany), 600 mg of grepafloxacin (Glaxo Wellcome, Hamburg, Germany), 500 mg of levofloxacin (Hoechst Marion Roussel GmbH, Bad Soden am Taunus, Germany), 400 mg of moxifloxacin (Bayer AG), and 200 mg of trovafloxacin (Pfizer GmbH, Karlsruhe, Germany). These dosages were based on the most frequent and recommended oral dosages for outpatient treatment.
Strenuous physical activity, smoking, alcohol, and stimulating beverages containing xanthine derivatives (tea, coffee, and soft drinks containing caffeine) were prohibited from 24 h before until 48 h after drug administration.
ECG.
ECG for controlling the Q-T interval was done before and 12 h after each medication.
Side effects.
Side effects were noted by spontaneous reporting and by questioning during examinations 12, 24, and 48 h after medication.
Sampling.
Blood samples were collected before and 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 36, and 48 h after medication through an indwelling cannula. Five milliliters of blood was taken for high-performance liquid chromatography (HPLC) analyses of all fluoroquinolones. After blood collection to obtain serum, the samples were stored at room temperature for about 30 min. All samples were centrifuged for 10 min at 1,000 × g and 21°C to separate the serum, which was then shock frozen at −20°C.
Urine samples were collected predose and at 0 to 3, 3 to 6, 6 to 12, 12 to 24, and 24 to 48 h after medication. Urine volumes were measured at these intervals, and two 5-ml aliquots for HPLC measurements were transferred to sterile tubes and shock frozen at −20°C. All samples (serum and urine) were protected against light during processing and storage.
HPLC.
Concentrations of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin, and trovafloxacin in serum and urine were determined by a validated HPLC method involving protein precipitation. For the analysis of gatifloxacin, grepafloxacin, and moxifloxacin, trovafloxacin was added as an internal standard. The extract was then chromatographed and quantified by fluorimetric detection. Urine samples were diluted only for the mobile phase. Details of the analysis of ciprofloxacin, levofloxacin, and trovafloxacin have been described previously (4, 5, 7). HPLC measurements for gatifloxacin, moxifloxacin, and grepafloxacin were similar to those for ciprofloxacin. Each method was validated. The detection limits were between 0.02 and 0.06 mg/liter in serum and 0.99 and 9.0 mg/liter in urine. The limits of quantitation ranged between 0.03 and 0.11 mg/liter in serum and 1.5 and 18.1 mg/liter in urine. Levels of precision within series were between 1.0 to 2.2% and 5.4 to 7.9% for serum and 1.1 to 2.0% and 2.2 to 4.8% for urine. Levels of precision between series ranged from 4.1 to 5.2% and 1.6 to 12.8% in serum and from 1.7 to 2.9% and 5.0 to 11.2% in urine.
Pharmacokinetic analysis.
The serum and urine data were analyzed with an open two-compartment model (peak concentration [Cmax], time to peak concentration [Tmax], time between drug administration and beginning of absorption [Tlag], and terminal half-life) chosen according to the Schwarz criterion (12, 13, 15). All other parameters were analyzed noncompartmentally (total areas under the curve [AUCtot], volume of distribution at steady state [Vss], mean residence time [MRT], and urinary recovery). The dose-dependent parameters (AUCtot and Cmax) were based on a dose per 70 kg of body weight. The main pharmacokinetic parameters (Cmax and AUCtot) of the different fluoroquinolones were additionally calculated for an average dose of 200 mg per 70 kg.
The calculations were elaborated using REVOL (14) for nonlinear regression analysis and Microsoft Excel for pharmacokinetic analysis.
Statistical analysis.
All fluoroquinolones were compared by variance analysis with the Student-Newman-Keuls procedure for multiple comparisons of sample means. P values of <0.05 were considered significant; P values of <0.01 were considered highly significant.
RESULTS
Pharmacokinetics.
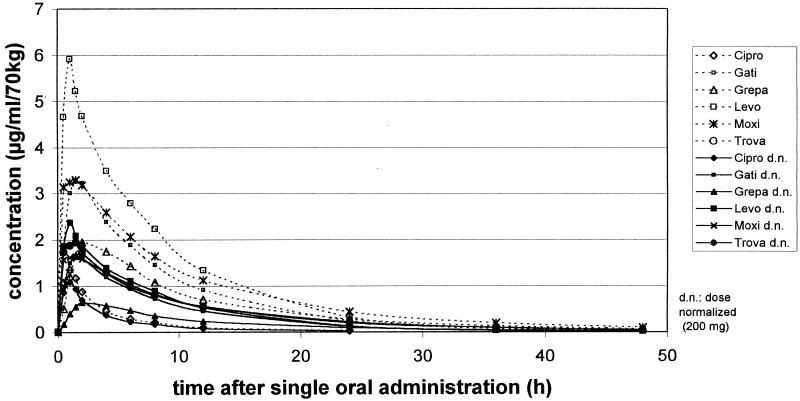
The pharmacokinetic data for all six fluoroquinolones, determined by HPLC, are shown in Fig. 1 and listed in Table 1. Levofloxacin showed the highest Cmax, 6.1 ± 1.3 μg/ml/70 kg, at 0.8 ± 0.4 h (Tmax) after medication, followed by moxifloxacin (Cmax, 4.3 ± 1.6 μg/ml; Tmax, 1.0 ± 0.7 h) and gatifloxacin (Cmax, 3.4 ± 0.7 μg/ml; Tmax, 1.5 ± 0.6 h). The lowest concentration in serum was observed for ciprofloxacin, 1.5 ± 0.4 μg/ml, after 0.8 ± 0.3 h. The AUCtot for ciprofloxacin (5.8 ± 1.2 μg · h/ml) was also significantly lower than those for the newer fluoroquinolones (P < 0.01). The longest half-life was observed for grepafloxacin (12.1 ± 3.9 h), followed by trovafloxacin (10.3 ± 3.4 h). The Tlag ranged between 0.12 ± 0.16 h (trovafloxacin) and 0.34 ± 0.06 h (grepafloxacin). Total urinary recovery, defined as the percentage of the administered dose over 48 h, was highest for gatifloxacin (77% ± 6%) and levofloxacin (76% ± 12%) and lowest for trovafloxacin (9.3% ± 2.5%).
FIG. 1.
Mean concentrations in serum of all six fluoroquinolones calculated for the original doses and normalized doses. Cipro, ciprofloxacin; Gati, gatifloxacin; Grepa, grepafloxacin; Levo, levofloxacin; Moxi, moxifloxacin; Trova, trovafloxacin.
TABLE 1.
Pharmacokinetic parameters for six fluoroquinolones tested in 12 volunteers eacha
| Drug (mg) | Cmax (μg/ml/70 kg) | Cmax (μg/ml/70 kg), dose normalized | Tmax (h) | Tlag (h) | Half-life (h) | MRT (h) | AUCtot (μg · h/ml/70 kg) | AUCtot (μg · h/ml/70 kg), dose normalized | Total urinary recovery (% of dose) | Renal clearance (ml/min/1.73 m2) | Vss (liters/70 kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin (250) | 1.5 ± 0.43 | 1.2 ± 0.34 | 0.78 ± 0.33 | 0.27 ± 0.17 | 5.37 ± 0.82 | 5.8 ± 0.94 | 5.75 ± 1.25 | 4.6 ± 1.0 | 40.8 ± 7.48 | 266 ± 40.6 | 231 ± 61.8 |
| Gatifloxacin (400) | 3.42 ± 0.74* | 1.71 ± 0.37 | 1.49 ± 0.65 | 0.21 ± 0.19 | 6.52 ± 0.87 | 9.28 ± 1.12* | 30 ± 3.8* | 15 ± 1.9* | 76.9 ± 5.6 | 153 ± 21 | 110 ± 20 |
| Grepafloxacin (600) | 1.98 ± 0.52* | 0.66 ± 0.17 | 2.77 ± 1.11 | 0.34 ± 0.06 | 12.12 ± 3.92* | 14.15 ± 2.67* | 23.5 ± 4.73* | 7.85 ± 1.58* | 9.36 ± 2.91 | 35.2 ± 6.9 | 306 ± 54.3 |
| Levofloxacin (500) | 6.21 ± 1.34* | 2.48 ± 0.53* | 0.8 ± 0.38 | 0.33 ± 0.15 | 6.95 ± 0.81 | 8.65 ± 0.8** | 44.8 ± 4.4* | 17.9 ± 1.76* | 75.9 ± 11.6 | 124 ± 19.1 | 88 ± 9.92 |
| Moxifloxacin (400) | 4.34 ± 1.61* | 2.17 ± 0.81* | 1.02 ± 0.72 | 0.18 ± 0.16 | 9.15 ± 1.62* | 12.5 ± 2.03* | 39.3 ± 5.35* | 19.7 ± 2.67* | 19.9 ± 4.55 | 30.5 ± 6.18 | 122 ± 19.6 |
| Trovafloxacin (200) | 2.09 ± 0.58 | 2.09 ± 0.58* | 0.95 ± 0.61 | 0.12 ± 0.16 | 10.3 ± 3.4* | 13.2 ± 5.37* | 19.5 ± 3.1* | 19.5 ± 3.1* | 9.27 ± 2.47 | 14.2 ± 3.5 | 129 ± 58 |
Drugs were given orally. Data were determined by HPLC for original doses and for normalized doses (200 mg) and are reported as means and standard deviations. P values for results that were statistically significant compared to those for ciprofloxacin were <0.01 (*) and <0.05 (**).
A comparison of Cmax and AUCtot calculated for an average dose of 200 mg is given in Table 1; mean concentrations in serum are shown in Fig. 1. The highest Cmaxs (in micrograms per milliliter per 70 kg) for an average 200-mg dose were 2.5 ± 0.5 for levofloxacin, 2.2 ± 0.8 for moxifloxacin, and 2.1 ± 0.6 for trovafloxacin. The AUCtot (in microgram-hours per milliliter per 70 kg) for moxifloxacin (19.5 ± 2.7), trovafloxacin (19.5 ± 3.1), and levofloxacin (17.9 ± 1.8) were significantly higher (P < 0.01) than that for ciprofloxacin (4.6 ± 1.0).
Side effects.
The overall tolerance of the six fluoroquinolones was good. Most side effects were registered with trovafloxacin. Nine volunteers (six women and three men) complained of mild to moderate CNS effects (dizziness, headache, and lack of concentration), three complained of gastrointestinal side effects (nausea and flatulence), and one complained of dry nasal mucosa. The main side effects with the other fluoroquinolones (n = 9) were headache and gastrointestinal disturbances (flatulence and diarrhea). The side effects were mild to moderate, in no case severe, and no volunteer had to be withdrawn from the study. No changes in ECG (Q-T interval), clinical examination, or vital and laboratory parameters (clinical chemistry, hematology, and urinalysis) were observed.
DISCUSSION
Most of our pharmacokinetic results for all six fluoroquinolones were in reasonable concurrence with published studies (6–11, 14, 16–18), except in the following parameters. For ciprofloxacin we found a higher AUCtot (5.75 ± 1.25 versus 4.23 ± 1.1) (6), and for gatifloxacin we found a shorter half-life (6.52 ± 0.87 versus 8.6 ± 1.4 h) (14). In our study, grepafloxacin had a higher Cmax (1.98 ± 0.52 versus 1.41 ± 0.28 μg/ml) than was described earlier (9, 10). Our findings for moxifloxacin were similar to those of Wise et al. (18) but differed from those of Stass et al. (16). For trovafloxacin, we noted a remarkably lower AUCtot (19.5 ± 3.1 versus 30.4 ± 8.1 μg/ml) (11, 17); also, the Tmax was significantly shorter (0.95 ± 0.61 versus 2.3 ± 1.0 h) (17).
Compared to data for ciprofloxacin as a standard fluoroquinolone, the peak Cmaxs of gatifloxacin, levofloxacin, and moxifloxacin were significantly higher (P < 0.01) and the AUCtot for all newer fluoroquinolones were significantly higher (P < 0.01).
Based on an average dose of 200 mg, levofloxacin had the highest Cmax (2.48 ± 0.534 μg/ml), followed by moxifloxacin (2.17 ± 0.805 μg/ml) and trovafloxacin (2.09 ± 0.58 μg/ml), but the highest AUCtot were found for moxifloxacin (19.7 ± 2.67 μg · h/ml/70 kg) and trovafloxacin (19.5 ± 3.1 μg · h/ml/70 kg). The Cmaxs of levofloxacin, moxifloxacin, and trovafloxacin were significantly higher than that of ciprofloxacin (P < 0.01), and the AUCtot for all newer fluoroquinolones were significantly higher (P < 0.01).
As is well known, longer elimination half-lives afford once-daily dosing of trovafloxacin, moxifloxacin, grepafloxacin, and gatifloxacin while maintaining levels in serum above the MICs for dominant pathogens. The urinary recovery of the unchanged drug (percentage of the administered dose) ranged between 9.27% ± 2.47% for trovafloxacin and 76.9% ± 5.6% for gatifloxacin, depending on the different elimination routes (renal versus hepatic routes).
The overall tolerance of the fluoroquinolones administered in our study was good. Most of the adverse effects were observed for trovafloxacin (mainly affecting the CNS). No volunteer had to be withdrawn from the study. No changes in vital signs, clinical chemistry, hematology, or urinalysis were observed in relation to the study medication. Also, no changes in Q-T interval or other changes in ECG were noted after a single oral administration of each of the six fluoroquinolones.
In conclusion, our results demonstrate that the newer fluoroquinolones gatifloxacin, moxifloxacin, grepafloxacin, trovafloxacin, and levofloxacin offer more favorable pharmacokinetic parameters (significantly higher AUCtot and Cmaxs and longer elimination half-lives) than the standard fluoroquinolone ciprofloxacin.
ACKNOWLEDGMENTS
The excellent technical assistance of H. Hartwig, E. Borner, M. Rau, and G. Schreiber is gratefully acknowledged.
REFERENCES
- 1.Andriole V T. The quinolones: prospects. In: Andriole T, editor. The quinolones. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1998. pp. 417–429. [Google Scholar]
- 2.Bergan T. Pharmacokinetics of the fluoroquinolones. In: Andriole T, editor. The quinolones. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1998. pp. 143–182. [Google Scholar]
- 3.Borner K, Hartwig H, Lode H. Determination of trovafloxacin in human body fluids by high-performance liquid chromatography. J Chromatogr A. 1999;846:175–180. doi: 10.1016/s0021-9673(99)00247-2. [DOI] [PubMed] [Google Scholar]
- 4.Borner K, Lode H, Höffken G, Prinzing C, Glatzel P, Wiley R. Liquid chromatographic determination of ciprofloxacin and some metabolites in human body fluids. J Clin Chem Clin Biochem. 1986;24:325–331. doi: 10.1515/cclm.1986.24.5.325. [DOI] [PubMed] [Google Scholar]
- 5.Borner K, Hartwig H, Lode H, Höffken G. Chromatographische Bestimmung von Ofloxacin in Körperflüssigkeiten. Fresenius' Z Anal Chem. 1986;324:355. [Google Scholar]
- 6.Borner K, Höffken G, Lode H, Koeppe P, Prinzing C, Glatzel P, Wiley R, Olschewski P, Sievers B, Reinitz D. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986;5:179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- 7.Chien S C, Chow A T, Natarajan J, Williams R R, Wong F A, Rogge M C, Nayak R K. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:1562–1565. doi: 10.1128/aac.41.7.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Child J, Andrews J M, Wise R. Pharmacokinetics and tissue penetration of the new fluoroquinolone grepafloxacin. Antimicrob Agents Chemother. 1995;39:513–515. doi: 10.1128/aac.39.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efthymiopiulos C. Pharmacokinetics of grepafloxacin. J Antimicrob Chemother. 1997;40(Suppl. A):35–42. doi: 10.1093/jac/40.suppl_1.35. [DOI] [PubMed] [Google Scholar]
- 11.Haria M, Lamb H M. Trovafloxacin. Drugs. 1997;54:435–445. doi: 10.2165/00003495-199754030-00006. [DOI] [PubMed] [Google Scholar]
- 12.Koeppe P, Hamann C M. REVOL—non-linear regression based on strategy of evolution. EDV Med Biol. 1978;9:112–117. [Google Scholar]
- 13.Koeppe P. New regression function for absorption kinetics. Drug Res. 1988;38:1375–1377. [PubMed] [Google Scholar]
- 14.Lober S, Ziege S, Rau M, Schreiber G, Mignot A, Koeppe P, Lode H. Pharmacokinetics of gatifloxacin and interaction with an antacid containing aluminium and magnesium. Antimicrob Agents Chemother. 1999;43:1067–1071. doi: 10.1128/aac.43.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;2:461–464. [Google Scholar]
- 16.Stass H, Dahlhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxyquinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng R, Dogolo L C, Willavize S A, Friedman H L, Vincent J. Oral bioavailability of trovafloxacin with and without food in healthy volunteers. J Antimicrob Chemother. 1997;39(Suppl. B):87–92. doi: 10.1093/jac/39.suppl_2.87. [DOI] [PubMed] [Google Scholar]
- 18.Wise R, Andrews J M, Marshall G, Hartman G. Pharmacokinetics and inflammatory fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother. 1999;43:1508–1510. doi: 10.1128/aac.43.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]